Abstract
Resurgence is relapse of an extinguished operant response following the removal of alternative reinforcement. In animal models of resurgence to date, rats have been food deprived and food is used as the source of alternative reinforcement. Thus, when the alternative reinforcer is removed, the only remaining source of food during experimental sessions is no longer available. Acute food deprivation is known to produce reinstatement of drug seeking, thus such deprivation has been suggested a potential mechanism of resurgence. The present experiments examined whether resurgence of sucrose and cocaine seeking could be obtained with rats that were not food deprived. Free feeding rats were trained to press a lever for either sucrose (Exp 1) or cocaine infusions (Exp 2). Next, lever pressing was extinguished and an alternative response (nose poking) was reinforced with sucrose. When nose poking was also placed on extinction, resurgence of both sucrose and cocaine seeking were observed. Thus, resurgence of both sucrose and cocaine seeking can be obtained in rats that are not food restricted and it appears unlikely that an acute hunger state is responsible for resurgence. In addition, the present procedures for studying resurgence in the absence of interpretive complexities introduced by the use of food-deprivation may prove useful for further investigations of the neurobiological mechanisms of resurgence.
Keywords: resurgence, relapse, drug self-administration, alternative reinforcement, extinction, operant behavior
An increase in an extinguished operant behavior generated by loss of an alternative source of reinforcement is known as resurgence [1] [2] [3] [4]. Alternative reinforcement is a common component of behavioral treatments for substance abuse [5], and suspension of such alternative reinforcement when treatment ends is often associated with relapse [6] [7] [8]. Furthermore, loss of non-drug sources of reinforcement (e.g., social, vocational) are often cited as sources of relapse in human substance abusers [9] [10] [11] [12]. Resurgence of drug-maintained behavior of rats has been demonstrated with alcohol [2] and cocaine [13] self-administration when an alternative source of food reinforcement is removed. As a result, the resurgence paradigm has been suggested as an animal model of relapse to drug seeking induced by loss of alternative non-drug reinforcement [2] [13] [14] [15].
To date, all non-human experiments on resurgence of drug [2] [13] [16] or food [1] [17] [18] [19] [20] seeking have used food-deprived subjects. In the resurgence test when the alternative reinforcement is removed, food is no longer available during the experimental session. Both acute and chronic food deprivation stress are known to generate relapse of drug-seeking [21] [22] [23], and it has been suggested that an acute hunger state could be the source of the resurgence effect [24]. Thus, the goal of the present experiments was to examine whether resurgence of sucrose (Experiment 1) and cocaine (Experiment 2) seeking could be obtained with rats that were not food deprived and to provide a procedure for examining resurgence that is free of the interpretive complexities introduced by food-deprivation
In Experiment 1 ten male Long-Evans rats (Charles River, Portage, MI, USA) were used. Rats were 71–80 days old upon arrival and were fed Harlan Teklad Rodent Diet 8604 ad libitum. Rats were allowed two weeks to adjust to handling and colony conditions before beginning the study. Sessions took place in four Med Associates operant chambers (St. Albans, Vermont, USA), described elsewhere [25]. Each chamber was equipped with two levers on either side of a food receptacle equipped with photobeam sensors to detect head entries. A small opening in the receptacle allowed a 0.1-ml dipper cup to be accessed for 4 s when the dipper arm was elevated upon completion of the scheduled response requirement. The 45-ml solution tray contained a 32% sucrose and water mixture that was newly mixed each day to ensure consistency in taste. The chamber wall opposite the levers and food aperture housed five small holes equipped with photobeams to detect nose pokes.
Prior to the start of the experiment rats were trained to consume the sucrose reinforcer for the dipper. Sucrose was delivered on a variable-time (VT) 60-s schedule in 45-min sessions. Throughout the experiment, when the dipper arm was elevated (always 4-s access), the food receptacle was illuminated and any previously lit stimulus was turned off. Only the food receptacle light was used during magazine training. Training lasted until all rats completed a session with head entries on greater than 80% of sucrose deliveries.
