Abstract
Background
Longitudinal studies of illness progression in Major Depressive Disorder (MDD) indicate that the onset of subsequent depressive episodes becomes increasingly decoupled from external stressors. A possible mechanism underlying this phenomenon is that multiple episodes induce long-lasting neurobiological changes that confer increased risk for recurrence. Prior morphometric studies have frequently reported volumetric reductions in MDD—especially in medial prefrontal cortex (mPFC) and the hippocampus— but few studies have investigated whether these changes are exacerbated by prior episodes.
Methods
We used structural magnetic resonance imaging (sMRI) to examine relationships between number of prior episodes, current stress, and brain volume and cortical thickness in a sample of 103 medication-free depressed patients and never-depressed controls. Volumetric analyses of the hippocampus were performed using a recently-validated subfield segmentation approach, while cortical thickness estimates were obtained using Vertex-Based Cortical Thickness (VBCT). Participants were grouped on the basis of the number of prior depressive episodes as well as current depressive state.
Results
Number of prior episodes was associated with both lower reported stress levels as well as reduced volume in the dentate gyrus. Cortical thinning of the left medial prefrontal cortex (mPFC) was associated with a greater number of prior depressive episodes, but not current depressive state.
Conclusions
Collectively, these findings are consistent with preclinical models suggesting that the dentate gyrus and mPFC are especially vulnerable to stress exposure, and provide evidence for morphometric changes that are consistent with stress-sensitization models of recurrence in MDD.
Keywords: MRI, Major Depression, Hippocampus, mPFC, Dentate Gyrus, MAGeT Brain
INTRODUCTION
Major Depressive Disorder (MDD) is a debilitating disease that affects over 20 million Americans every year (1), drains billions of dollars from the economy (2), and has recently become the second leading cause of disability worldwide (3). A substantial portion of these staggering societal costs is attributable to the episodic course of the disorder; whereas individuals with one prior episode have a 60% chance of a recurrence, the likelihood of an additional episode after 3-4 episodes hovers around 90% (4, 5). Consequently, understanding the mechanisms that underlie the development of subsequent major depressive episodes (MDEs) is crucial for alleviating the impact of this devastating disorder on public health.
Over the last several decades, accruing evidence suggests that while stressful life events play a central role in triggering the onset of an initial MDE, their role in episode onset progressively diminishes as the number of episodes increases (6, 7). Thus, in several large-sample prospective studies, individuals who developed a first depressive episode over the study period reported significantly higher levels of chronic stress as compared to those who experienced an MDE recurrence (8-10). Along similar lines, epidemiological research has shown that the predictive validity of reported stress-levels prior to MDE onset declines monotonically with each successive episode (9, 11-13).
These findings raise the possibility that MDD illness progression is linked to specific biological changes that may mediate the interplay between external stressors and recurrence. One candidate mechanism is structural abnormalities within the medial prefrontal cortex (mPFC) and the hippocampus. These regions are known to regulate behavioral and neuroendocrine responses to stress, and can be damaged by excessive exposure to stress-induced release of steroidal and inflammatory signaling molecules (11-13). In depressed patients, numerous Magnetic Resonance Imaging (MRI) studies and meta-analyses have found evidence for diminished grey matter volume in aspects of mPFC, including rostral and dorsal subdivisions of the anterior cingulate cortex (ACC) as well as subgenual and subcallosal cortex, and limbic regions such as the hippocampus and amygdala (14-17). Post-mortem studies also show evidence for structural alterations in these regions, including decreased cellular density (18-20) and reduced expression of critical proteins involved in neurogenesis and synaptic plasticity (24-26). Further implicating these areas, similar structural differences were reported in a large sample of never-depressed individuals with a high polygenic risk score for MDD, suggesting that these differences may partly reflect a biological diathesis for MDD (21).
While such effects are generally present on the aggregate level, it is unclear whether they relate to the mere presence of a depressive state, a biological diathesis, or an accumulation effect of prior depressive episodes. Prior cross-sectional and longitudinal studies have suggested that volumetric changes associated with MDD fluctuate with state (22, 23), but also depend on prior number of episodes (24-27). However, the relative contribution of state and depressive history remains unclear, partly due to a prior emphasis on group comparisons rather than dimensional approaches (28, 29).
Consequently, the goal of the current study was to evaluate differences in brain morphology and current stress levels across never-depressed and currently depressed individuals with varying numbers of prior MDEs. This approach is particularly relevant for understanding the biological mechanisms underlying the relationship between stress and recurrence; in particular, if stress-induced abnormalities in specific brain regions mediate the increased risk for subsequent depressive episodes, individuals with more past depressive episodes should exhibit greater structural deficits as well as diminished levels of perceived stress.
To address these questions, we analyzed structural MRI images of 103 depressed and never-depressed individuals using whole-brain Vertex-Based Cortical Thickness (VBCT) and a recently-developed methodology for high-quality segmentation of hippocampal subfields (30, 31). To test for the specificity of associations with hippocampal subfields, we also examined amygdala volume, which has been implicated in MDD (32) and is generally correlated with hippocampal volume (21, 33). Our primary hypotheses were that 1) current stress levels would be greatest in individuals reporting few depressive episodes relative to controls and individuals with a high number of episodes, and 2) the number of episodes would be associated with progressive reductions of cortical and limbic areas known to be vulnerable to stress (i.e., the mPFC and hippocampus).
METHODS
Participants
A full description of sample characteristics is provided Table 1. A total of 103 participants were included in this study, including 51 healthy controls (49% female) and 52 unmedicated subjects with a current diagnosis of MDD (54% female). There were no differences between the currently depressed MDD subjects and never depressed controls in terms of age (t101 = −1.55, p = 0.13), sex (χ2(1) = 0.24, p = 0.62), % Caucasian (χ2(1) = 0.027, p = 0.87), years of education (t100 = 0.62, p = 0.54), employment status (χ2(1) = 5.5, p = 0.14) or marital status (χ2(1) = 5.5, p = 0.14). MDD subjects were recruited through a combination of ongoing treatment studies and community recruitment. Healthy controls were recruited from the community. For all subjects, exclusion criteria included any history of bipolar disorder, ADHD, psychosis, or substance dependence. Additionally, subjects were excluded if they had any evidence of substance abuse within the last year. Subjects were additionally excluded if they had any condition that would interfere with an MRI scan (e.g., claustrophobia, cochlear implant, cardiac pacemaker). Controls were additionally required to be free of any current or past history of Axis I disorders. Depressed patients were required to meet full criteria for current MDD as assessed by a Structured Clinical Interview (SCID; (34)) as well as present a score of 16 or higher on the 21-item Hamilton Depression Rating Scale (HDRS; (35)) at time of initial intake. Additionally, MDD subjects were required to be free of any use of psychotropic medications for at least two weeks (6 weeks for fluoxetine; 6 months for dopaminergic drugs or neuroleptics) before the scan. All procedures were reviewed and approved by the Committee on the Use of Human Subjects at Harvard University and the Partners Human Research Committee IRB, and all participants provided written informed consent.
