Abstract
Background
Atherosclerotic animal models show increased recruitment of inflammatory cells to the heart following myocardial infarction (MI), which impacts ventricular function and remodeling.
Objective
To determine whether increased myocardial inflammation following MI also contributes to arrhythmias.
Methods
MI was created in 3 mouse models: 1) atherosclerotic (ApoE−/− on atherogenic diet; n=12), 2) acute inflammation (wild-type [WT] given daily lipopolysaccharide [LPS], 10µg/day; n=7), and 3) WT (n=14). Sham-operated (n=4) mice were also studied. Four days post-MI, an inflammatory protease-activatable fluorescent probe (Prosense680) was injected intravenously to quantify myocardial inflammation on day 5. Optical mapping with voltage-sensitive dye was performed on day 5 to assess electrophysiology and arrhythmia susceptibility.
Results
Inflammatory activity (Prosense680 fluorescence) was increased approximately 2-fold in ApoE+MI and LPS+MI hearts versus WT+MI (p<0.05) and 3-fold versus Sham (p<0.05). ApoE+MI and LPS+MI hearts also had prolonged action potential duration, slowed conduction velocity, and increased susceptibility to pacing-induced arrhythmias (56% and 71%; vs. 13% for WT+MI and 0% for Sham, respectively, p<0.05 for ApoE+MI and LPS+MI groups versus both WT+MI and Sham). Increased macrophage accumulation in ApoE+MI and LPS+MI hearts was confirmed with immunofluorescence. Macrophages were associated with areas of connexin-43 (Cx43) degradation and a 2-fold decrease in Cx43 expression was found in ApoE+MI versus WT+MI hearts (p<0.05). ApoE+MI hearts also had a 3-fold increase in interleukin-1β expression, an inflammatory cytokine known to degrade Cx43.
Conclusions
Underlying atherosclerosis exacerbates post-MI electrophysiological remodeling and arrhythmias. LPS+MI hearts fully recapitulate the atherosclerotic phenotype, suggesting myocardial inflammation as a key contributor to post-MI arrhythmia.
Keywords: arrhythmia, atherosclerosis, optical mapping, myocardial infarction, inflammation
Introduction
Nearly every 30 seconds, an American has a new or recurrent coronary event leading to myocardial infarction (MI).1 Post-MI patients have a life-long risk of ventricular arrhythmias and sudden cardiac death that is approximately four times higher than the general population.1 Arrhythmias arise not only from death of cardiomyocytes and fibrotic scar replacement, but also because surviving myocytes near the infarct (border zone) undergo dramatic electrophysiological remodeling, leading to altered action potential (AP) and conduction properties. Post-MI electrophysiological remodeling and mechanisms of arrhythmogenesis have been extensively characterized in animal models of MI ranging from rodents2,3 to canines4,5 and from acute ischemia6,7 to chronic,8 healed MI. However, most studies to date have been performed in otherwise healthy animals despite the fact that advanced atherosclerosis is the primary underlying cause of MI.
Atherosclerosis is a chronic inflammatory disease9 and the leading cause of MI. Atherosclerotic mouse models have a 14-fold increase in circulating monocytes compared to healthy mice,10 which leads to an augmented post-MI inflammatory response characterized by elevated serum cytokines, increased recruitment and retention of inflammatory cells in the infarct, and increased protease activity in the myocardium.11,12 This elevated pro-inflammatory state has important functional consequences. For example, increased levels of circulating monocytes following MI are associated with decreased functional recovery and adverse left ventricular (LV) remodeling.13,14 Elevated serum cytokines are also associated with accelerated progression to heart failure.15,16 In addition to compromised pump function, elevated post-MI inflammation may also impact electrophysiological remodeling and susceptibility to arrhythmia through the actions of inflammatory cytokines and proteases, such as interleukin-1β (IL-1β) and matrix metalloproteinase-7 (MMP-7). Both of these factors have been shown to degrade connexin43 (Cx43) following MI,17,18 leading to slowed conduction and an increased propensity to arrhythmia.