Next during training rats earned sucrose presentations contingent upon pressing a lever, the location of which (left or right) was counterbalanced and signaled by LEDs above the lever. During training, the ratio requirement doubled after every five sucrose deliveries from a fixed-ratio (FR) 1 to an FR 8. Next, the rats experienced a variable-interval (VI) 15-s then 30-s schedule. Training sessions began using the last schedule of reinforcement in place in the prior day’s session. Session duration was 30 minutes less time for dipper delivery in training and all subsequent phases. Phase 1 began in the session following exposure to the VI 30-s schedule. Training lasted between 1 and 7 sessions.
In Phase 1 (Target Reinforced) rats received 50 sessions of training in which sucrose was available for presses to the target lever on a VI 45-s schedule. Presses to the inactive lever and alternative nose poke were counted but had no consequences. In Phase 2 (Extinction + Alternative Reinforcement), presses to the target lever and inactive lever were recorded but had no effect. Nose pokes into the illuminated left-most nose poke aperture produced sucrose deliveries on a VI 10-s schedule. Phase 2 lasted 10 sessions. In Phase 3 (Resurgence Test), sucrose was no longer available for the target or alternative response during this phase, although the solution was present in the dipper tray to keep olfactory cues consistent across phases. Phase 3 lasted four sessions.
In the last session of Phase 1, mean total target lever responses, inactive lever responses, alternative nose pokes, and sucrose deliveries were 204.7 (SD = 152.44), 1.98 (SD = 2.28), 1.70 (SD = 2.06), and 61.90 (SD = 20.15), respectively. At the end of Phase 2 (Extinction + Alternative Reinforcement) total target responses had decreased to 6.60 (SD = 5.85) presses and the number of alternative responses increased to 62.00 (SD = 20.11) pokes, resulting in an average of 61.90 sucrose deliveries (SD = 20.15). Total inactive responses remained low at 0.9 (SD = 1.37) presses.
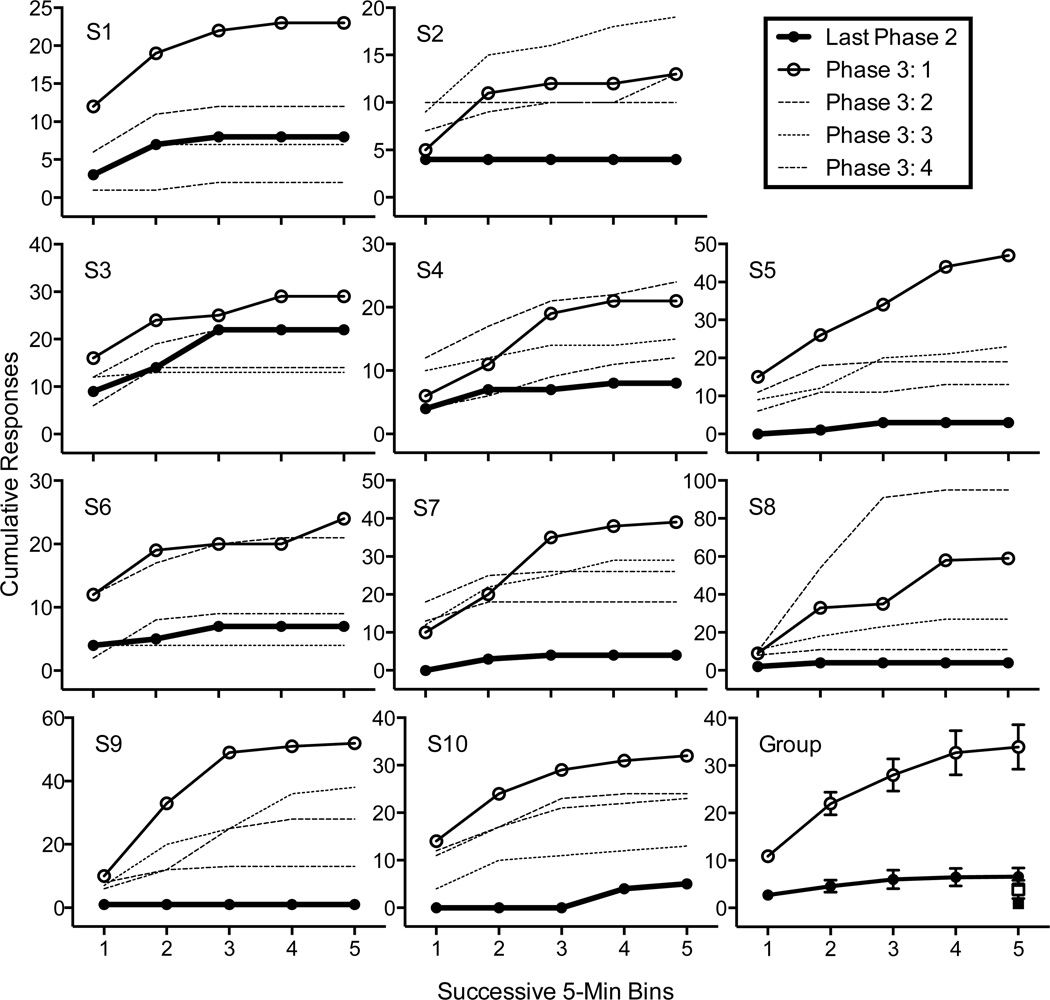