Table 1. Sample Demographics.
| Healthy Controls (N = 51) |
MDD (N = 52) |
||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p-value | |
|
|
|
|
|||
| % Female | 49% | - | 54% | - | 0.62 |
| Age | 36.8 | 14.1 | 40.9 | 12.8 | 0.13 |
| % Caucasian | 74% | - | 73% | - | 0.87 |
| Years of Education | 15.6 | 2.1 | 15.3 | 2.2 | 0.54 |
| % Unemployed | 26% | - | 45% | - | 0.14 |
| BDI-II | 2.5 | 3.2 | 25.0 | 10.5 | < 0.0001 |
| HDRS (17-item) | - | - | 18.0 | 4.0 | - |
| Number of Episodes | - | - | 3.6 | 3.3 | - |
| N with Comorbid Conditions | |||||
| Panic Disorder | - | - | 1 | - | - |
| Generalized Anxiety | - | - | 1 | - | - |
| Social Phobia | - | - | 1 | - | - |
| Specific Phobia | - | - | 2 | - | - |
| OCD | - | - | 1 | - | - |
| Body-Dysmorphic | - | - | 1 | - | - |
Measure of Recent Stress
To assess recent levels of stress, all subjects were administered the Perceived Stress Scale (PSS). The PSS is a brief self-report measure that has been well-validated as a measure of the perceived intensity and tolerability of daily-life stressors over the previous month (36). The PSS includes items that ask subjects to rate the perceived predictability and controllability of these stressors, as well has how overwhelmed they felt. Examples items include: “In the last month, how often have you felt that you were unable to control the important things in your life?” or “In the last month, how often have you found that you could not cope with all the things that you had to do?”. Participants rated their response to each item using a 0-4 scale where 0 is defined as “never” and 4 is defined as “very often”. Total scores for each subject were then generated by summing across the total number of items, resulting in a total range of 0-56.
Number of Prior Major Depressive Episodes (MDEs)
During the clinical interview, all MDD subjects reported the number of episodes they had previously experienced, which ranged from 1 to 15 prior episodes (including the current episode). As the distribution of the number of episodes was skewed to the right, the MDD sample was divided into groups of individuals with one episode (n=21), 2-4 episodes (n=12) and 5 or more episodes (n = 21). This variable was then used as a predictor of structural changes across all subjects (i.e., including controls), and ranged from 0 (healthy controls) to 3 (MDD subjects with ≥5 MDEs). As an alternative approach to normalizing the number of episodes variable, we also used a logarithmic transform. This produced a variable that was highly correlated with the sub-group approach (r = 0.98). However, the grouping approach is preferable, as it is less sensitive to variability in retrospective report, which can be subject to bias.
Procedure
All subjects were recruited via advertising within the community. When subjects responded to ads, a trained research assistant administered a phone screening to assess the presence of general inclusion/exclusion criteria. Subjects deemed eligible were scheduled for an initial clinical assessment session, during which the SCID was administered by a certified master’s level clinician or psychiatrist, and self-report questionnaires were completed. Subjects meeting study inclusion returned for a second session, which included an MRI scan. During the MRI, structural and functional scans were acquired.
MRI Data Acquisition
Imaging data were acquired using a 1.5 Tesla Symphony/Sonata scanner (Siemens Medical Systems, Iselin, N.J.). For the purposes of morphometric analysis, a T1-weighted MPRAGE image was acquired with the following parameters: TR = 2730 ms; TE = 3.39 ms; FOV = 256 mm; voxel size = 1×1×1.33; 128 slices.
Hippocampal Subfields/Amygdala Segmentation
Hippocampal and amygdala segmentations of MR data were performed using the Multiple Automatically Generated Templates for different Brains (MAGeT Brain), a recently published modified multi-atlas algorithm (30, 37, 38). In more traditional multi-atlas segmentation algorithms, an atlas library is used in order to obtain several representations of the underlying neuroanatomy of interest. Typically these libraries contain between 20-80 atlases that have been laboriously manually delineated by neuroanatomical experts (39-41). These methods are limited, however, by the specific demographics of the atlas library at hand, and may be difficult to adapt to new datasets (for example, using a library of young healthy controls to segment a population suffering from a neurodegenerative disorder). Further, these methods are not easily used with atlases that are somehow unique or time consuming to develop (such as atlases derived from reconstructed serial histological data (42) or high-resolution magnetic resonance imaging data) (30). Instead of utilizing multiple input atlases, MAGeT Brain uses the variability inherent in any dataset in order to limit the number of manually labeled atlases required as input (37, 38). The process starts by using 5 high-resolution atlases of the hippocampus, the hippocampal subfields, and the amygdala as inputs. A subset of the dataset to be segmented is then taken and used as a “template library”. For the purpose of the work presented here 10 controls and 11 MDD subjects were used in the template library. Each of the manually labeled atlases is then nonlinearly warped to each subject in the template library, yielding 5 different possible labels for the different neuroanatomical structures. Each subject to be segmented is then nonlinearly warped to each of the subjects in the template library, and each of the 5 labels from each subject’s template library is warped to fit each subject. This yields a total of 105 candidate labels for each subject that are then fused using a “majority-vote” by taking the most frequently occurring label at every voxel (38). This algorithm has been shown to have limited proportional bias in its estimation of hippocampal volume and subfield segmentations for MRI data acquired at 3T were also shown to be accurate.
To this end, five high-resolution atlases of the hippocampus and its subfields were used as input for the automated segmentation (30). The amygdala was manually segmented in the same five high-resolution T1-weighted images following a previously established protocol for manual segmentation of the amygdala (43). All segmentations were checked visually by a trained observer (MTMP) prior to analysis, based on fifteen representative slices encompassing the individual segmentations (Figure 2). After strict quality control, 99 subjects remained for hippocampal subfield analysis. For purposes of methodological comparison, the relationships between hippocampal volume estimates produced by MAGeT and those generated through standard FreeSurfer sub-cortical volume segmentation (see below) are also reported.
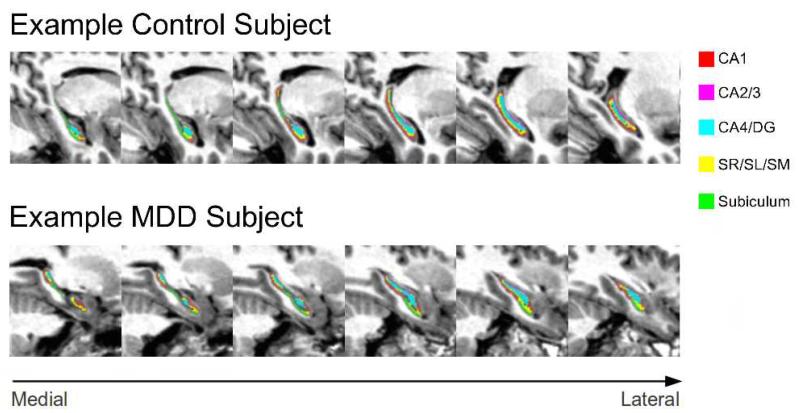
Figure 2.
Examples of representative hippocampal subfield segmentations for MDD and Control subjects.
Group-Level Analysis of Hippocampal Sub-field and Amygdalar Volumes
For the remaining 99 subjects, extracted estimates of hippocampal volume for each sub-field were analyzed using linear mixed-effect models with hemisphere as the repeated variable and age, sex and total intracranial volume included as additional covariates. All linear mixed-effects model analyses were performed using SPSS 21 (IBM, Armok, NY).
Vertex-Based Cortical Thickness (VBCT)
VBCT was estimated using FreeSurfer with a processing stream that has previously been described in detail (44). Briefly, the T1-weighted image was preprocessed and segmented to separate cortical grey matter from white matter and subcortical structures. The white-gray boundary was then tessellated to form a triangular mesh defining the cortical surface. This mesh was then deformed following intensity gradients to optimally locate the white-gray and gray-pial surfaces, and cortical thickness was defined as the shortest distance between the two surfaces at each vertex (45). Additionally, the local curvature of the gray-white surface was calculated and used to drive a nonlinear registration to a common template, which aligned the VBCT maps across subjects for the group analysis (46). The outputs of this automated workflow were visually inspected, and any defects were manually corrected. Consistent with other cortical thickness studies in psychiatric populations (e.g. (41, 42)), the VBCT maps were smoothed along the cortical surface with an approximate 15mm FWHM Gaussian kernel to account for anatomical variability and improve the normality of error distributions. A mass-univariate random effects multiple regression was then performed on the resulting maps with an additive model that included Number of Episodes as a regressor of interest while controlling for age and sex. All 103 subjects were included. Clusters were formed with an uncorrected height threshold of p < 0.05, and correction for multiple comparisons was achieved by using a Monte Carlo simulation of the cluster size distribution under the null hypothesis to threshold the resulting clusters at p < 0.05, corrected (47).
RESULTS
Relationships between reported current stress and number of depressive episodes
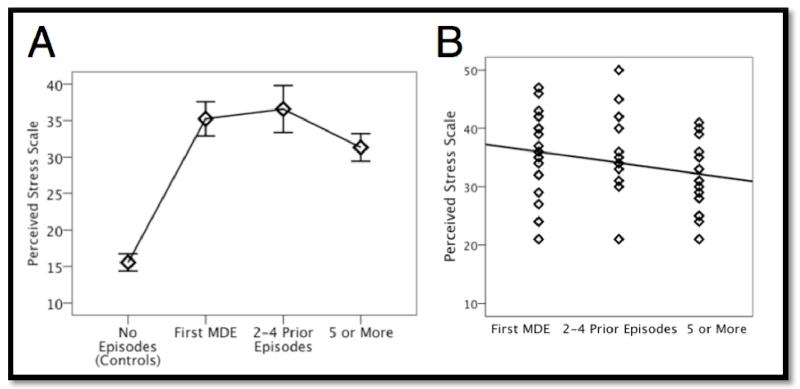
PSS data were unavailable for one control and two MDD subjects. MDD patients reported significantly higher PSS scores (M = 34.2, SD = 7.2) as compared to controls (M = 15.6, SD = 6.0) (t98 = −14.10, p < 0.001). As would be predicted by the stress-sensitization model, as the number of depressive episodes increased, PSS scores began to decline, creating an inverted U shape curve across the entire sample. When comparing linear vs. quadratic fits across the sample, the R2 of the model including a quadratic term (R2 = 0.68, p < 0.001) was stronger than that of the linear model (R2 = 0.41, p < 0.001) (Fig 1a). Importantly, when assessing the MDD group alone, the number of episodes regressor showed a significant inverse relationship to perceived stress (b = −0.24, p < 0.05 (one-tailed)), indicating that increasing number of prior depressive episodes was associated with decreased PSS scores (Fig 1b). Of note, the number of episodes was not associated with differences in average BDI scores (F(2, 48) = 1.57, p = 0.22)
Figure 1.
Linear and quadratic relationship between recent stress levels and number of prior episodes. A. Across all subjects, a quadratic model had a significantly better fit (R2 = 0.68, p < 0.001) than the linear model (R2 = 0.41, p <0.001). Error bars represent ±95% confidence interval. B. Within currently depressed patients, PSS showed a significant inverse relationship to with number of episodes (b = −0.24, p < 0.05 (one-tailed)).
Relationships between hippocampal subfield volume and number of depressive episodes
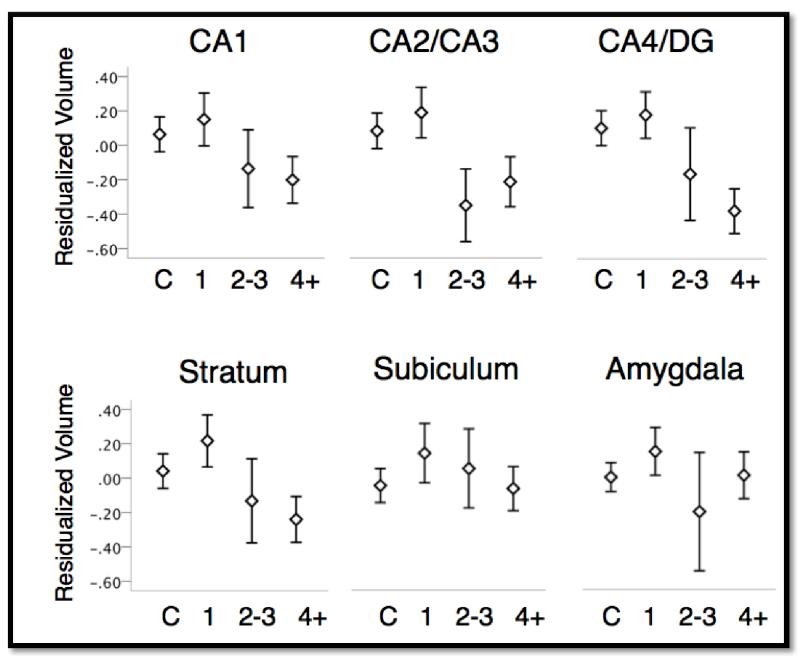
Full results of hippocampal volume in relationship to number of episodes across all subjects (i.e., including controls), as well as within the MDD group alone are reported in Table 2. Whole hippocampus volume showed general agreement across the subfield-segmentation and standard FreeSurfer segmentation for both hemispheres (left: r = 0.857, p < .001; right: r = 0.860, p < 0.001). Across all participants, only the dentate gyrus was associated with a significant reduction in volume as the number of episodes increased (b = −8.13, p = 0.011), though area CA2/CA3 exhibited trend-level significance (b = −2.65, p = 0.054). Within the MDD group alone, however, all five sub-regions showed significant declines in volume as a function of multiple episodes, with the strongest effects in the dentate gyrus and stratum (both p’s < 0.0005). The significance of these within-group effects was unchanged when BDI or PSS scores were controlled for, and there were no subfields that showed a significant interaction between BDI scores and number of episodes (all p’s > 0.28). Finally, we also tested for interactions with gender and number of episodes, but found no evidence of a significant interaction for any subfield (all p’s > 0.20).