Figure 1 shows cumulative sucrose-lever response across successive 5-min bins in the last session of Phase 2 and the four sessions of Phase 3 (Resurgence Test). For all rats, sucrose-lever responding was greater during the first session of Phase 3 than in the last session of Phase 2. Response output varied across Phase 3 sessions, but generally remained elevated relative to last session of Phase 2. The bottom right panel of Figure 1 shows mean cumulative sucrose-lever responses and total inactive-lever responses for the last session of Phase 2 and the first session of Phase 3. The conclusion that resurgence was obtained in the first session of Phase 3 is further supported by a 2 (last Phase 2 versus first Phase 3) × 5 (Bin) repeated-measures ANOVA documenting significant main effects of Phase [F(1, 5) = 6.72, p < .05] and Bin [F(8, 40) = 24.94, p < .001] and a Phase × Bin interaction [F(8, 40) = 3.96, p < .01]. Resurgence was specific to sucrose-lever responding as there was no significant increase in inactive lever pressing between the last session of Phase 2 and the first sessions of Phase 3 [t(9)=1.53, ns].
Figure 1.
Cumulative sucrose-lever presses in the last session of Phase 2 (Extinction + Alternative Reinforcement) and all sessions of Phase 3 (Resurgence Test) for individual rats. The bottom-right panel shows group mean data for the last session of Phase 2 and the first session of Phase 3. Filled and empty squares represent total inactive lever presses for corresponding sessions in Phases 2 and 3, respectively.
In Experiment 2 six experimentally naïve male Long Evans rats (Charles River, Portage, MI) served. Rats were housed individually in a temperature- and humidity-controlled colony room with a 12:12 hr light/dark cycle (lights on at 7:00 AM) and had unrestricted access to food and water. Each rat was approximately 90 days of age at the time of surgery (see below). Animal care and all procedures detailed below were conducted in accordance with guidelines set forth by Utah State University’s Institutional Animal Care and Use Committee. Four modular operant self-administration chambers like those used in Experiment and also equipped for IV drug self-administration were used. Tygon tubing encased in a metal-spring tether was inserted through a 4 cm diameter hold cut in the center of the roof of each camber. At all times, the Tygon tubing and tether were attached to rats’ back-mounted cannulae. The other end of the tubing attached to a stainless-steel swivel (Instech, Plymouth Meeting, PA) suspended ~15 cm above the roof of the chamber. Another 60 cm of Tygon tubing attached the swivel to a 60-ml syringe mounted on an infusion pump (Med Associates) positioned outside the sound-attenuating cabinet.