Table 2. Results from Linear Mixed Models Analysis of Effects of Number of Episodes on Hippocampal Subfield Volume.
All models include, sex, age and TBV as covariates. Model results shown for each subfield as examined across all subjects and within MDD subjects
| Model Tested |
Beta (Unstandardized) |
SE |
p-value |
|---|---|---|---|
| Number of Episodes (All Subjects) | |||
| CA1 | −6.31 | 4.56 | 0.167 |
| CA2-3 | −2.65 | 1.36 | 0.054 |
| CA4/Dentate Gyrus* | −8.13 | 3.15 | 0.011 |
| Stratum | −5.25 | 3.74 | 0.162 |
| Subiculum | 0.38 | 2.71 | 0.887 |
| Whole Hippocampus | −22.36 | 13.09 | 0.089 |
| Number of Episodes (MDD Only) | |||
| CA1 ** | −27.81 | 8.07 | 0.00086 |
| CA2-3* | −6.11 | 2.59 | 0.02028 |
| CA4/Dentate Gyrus*** | −23.19 | 5.74 | 0.00011 |
| Stratum*** | −25.64 | 6.67 | 0.00023 |
| Subiculum* | −12.69 | 5.14 | 0.01534 |
| Whole Hippocampus*** | −95.72 | 22.61 | 0.00006 |
p < 0.05
p < 0.005
p <.0005
Upon further examination of the data, we observed a general pattern across sub-field volume such that first-episode MDD subjects typically exhibited slightly enlarged hippocampal volumes as compared to controls. This pattern was present across all regions. To test whether this represented a significant increase in volume, we repeated the above analyses while restricting our sample to healthy controls and first-episode MDD patients. No subfields showed a significant difference (all p’s >0.41).
Amygdala Volume Analysis
Like the hippocampus, volumetric changes in the amygdala have also been implicated in depression (32), making the amygdala a useful control region for examination of the specificity of the association between repeated episodes and hippocampal subfield volume. For both groups, amygdala and hippocampal volumes were highly correlated (Controls: r = 0.80, p < 0.001; MDD: r = 0.72, p < 0.001). Across all subjects, however, we did not observe any association with number of episodes and amygdala volume (b = −1.09, p = 0.86), nor did we observe any association within the MDD group alone (b = −20.09, p = 0.14). This was unchanged when BDI and PSS scores were controlled for. Additionally, we observed no significant difference between controls and first-episode MDD patients (b = 10.80, p = 0.49).
Whole-Brain VBCT Analysis
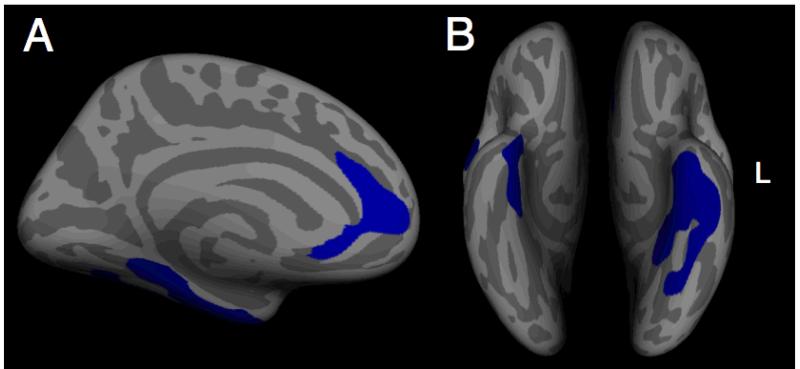
For cortical thickness, the number of prior episodes was associated with significant decreases in left medial prefrontal cortex, including aspects of Broadmann’s areas 24 and 25, bilateral parahippocampal gyrus, and bilateral portions of motor and premotor cortex (Fig 4 A) (Table 2). No other regions showed a significant negative association with prior depressive episodes, and there were no regions characterized by increased cortical thickness as a function of numbers of MDEs. These results were unchanged when controlling for both depression symptom severity as assessed by the BDI or perceived stress as measured by the PSS. Neither the BDI nor the PSS showed any significant associated with cortical thickness. Additionally, no region showed a significant interaction between gender and number of episodes.
Figure 4.
Areas showing an association between cortical thickness and number of depressive episodes across all subjects, cluster-corrected. Regions shown include the left medial prefrontal cortex (A) as well as bilateral parahippocampal gyrus and medial temporal cortex (B).
DISCUSSION
The overarching goal of the present study was to evaluate changes in grey matter morphometry as a function of illness progression in MDD. Our findings are broadly consonant with sensitization models of recurrence. As expected, reported perceived stress levels were lower in individuals with multiple episodes as compared to first episode patients, though still higher than never-depressed controls. Moreover, we observed that number of prior MDEs was a strong predictor of structural changes in two key brain areas associated with both depression and stress: the hippocampus and mPFC.
The identification of both hippocampal and medial prefrontal regions as showing a relationship to number of episodes is consistent with both theoretical models and preclinical evidence relating stress with structural microdamage in these areas. Both regions express high numbers of glucocorticoid receptors, which are believed to play a critical role in mediating negative-feedback regulation of glucocorticoid release during stress (48, 49). In animal models, chronic stress exposure as well as local corticosteroid injections produce structural alterations in these regions, including de-arborization and loss of dendritic spines (50-53). This stress-induced microdamage has been linked to behavioral changes that mimic aspects of a depressive state, including impaired working memory, decision-making and goal-directed behavior (54-56). In humans, similar relationships have been observed among stress, cortisol, glutamate pathways and grey-matter volume in these regions in both depressed and non-depressed samples (57-61).
Prior studies have indicated that hippocampal volume is sensitive to course of illness in MDD, with initial reports suggesting that volumetric deficits in the hippocampus were inversely related to both number of episodes (25) and duration of untreated illness (24). Further research confirmed the sensitivity of this structure to clinical course, with evidence that reduced hippocampal volumes were partially remediated by antidepressant treatment (22, 26, 62), as well as a remitted state without treatment (22). These past studies have not, however, examined the relationship between number of prior episodes and subfields within the hippocampus. While our analysis of hippocampal subfields suggested that number of prior episodes was broadly associated with reduced volumes among currently depressed patients, it is notable that the strongest effects for both within and between group analyses were found in the dentate gyrus. This region is believed to be the primary site of newly-developing cells (63), which may render it especially vulnerable to the noxious effects of glucocorticoids and inflammation (13, 64). Damage to this region may underlie well-documented impairments in memory functioning in MDD (23, 65, 66), which have also been strongly linked to number of prior episodes (67). Indeed, a recent study found that hippocampal subfield volume—especially in the dentate gyrus—were correlated with memory performance in healthy older adults (68).
Whole-brain VBCT analysis revealed an association with the number of episodes and decreased cortical thickness in the left mPFC, including aspects of rostral and subgenual anterior cingulate, as well as reductions in bilateral parahippocampal gyrus and surrounding temporal cortex. The mPFC is of particular interest given its key role in mediating adaptive vs. “learned helpless” responses to stress (69). In particular, de-activation of medial prefrontal projections to key midbrain monoaminergic nuclei can result in learned helplessness behavior following stress exposure in rodents (70, 71). Similarly in humans, function and structure of this region has consistently been related to regulation of negative affect (72-75). The laterality of this effect is also notable, given long-standing evidence for prefrontal hemisphereic differences in MDD, including a meta analysis showing asymmetry in the magnitude of volumetric reductions in left vs. right prefrontal cortex (15), reduced dACC white matter integrity (76), and hypo-recruitment of left prefrontal EEG signals (76-79).
Taken together, these results highlight structural damage to mPFC as being a critical factor in risk for recurrence. Such damage may occur as a consequence of prior MDEs, consistent with stress-sensitization models. Alternatively, naturally occurring variation in cortical thickness of mPFC may reflect a biological diathesis that confers risk towards multiple depressive episodes. Consistent with this latter interpretation, similar patterns of cortical thinning in mPFC have been observed in never-depressed individuals with elevated polygenic risk for MDD (21). Given the cross-sectional nature of our study, we are unable to speculate on the direction of causality. In either case, however, these findings isolate the structural integrity of the mPFC as a potential bulwark against MDE relapse, as individuals with reduced thickness in this region reported more prior episodes despite lower levels of recent stress.
The study has some limitations. First, our subjects were scanned on a 1.5T scanner, which has reduced sensitivity as compared to images acquired at higher field strengths. Second, samples sizes within the number of episodes categories were modest, with one cell as low as 12 participants, although the concern of low power is somewhat tempered by focus on linear trend analysis across all categories. Second, the cross-sectional nature of study limits our ability to fully characterize the fluctuations in structure that may occur as individuals move in and out of depressive episodes. Finally, we relied on retrospective report regarding the number of episodes. While this metric has been used in prior studies, retrospective reports can be subject to biases. We attempted to limit such biases by grouping the number of episodes into several categories, so as to minimize the effect of inaccurate recall; this approach also helped to normalize the distribution of scores.
Conclusions
In sum, this work provides important evidence for stress-sensitization models of illness progression in MDD, and points to pathophysiological correlates of the apparent decoupling between external stressors and subsequent episodes. These results suggest that stress-linked microdamage in mPFC may be a critical mechanism in this process, though the role of pre-morbid structural abnormalities cannot be ruled out. More generally, by providing a critical link between MDE history and animal models of structural degeneration, these findings help further our understanding of the pathophysiology of MDD. Finally, these results also have potential implications for treatment. In particular, they contribute to the growing literature suggesting that hippocampal volume may be a potential biomarker for depression (23). In addition, they highlight the dentate gyrus as a potential treatment target for novel compounds or cognitive retraining protocols that may help stimulate remediate volumetric reductions (68).
Figure 3.
Effects of number of episodes on volume of hippocampal subfields and amygdala (averaged across hemisphere). X-axis shows number of prior depressed episodes with “C” denoting never-depressed controls. Y-axis shows residualized volume after controlling for sex, age, and total brain volume. Error bars represent ± standard error of the mean.
Table 3. Results from Whole-Brain Analysis of Number of Episodes Effects on Cortical Thickness.
Sex and age are included as covariates.
| Talairach Coordinates | p-value (cluster) |
||||
|---|---|---|---|---|---|
| Region | x | y | z | z-score | |
|
|
|
|
|
||
| Effects of Prior MDEs (including controls) | |||||
| Right Pre-central Gyrus | 56 | 1 | 33 | −3.75 | 0.0001 |
| Left Middle Frontal Gyrus | −31 | 6 | 49 | −3.58 | 0.0024 |
| Left Parahippocampal Gyrus | −29 | −41 | −5 | −3.31 | 0.0001 |
| Right Parahippocampal Gyrus | 34 | −14 | −26 | −3.30 | 0.038 |
| Left Anterior Cingulate | −2 | 22 | 3.1 | −2.87 | 0.026 |
ACKNOWLEDGEMENTS
The authors are grateful to Elena L. Goetz, Jeffrey Birk, Sunny J. Dutra and Nancy Brooks Hall for their skilled assistance with this study. This study was supported by the W. Garfield Weston Foundation to MMC and the National Institute of Mental Health (NIMH) grant R01 MH068376 awarded to DAP as well as National Center for Complementary & Alternative Medicine (NCCAM) grant R21 AT002974 awarded to DAP and NCCAM R01 AT001638 awarded to MF. AJH and DGD were supported by grants K01 MH099232 and K99 MH094438, respectively.
Dr. Iosifescu has received over the past three years funding through Icahn School of Medicine at Mount Sinai from AstraZeneca, Brainsway, Euthymics, Neosync, Roche; and consulting fees from Avanir, CNS Response, Otsuka, Servier and Sunovion.