Prior to the experiment, rats underwent jugular-catheterization surgery which was preceded by injections of antibiotic (gentamicin, 2.0 mg/kg, IM), analgesic/anti-inflammatory (flunixin meglumine, 1.1 mg/kg, IP), and anesthetic (ketamine, 75 mg/kg, and xylaxine hydrochloride, 7.5 mg/kg, IM) drugs. An indwelling, back-mounted cannula (Plastics One, Roanoke, VA) attached to a silastic catheter (SAI-Infusions, Lake Villa, IL) was inserted through an incision in the rat’s lower back and fed subcutaneously to a 0.5 cm incision centered 2 cm below the rat’s shoulder blades. The catheter was fed subcutaneously from the upper-back incision to an incision made in the right ventral surface of the neck, then inserted into the right jugular vein. Following surgery, rats were given injections of flunixin meglumine (1.1 mg/kg, IP), gentamicin (2.0 mg/kg, IM), and electrolyte soltion (Ringer’s, 5 cc, SC) every 12 hr for 2–5 days. Rats began the procedure detailed below a minimum of five days post-surgery. Cocaine hydrochloride was dissolved in a sterile 0.9% saline solution to a concentration 2.56 mg/ml. Drug doses were adjusted by changing the activation duration of the fixed-speed (0.0527 ml/s) syringe pump.
Prior to beginning the experiment rats experienced several sessions of training to ensure they reliably drank a 32% sucrose solution from the 0.1 ml dipper when it operated. Dipper training proceeded as described for Experiment 1. Next, rats were trained to self-administer IV cocaine infusions. Sessions during self-administration training and all phases of the experiment detailed below were 45 min long and began with illumination of the house light and target-lever LEDs. During the first session of training, a single press (i.e., fixed-ratio [FR] 1) to the target lever produced a 1 mg/kg infusion of cocaine. Here and below, each cocaine infusion was accompanied by blackout of the chamber and activation of the Sonalert for the duration of the infusion. After a minimum of three sessions of self-administration under FR 1, the number of responses required per infusion was increased to 2, and then to 3 after a minimum of three additional sessions. The dose of cocaine then was decreased across sessions to 0.75 mg/kg, 0.5 mg/kg, and, finally, 0.32 mg/kg per infusion. This phase ended when the rats reached the 0.32 mg/kg dose.
In Phase 1 (Target Reinforced) of the experiment, rats self-administered 0.32 mg/kg infusions of cocaine according to an FR 3 schedule for presses to the target lever. Phase 1 continued for a minimum of 14 sessions and until responding over the last three sessions occurred at stable rates (i.e., absence of reliable trend). In Phase 2 (Extinction + Alternative Reinforcement), presses to the target lever and inactive lever were recorded but had no effect. In the first session each alternative response (i.e., nose poke into the illuminated left-most nose poke aperture) produced a sucrose delivery. In the second and third sessions, the number of nose pokes required was increased to two and then three head entries. Phase 2 continued for a minimum of 14 sessions and ended when target-lever responding was reduced to low levels (~20% of the rate during the last sessions of Phase 1) for each rat. Finally, in Phase 3 (Resurgence Test) sucrose was no longer available for either the target or alternative response during this phase, although the solution continued to be present in the dipper tray to keep olfactory cues consistent across phases. Phase 3 lasted four sessions.
In the last session of Phase 1, mean total target responses, inactive lever responses, alternative nose pokes, and cocaine infusions were 28.17 (SD = 11.51), 1.00 (SD = 0.63), 2.83 (SD = 4.58), and 8.83 (SD = 3.87), respectively. At the end of Phase 2 (Extinction + Alternative Reinforcement) total target responses had decreased to 5.66 (SD = 4.22) presses and the number of alternative responses increased to 269.50 (SD = 142.78) pokes, resulting in an average of 89.66 sucrose deliveries (SD = 47.67). Total inactive-lever responses remained low at 0.67 (SD = 0.81) presses.