Dr. Fava has received research support from Abbot Laboratories, Alkermes Inc., American Cyanamid, Aspect Medical Systems, AstraZeneca, BioResearch, BrainCells Inc., Bristol-Myers Squib, CeNeRx BioPharma, Cephalon, Clintara LLC, Covance, Covidien, Eli Lilly and Company, EnVivo Pharmaceuticals Inc., Euthymics Bioscience Inc., Forest Pharmaceuticals Inc., Ganeden Biotec, Inc., GlaxoSmithKline, Harvard Clinical Research Institute, Hoffman-LaRoche, Icon Clinical Research, i3 Innovus/Ingenix, Janssen R&D LLC, Jed Foundation, Johnson & Johnson Pharmaceutical Research & Development, Lichtwer Pharma GmbH, Lorex Pharmaceuticals, MedAvante, National Alliance for Research on Schizophrenia & Depression (NARSAD), National Center for Complementary and Alternative Medicine (NCCAM), National Institute of Drug Abuse (NIDA), National Institute of Mental Healt (NIMH), Neuralstem Inc., Novartis AG, Organon Pharmaceuticals, PamLab LLC, Pfizer Inc., Pharmacia-Upjohn, Pharmaceutical Research Associates Inc., Pharmavite® LLC, PharmoRx Therapeutics, Photothera, Roche Pharmaceuticals, RCT Logic, LLC (formerly Clinical Trials Solutions, LLC), Sanofi-Aventis US LLC, Shire, Solvay Pharmaceuticals Inc., Synthelabo, Wyeth-Ayerst Laboratories. Advisory/consulting from Abbott Laboratories, Affectis Pharmaceuticals AG, Alkermes Inc., Amarin Pharma Inc., Aspect Medical Systems, AstraZeneca, Auspex Pharmaceuticals, Bayer AG, Best Practice Project Management Inc., BioMarin Pharmaceuticals Inc., Biovail Corporation, BrainCells Inc., Bristol-Myers Squibb, CeNeRx BioPharma, Cephalon Inc., Cerecor, Clinical Trials Solutions, CNS Response Inc., Compellis Pharmaceuticals, Cypress Pharmaceutical Inc., DiagnoSearch Life Sciences (P) Ltd., Dinippon Sumitomo Pharma Co. Inc., Dov Pharmaceuticals Inc., Edgemont Pharmaceuticals Inc., Eisai Inc., Eli Lilly and Company, EnVivo Pharmaceuticals Inc., ePharmaSolutions, EPIX Pharmaceuticals Inc., Euthymics Bioscience Inc., Fabre-Kramer Pharmaceuticals Inc., Forest Pharmaceuticals Inc., GenOmind LLC, GlaxoSmithKline, Grunenthal GmbH, i3 Innovus/Ingenis, Janssen Pharmaceutica, Jazz Pharmaceuticals Inc., Johnson & Johnson Pharmaceutical Research & Development LLC, Knoll Pharmaceuticals Corp., Labopharm Inc., Lorex Pharmaceuticals, Lundbeck Inc., MedAvante Inc., Merck & Co., Inc., MSI Methylation Sciences Inc., Naurex Inc., Neuralstem Inc., Neuronetics Inc., NextWave Pharmaceuticals, Novartis AG, Nutrition 21, Orexigen Therapeutics Inc., Organon Pharmaceuticals, Otsuka Pharmaceuticals, Pamlab LLC., Pfizer Inc., PharmaStar, Pharmavite® LLC., PharmoRx Therapeutics, Precision Human Biolaboratory, Prexa Pharmaceuticals Inc., Puretech Ventures, PsychoGenics, Psylin Neurosciences Inc., Rexahn Pharmaceuticals Inc., Ridge Diagnostics Inc., Roche, Sanofi-Aventis US LLC., Sepracor Inc., Servier Laboratories, Schering-Plough Corporation, Solvay Pharmaceuticals Inc., Somaxon Pharmaceuticals Inc., Somerset Pharmaceuticals Inc., Sunovion Pharmaceuticals, Supernus Pharmaceuticals Inc., Synthelabo, Takeda Pharmaceutical Company Limited, Tal Medical Inc., Tetragenex Pharmaceuticals Inc., TransForm Pharmaceuticals Inc., Transcept Pharmaceuticals Inc., Vanda Pharmaceuticals Inc. Speaking/publishing from Adamed Co, Advanced Meeting Partners, American Psychiatric Association, American Society of Clinical Psychopharmacology, AstraZeneca, Belvoir Media Group, Boehringer Ingelheim GmbH, Bristol-Myers Squibb, Cephalon Inc., CME Institute/Physicians Postgraduate Press Inc., Eli Lilly and Company, Forest Pharmaceuticals Inc., GlaxoSmithKline, Imedex LLC, MGH Psychiatry Academy/Primedia, MGH Psychiatry Academy/Reed Elsevier, Novartis AG, Organon Pharmaceuticals, Pfizer Inc., PharmaStar, United BioSource Corp., Wyeth-Ayerst Laboratories. Equity holdings in Compellis, PsyBrain Inc. Royalty/patent or other income from patent for Sequential Parallel Comparison Design (SPCD), which are licensed by MGH to RCT Logic, LLC, and patent application for a combination of Scopolamine and Ketamine in Major Depressive Disorder (MDD). Copyright for the MGH Cognitive & Physical Functioning Questionnaire (CPFQ), Sexual Functioning Inventory (SFI), Antidepressant Treatment Response Questionnaire (ATRQ), Discontinuation-Emergent Signs & Symptoms (DESS), and SAFER, Lippincott, Williams & Wilkins, Wolkers Kluwer, World Scientific Publishing Co. Pte. Ltd.
Dr. Pizzagalli has received over the past three years, honoraria/consulting fees from Advanced Neuro Technology North America, AstraZeneca, Ono Pharma USA, Servier, and Shire for activities unrelated to this project.
Footnotes
FINANCIAL DISCLOSURES
Drs. Treadway, Dillon, Holmes, Chakravarty, Polli, and Gabrieli, Mr. Waskom, Mr. Park and Ms. Dutra report no biomedical financial interests to disclose.