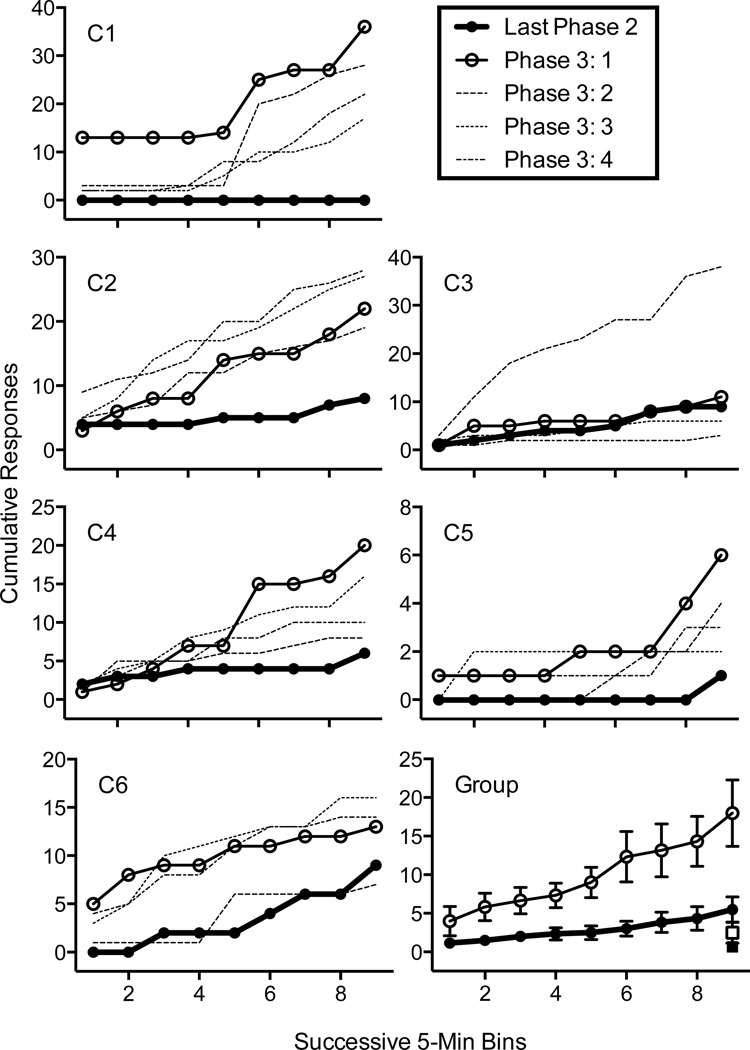
Figure 2 shows cumulative target presses on the cocaine lever across the last session of Phase 2 and the four sessions of Phase 3 (Resurgence Test). For all 6 rats, cocaine-lever responding was greater during the first session of Phase 3 than in the last session of Phase 2, although this difference was very small for Rat C3. Response output varied across Phase 3 sessions, but generally remained elevated relative to last session of Phase 2. The bottom right panel of Figure 1 shows the mean cumulative cocaine-lever responses and total inactive-lever responses for the last session of Phase 2 and the first session of Phase 3. The conclusion that resurgence was obtained in the first session of Phase 3 is further supported by a 2 (last Phase 2 versus first Phase 3) × 5 (Bin) repeated-measures ANOVA documenting significant main effects of Phase [F(1, 9) = 28.22, p < .001] and Bin [F(1.34, 12.10) = 32.06, p < .001—Greenhouse-Geisser df reduction] and a Phase × Bin interaction [F(1.33, 11.93) = 11.26, p < .01—Greenhouse-Geisser df reduction]. Resurgence was specific to target responding as there was no significant increase in inactive lever pressing between the last session of Phase 2 and the first sessions of Phase 3 [t(5)=1.38, ns].
Figure 2.
Cumulative cocaine-lever presses in the last session of Phase 2 (Extinction + Alternative Reinforcement) and all sessions of Phase 3 (Resurgence Test) for individual rats. The bottom-right panel shows group mean data for the last session of Phase 2 and the first session of Phase 3. Filled and empty squares represent total inactive lever presses for corresponding sessions in Phases 2 and 3, respectively.