REFERENCES
- 1.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kessler RC. The costs of depression. Psychiatr Clin North Am. 2012;35:1–14. doi: 10.1016/j.psc.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10:e1001547. doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.APA Practice guideline for the treatment of patients with major depressive disorder (revision) Am J Psychiatry. 2000;157:1–45. [PubMed] [Google Scholar]
- 5.Monroe SM, Harkness KL. Recurrence in major depression: a conceptual analysis. Psychol Rev. 2011;118:655–674. doi: 10.1037/a0025190. [DOI] [PubMed] [Google Scholar]
- 6.Monroe SM, Harkness KL. Life stress, the “kindling” hypothesis, and the recurrence of depression: considerations from a life stress perspective. Psychol Rev. 2005;112:417–445. doi: 10.1037/0033-295X.112.2.417. [DOI] [PubMed] [Google Scholar]
- 7.Post RM. Transduction of Psychosocial Stress Into the Neurobiology. Am J Psychiatry. 1992;149:999–1010. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- 8.Farmer A, Harris T, Redman K, Sadler S, Mahmood A, McGUFFIN P. Cardiff Depression Study A sib-pair study of life events and familiality in major depression. The British Journal of Psychiatry. 2000;176:150–155. doi: 10.1192/bjp.176.2.150. [DOI] [PubMed] [Google Scholar]
- 9.Stroud CB, Davila J, Hammen C, Vrshek-Schallhorn S. Severe and nonsevere events in first onsets versus recurrences of depression: Evidence for stress sensitization. J Abnorm Psychol. 2011;120:142. doi: 10.1037/a0021659. [DOI] [PubMed] [Google Scholar]
- 10.Ormel J, Oldehinkel AJ, Brilman EI. The interplay and etiological continuity of neuroticism, difficulties, and life events in the etiology of major and subsyndromal, first and recurrent depressive episodes in later life. Am J Psychiatry. 2001;158:885–891. doi: 10.1176/appi.ajp.158.6.885. [DOI] [PubMed] [Google Scholar]
- 11.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 12.Sorrells SF, Sapolsky RM. An inflammatory review of glucocorticoid actions in the CNS. Brain Behav Immun. 2007;21:259–272. doi: 10.1016/j.bbi.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- 14.Kempton MJ, Salvador Z, Munafo MR, Geddes JR, Simmons A, Frangou S, et al. Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Arch Gen Psychiatry. 2011;68:675–690. doi: 10.1001/archgenpsychiatry.2011.60. [DOI] [PubMed] [Google Scholar]
- 15.Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: A meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp. 2009 doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bora E, Fornito A, Yucel M, Pantelis C. Voxelwise meta-analysis of gray matter abnormalities in bipolar disorder. Biol Psychiatry. 2010;67:1097–1105. doi: 10.1016/j.biopsych.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 17.Bora E, Harrison BJ, Davey CG, Yucel M, Pantelis C. Meta-analysis of volumetric abnormalities in cortico-striatal-pallidal-thalamic circuits in major depressive disorder. Psychol Med. 2011;42:671–681. doi: 10.1017/S0033291711001668. [DOI] [PubMed] [Google Scholar]
- 18.Chana G, Landau S, Beasley C, Everall IP, Cotter D. Two-dimensional assessment of cytoarchitecture in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia: evidence for decreased neuronal somal size and increased neuronal density. Biol Psychiatry. 2003;53:1086–1098. doi: 10.1016/s0006-3223(03)00114-8. [DOI] [PubMed] [Google Scholar]
- 19.Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry. 2001;58:545–553. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- 20.Monkul E, Hatch JP, Nicoletti MA, Spence S, Brambilla P, Lacerda AL, et al. Fronto-limbic brain structures in suicidal and non-suicidal female patients with major depressive disorder. Mol Psychiatry. 2006;12:360–366. doi: 10.1038/sj.mp.4001919. [DOI] [PubMed] [Google Scholar]
- 21.Holmes AJ, Lee PH, Hollinshead MO, Bakst L, Roffman JL, Smoller JW, et al. Individual differences in amygdala-medial prefrontal anatomy link negative affect, impaired social functioning, and polygenic depression risk. J Neurosci. 2012;32:18087–18100. doi: 10.1523/JNEUROSCI.2531-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnone D, McKie S, Elliott R, Juhasz G, Thomas EJ, Downey D, et al. State-dependent changes in hippocampal grey matter in depression. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.150. [DOI] [PubMed] [Google Scholar]
- 23.MacQueen G, Frodl T. The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research&quest. Mol Psychiatry. 2010 doi: 10.1038/mp.2010.80. [DOI] [PubMed] [Google Scholar]
- 24.Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proceedings of the National Academy of Sciences. 2003;100:1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frodl T, Jager M, Smajstrlova I, Born C, Bottlender R, Palladino T, et al. Effect of hippocampal and amygdala volumes on clinical outcomes in major depression: a 3-year prospective magnetic resonance imaging study. J Psychiatry Neurosci. 2008;33:423–430. [PMC free article] [PubMed] [Google Scholar]
- 27.Yucel K, McKinnon MC, Chahal R, Taylor VH, Macdonald K, Joffe R, et al. Anterior cingulate volumes in never-treated patients with major depressive disorder. Neuropsychopharmacology. 2008;33:3157–3163. doi: 10.1038/npp.2008.40. [DOI] [PubMed] [Google Scholar]
- 28.Plomin R, Haworth CM, Davis OS. Common disorders are quantitative traits. Nat Rev Genet. 2009;10:872–878. doi: 10.1038/nrg2670. [DOI] [PubMed] [Google Scholar]
- 29.Pizzagalli DA, Treadway MT. Neuroimaging approaches to the study of major depressive disorder - From where to why. In: Gotlib IH, Hammen C, editors. Handbook of Depression. 4th edition Guilford Press; New York, NY: 2014. [Google Scholar]
- 30.Winterburn JL, Pruessner JC, Chavez S, Schira MM, Lobaugh NJ, Voineskos AN, et al. A novel in vivo atlas of human hippocampal subfields using high-resolution 3T magnetic resonance imaging. Neuroimage. 2013;74:254–265. doi: 10.1016/j.neuroimage.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Pipitone J, Park M, Winterburn JL, Lett TA, Lerch JP, Pruessner J, et al. Alzheimer’s Disease NeuroImaging Initiative. Bootstrapping multi-atlas hippocampal segmentation with MAGeT Brain. Neuroimage. Submitted. [Google Scholar]
- 32.Hamilton JP, Siemer M, Gotlib IH. Amygdala volume in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Mol Psychiatry. 2008;13:993–1000. doi: 10.1038/mp.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grieve SM, Clark CR, Williams LM, Peduto AJ, Gordon E. Preservation of limbic and paralimbic structures in aging. Hum Brain Mapp. 2005;25:391–401. doi: 10.1002/hbm.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition.(SCID-I/P) Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- 35.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 37.Chakravarty MM, Steadman P, Eede MC, Calcott RD, Gu V, Shaw P, et al. Performing label-fusion-based segmentation using multiple automatically generated templates. Hum Brain Mapp. 2013;34:2635–2654. doi: 10.1002/hbm.22092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pipitone J, Park MTM, Winterburn J, Lett TA, Lerch JP, Pruessner JC, et al. Multi-atlas segmentation of the whole hippocampus and subfields using multiple automatically generated templates. Neuroimage. 2014 doi: 10.1016/j.neuroimage.2014.04.054. [DOI] [PubMed] [Google Scholar]