The data from these experiments provide evidence that resurgence of both sucrose and cocaine seeking can be obtained in rats that are not food restricted. Thus, it appears unlikely that an acute hunger state is responsible for resurgence [24]. However, the fact that resurgence of sucrose and cocaine seeking are likely not due to food-deprivation induced stress, does not rule out other potential sources of stress in generation of the effect. For example, the loss of expected reinforcement per se could generate increases in responding via a mechanism that is at least in part stress mediated [13]--a possibility that is consistent with the finding that the adrenergic alpha-2 receptor agonist clonidine reduces resurgence of food seeking [17]. Nevertheless, next to nothing is known about the neurobiological mechanisms mediating resurgence. The present procedures for studying resurgence of both sucrose and cocaine seeking in the absence of interpretive complexities introduced by the use of food-deprivation may prove useful for further investigation of such mechanisms.
Highlights.
Loss of an alternative reward induced relapse of sucrose and cocaine seeking in free-feeding rats
It is unlike that an acute hunger state is responsible for resurgence
These procedures may be useful for examining the neurobiology of resurgence
Acknowledgements
The authors thank Rusty Nall, Ciara Marshall, Casey Frye, & Paul Cunningham for their assistance in conducting this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leitenberg H, Rawson RA, Mulick JA. Extinction and reinforcement of alternative behavior. Journal of Comparative and Physiological Psychology. 1975;88:640–652. [Google Scholar]
- 2.Podlesnik CA, Jimemez-Gomez C, Shahan TA. Resurgence of alcohol seeking produced by discontinuing non-drug reinforcement as an animal model of drug relapse. Behavioural Pharmacology. 2006;17:369–374. doi: 10.1097/01.fbp.0000224385.09486.ba. [DOI] [PubMed] [Google Scholar]
- 3.Shahan TA, Sweeney MM. A model of resurgence based on behavioral momentum theory. Journal of the Experimental Analysis of Behavior. 2011;95:91–108. doi: 10.1901/jeab.2011.95-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winterbauer NE, Bouton ME. Mechanisms of resurgence of an extinguished instrumental behaviora. Journal of Experimental Psychology: Animal Behavior Processes. 2010;36:343–353. doi: 10.1037/a0017365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higgins ST, Heil SH, Lussier J. Clinical implications of reinforcement as a determinant of substance use disorders. Annual Review of Psychology. 2004;55:431–461. doi: 10.1146/annurev.psych.55.090902.142033. [DOI] [PubMed] [Google Scholar]
- 6.Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: A meta-analysis. Addiction. 2006;101:1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- 7.Silverman K, Wong CJ, Umbricht-Schneiter A, Montoya ID, Schuster CR, Preston KL. Broad beneficial effects of cocaine abstinence reinforcement among methadone patients. Journal of Consulting and Clinical Psychology. 1998;66:811–824. doi: 10.1037//0022-006x.66.5.811. [DOI] [PubMed] [Google Scholar]
- 8.Silverman K, Chutuape M, Bigelow GE, Stitzer ML. Voucher-based reinforcement of cocaine abstinence in treatment-resistant methadone patients: Effects of reinforcement magnitude. Psychopharmacology. 1999;146:128–138. doi: 10.1007/s002130051098. [DOI] [PubMed] [Google Scholar]
- 9.Falba T, Teng H, Sindelar JL, Gallo WT. The effect of involuntary job loss on smoking intensity and relapse. Addiction. 2005;100:1330–1339. doi: 10.1111/j.1360-0443.2005.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallo WT, Bradley EH, Siegel M, Kasl SV. The impact of involuntary job loss on subsequent alcohol consumption by older workers: Findings from the Health and Retirement Survey. The Journal of Gerontology: Series B: Psychological Sciences and Social Sciences. 2001;56B:S3–S9. doi: 10.1093/geronb/56.1.s3. [DOI] [PubMed] [Google Scholar]
- 11.Temple JL, Legierski CM, Giacomelli AM, Salvy S, Epstein LH. Overweight children find food more reinforcing and consume more energy than do nonoverweight children. American Journal of Clinical Nutrition. 2008;87:1121–1127. doi: 10.1093/ajcn/87.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vuchinich RE, Tucker JA. Alcoholic relapse, life events, and behavioral theories of choice: A prospective analysis. Experimental and Clinical Psychopharmacology. 1996;4:19–28. [Google Scholar]
- 13.Quick SL, Pyszczynski AD, Colston KA, Shahan TA. Loss of alternative non-drug reinforcement induces relapse of cocaine-seeking in rats: Role of dopamine D1 receptors. Neuropsychopharmacology. 2011;36:1015–1020. doi: 10.1038/npp.2010.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marchant NJ, Li X, Shaham Y. Recent developments in animal models of drug relapse. Current Opinion in Neurobiology. 2013;23:675–683. doi: 10.1016/j.conb.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peck JA, Ranaldi R. Drug abstinence: Exploring animal models and behavioral treatment strategies. Psychopharmacology. 2014;231:2045–2058. doi: 10.1007/s00213-014-3517-2. [DOI] [PubMed] [Google Scholar]
- 16.Pyszczynski AD, Shahan TA. Loss of nondrug reinforcement in one contest produces alcohol seeking in another context. Behavioural Pharmacology. 2013;24:496–503. doi: 10.1097/FBP.0b013e328364502a. [DOI] [PubMed] [Google Scholar]
- 17.Pyszczynski AD, Shahan TA. Examination of the role of Dopamine D2 and Adrenergic α2 Receptors in Resurgence. Behavioural Brain Research. 2014;271:122–128. doi: 10.1016/j.bbr.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sweeney MM, Shahan TA. Behavioral momentum and resurgence: Effects of time in extinction and repeated resurgence tests. Learning & Behavior. 2013;41:414–424. doi: 10.3758/s13420-013-0116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sweeney MM, Shahan TA. Effects of high, low, and thinning rates of alternative reinforcement on response elimination and resurgence. Journal of the Experimental Analysis of Behavior. 2013;100:102–116. doi: 10.1002/jeab.26. [DOI] [PubMed] [Google Scholar]
- 20.Winterbauer NE, Lucke S, Bouton ME. Some factors modulating the strength of resurgence after extinction of an instrumental behavior. Learning and motivation. 2013;44:60–71. doi: 10.1016/j.lmot.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carroll ME. The role of food deprivation in the maintenance and reinstatement of cocaine-seeking behavior in rats. Drug and Alcohol Dependence. 1985;16:95–109. doi: 10.1016/0376-8716(85)90109-7. [DOI] [PubMed] [Google Scholar]
- 22.Shalev U, Highfield D, Yap J, Shaham Y. Stress and relapse to drug seeking in rats: studies on the generality of the effect. Psychopharmacology. 2000;150:337–346. doi: 10.1007/s002130000441. [DOI] [PubMed] [Google Scholar]
- 23.Shalev U, Marinelli M, Baumann MH, Piazza PV, Shaham Y. The role of corticosterone in food deprivation-induced reinstatement of cocaine seeking in the rat. Psychopharmacology. 2003;168:170–176. doi: 10.1007/s00213-002-1200-5. [DOI] [PubMed] [Google Scholar]
- 24.Shaham Y, Pickens C, Theberge F. F1000Prime Recommendation of [Quick S. L. et al. (2011). Neuropsychopharmacology, 36, 1015–1020.] F1000Prime. 2011 Mar 31; 2011. [Google Scholar]
- 25.Quick SL, Shahan TA. Behavioral momentum of cocaine self-administration: effects of frequency of reinforcement on resistance to extinction. Behavioural Pharmacology. 2009;20:337–345. doi: 10.1097/FBP.0b013e32832f01a8. [DOI] [PubMed] [Google Scholar]