- 39.Collins DL, Pruessner JC. Towards accurate, automatic segmentation of the hippocampus and amygdala from MRI by augmenting ANIMAL with a template library and label fusion. Neuroimage. 2010;52:1355–1366. doi: 10.1016/j.neuroimage.2010.04.193. [DOI] [PubMed] [Google Scholar]
- 40.Heckemann RA, Keihaninejad S, Aljabar P, Rueckert D, Hajnal JV, Hammers A. Improving intersubject image registration using tissue-class information benefits robustness and accuracy of multi-atlas based anatomical segmentation. Neuroimage. 2010;51:221–227. doi: 10.1016/j.neuroimage.2010.01.072. [DOI] [PubMed] [Google Scholar]
- 41.Aljabar P, Heckemann RA, Hammers A, Hajnal JV, Rueckert D. Multi-atlas based segmentation of brain images: atlas selection and its effect on accuracy. Neuroimage. 2009;46:726–738. doi: 10.1016/j.neuroimage.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 42.Chakravarty MM, Bertrand G, Hodge CP, Sadikot AF, Collins DL. The creation of a brain atlas for image guided neurosurgery using serial histological data. Neuroimage. 2006;30:359–376. doi: 10.1016/j.neuroimage.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 43.Entis JJ, Doerga P, Barrett LF, Dickerson BC. A reliable protocol for the manual segmentation of the human amygdala and its subregions using ultra-high resolution MRI. Neuroimage. 2012;60:1226–1235. doi: 10.1016/j.neuroimage.2011.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 45.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hagler DJ, Jr, Saygin AP, Sereno MI. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage. 2006;33:1093–1103. doi: 10.1016/j.neuroimage.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chrousos GP, Gold PW. A healthy body in a healthy mind--and vice versa--the damaging power of “uncontrollable” stress. J Clin Endocrinol Metab. 1998;83:1842–1845. doi: 10.1210/jcem.83.6.4908. [DOI] [PubMed] [Google Scholar]
- 49.Radley JJ, Sawchenko PE. A common substrate for prefrontal and hippocampal inhibition of the neuroendocrine stress response. J Neurosci. 2011;31:9683–9695. doi: 10.1523/JNEUROSCI.6040-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- 51.Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- 52.Cerqueira JJ, Pego JM, Taipa R, Bessa JM, Almeida OF, Sousa N. Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J Neurosci. 2005;25:7792–7800. doi: 10.1523/JNEUROSCI.1598-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 54.Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, et al. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325:621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- 55.Cerqueira JJ, Mailliet F, Almeida OF, Jay TM, Sousa N. The prefrontal cortex as a key target of the maladaptive response to stress. J Neurosci. 2007;27:2781–2787. doi: 10.1523/JNEUROSCI.4372-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soares JM, Sampaio A, Ferreira LM, Santos NC, Marques F, Palha JA, et al. Stress-induced changes in human decision-making are reversible. Transl Psychiatry. 2012;2:e131. doi: 10.1038/tp.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Castro-Fornieles J, Bargallo N, Lazaro L, Andres S, Falcon C, Plana MT, et al. A cross-sectional and follow-up voxel-based morphometric MRI study in adolescent anorexia nervosa. J Psychiatr Res. 2009;43:331–340. doi: 10.1016/j.jpsychires.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 59.Treadway MT, Grant MM, Ding Z, Hollon SD, Gore JC, Shelton RC. Early adverse events, HPA activity and rostral anterior cingulate volume in MDD. PLoS ONE. 2009;4:e4887. doi: 10.1371/journal.pone.0004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Drevets WC, Price JL, Simpson JR, Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 61.Deschwanden A, Karolewicz B, Feyissa AM, Treyer V, Ametamey SM, Johayem A, et al. Reduced Metabotropic Glutamate Receptor 5 Density in Major Depression Determined by [11C]ABP688 PET and Postmortem Study. Am J Psychiatry. 2011;168:727–734. doi: 10.1176/appi.ajp.2011.09111607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schermuly I, Wolf D, Lieb K, Stoeter P, Fellgiebel A. State dependent posterior hippocampal volume increases in patients with major depressive disorder. J Affect Disord. 135:405–409. doi: 10.1016/j.jad.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 63.Eriksson PS, Perfilieva E, Bjoork-Eriksson T, Alborn A-M, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 64.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 65.Burt DB, Zembar MJ, Niederehe G. Depression and memory impairment: a meta-analysis of the association, its pattern, and specificity. Psychol Bull. 1995;117:285. doi: 10.1037/0033-2909.117.2.285. [DOI] [PubMed] [Google Scholar]
- 66.Bremner JD, Vythilingam M, Vermetten E, Vaccarino V, Charney DS. Deficits in hippocampal and anterior cingulate functioning during verbal declarative memory encoding in midlife major depression. Am J Psychiatry. 2004;161:637–645. doi: 10.1176/appi.ajp.161.4.637. [DOI] [PubMed] [Google Scholar]
- 67.Gorwood P, Corruble E, Falissard B, Goodwin G. Toxic effects of depression on brain function: impairment of delayed recall and the cumulative length of depressive disorder in a large sample of depressed outpatients. Am J Psychiatry. 2008;165:731–739. doi: 10.1176/appi.ajp.2008.07040574. [DOI] [PubMed] [Google Scholar]
- 68.Engvig A, Fjell AM, Westlye LT, Skaane NV, Sundseth O, Walhovd KB. Hippocampal subfield volumes correlate with memory training benefit in subjective memory impairment. Neuroimage. 2012;61:188–194. doi: 10.1016/j.neuroimage.2012.02.072. [DOI] [PubMed] [Google Scholar]
- 69.Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- 70.Amat J, Paul E, Watkins LR, Maier SF. Activation of the ventral medial prefrontal cortex during an uncontrollable stressor reproduces both the immediate and long-term protective effects of behavioral control. Neuroscience. 2008;154:1178–1186. doi: 10.1016/j.neuroscience.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maier SF, Watkins LR. Role of the medial prefrontal cortex in coping and resilience. Brain Res. 2010;1355:52–60. doi: 10.1016/j.brainres.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dillon DG, Pizzagalli DA. Evidence of successful modulation of brain activation and subjective experience during reappraisal of negative emotion in unmedicated depression. Psychiatry Res. 2013;212:99–107. doi: 10.1016/j.pscychresns.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Diener C, Kuehner C, Brusniak W, Ubl B, Wessa M, Flor H. A meta-analysis of neurofunctional imaging studies of emotion and cognition in major depression. Neuroimage. 2012;61:677–685. doi: 10.1016/j.neuroimage.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 74.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 75.Mak AK, Wong M, Han S-h, Lee T. Gray matter reduction associated with emotion regulation in female outpatients with major depressive disorder: a voxel-based morphometry study. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1184–1190. doi: 10.1016/j.pnpbp.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 76.Abe O, Yamasue H, Kasai K, Yamada H, Aoki S, Inoue H, et al. Voxel-based analyses of gray/white matter volume and diffusion tensor data in major depression. Psychiatry Research: Neuroimaging. 2010;181:64–70. doi: 10.1016/j.pscychresns.2009.07.007. [DOI] [PubMed] [Google Scholar]