We therefore hypothesized that atherosclerosis and its accompanying pro-inflammatory state may increase post-MI electrophysiological remodeling and arrhythmias and that Cx43 degradation by inflammatory factors may be a key contributor to arrhythmogenesis. To test this hypothesis, we developed a novel multi-functional imaging approach to visualize and quantify the intensity and spatial extent of myocardial inflammation with concomitant high-speed, high-resolution optical mapping of electrophysiological activity in atherosclerotic and wild-type (WT) mouse models of MI. Because atherosclerosis is also associated with hyperlipidemia and hypercholesterolemia, which may have independent effects on cardiac electrophysiology,19 we further tested our inflammatory hypothesis in a post-MI model of acute, systemic inflammation induced by daily administration of lipopolysaccharide (LPS).
Methods
An expanded Methods section can be found in the online-only Data Supplement.
All procedures involving animals were approved by the Animal Care and Use Committee of the University of California, Davis and adhered to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. MI was performed in WT C57BL/6 male mice (16–24 weeks of age, n=25 or apolipoprotein E deficient male mice on a C57BL/6 background (ApoE−/−, n=12), from Jackson Laboratories. ApoE−/− mice were approximately 20 weeks of age following 12.0±1.6 weeks on atherogenic diet (Harlan TD.88137). MI was created using previously described procedures20 with 45 min of ischemia followed by reperfusion. Briefly, mice were anesthetized with an intraperitoneal (I.P.) injection of pentobarbital sodium (70 mg/kg), intubated, and ventilated. Isoflurane was used to maintain anesthesia. The chest was opened and the left anterior descending coronary artery (LAD) was ligated. A lead I electrocardiogram (ECG) was monitored and ligation was confirmed by ST elevation (Supplemental Figure 1). Sham-operated mice (n=4) were created without tying the suture but passing it under the LAD. Mice were allowed to recover for 5 days. Starting four hours post-MI, a subset of WT mice (n=7) received daily injections of LPS (10 µg/day, I.P.) to stimulate monocyte/macrophage activation and a heightened post-MI inflammatory response. This dose of LPS is a low, sub-septic concentration similar to what would be seen in atherosclerosis and is approximately 10-fold lower than sepsis. For visualization and quantification of inflammatory activity, all animals were given an intravenous injection of ProSense680 (0.5mL/kg, PerkinElmer) 24 hours prior to sacrifice. ProSense680 is an activatable fluorescent agent that only fluoresces when cleaved by specific proteases (cathepsin B, K, L, and S)21 highly expressed during inflammation. Previous studies in mouse models of MI have validated ProSense as a reporter of myocardial inflammation,12,22 with a majority of the ProSense fluorescence attributable to pro-inflammatory macrophages within the infarct.11
On day 5 post-MI, mice were anesthetized with pentobarbital sodium (150 mg/kg, I.P.) containing 120 IU heparin. Hearts were rapidly excised and cannulated for Langendorff perfusion. Blebbistatin (10–20 µM, Tocris Bioscience, Ellisville, MO) was added to the perfusate to reduce motion artifacts during optical recordings. Hearts were stained with voltage-sensitive dye (di-4-ANEPPS, 5 µL of 5 mg/mL in DMSO, Molecular Probes). Baseline electrophysiological parameters were determined during LV epicardial pacing at a pacing cycle length (PCL) of 150ms. Effective refractory period (ERP) and arrhythmia propensity were determined using an S1–S2 pacing protocol. Optical mapping data analysis was performed as previously described.23
Following optical mapping experiments, hearts were transferred to a Maestro II imaging system (Caliper Life Sciences Corp.) for fluorescence reflectance imaging (FRI) of ProSense680 (see Online Data Supplement for imaging and quantification details). Hearts were then embedded in optimal cutting temperature (O.C.T.) medium, frozen, and sectioned for fluorescence microscopy of residual ProSense680 and immunohistochemistry for Cx43, the adherens junctional protein, N-cadherin (N-Cad), and/or the macrophage marker, CD68. A subset of hearts (ApoE−/−, n=3; WT, n=3) were snap-frozen and protein was extracted for western blot for Cx43, IL-1β, and Nav1.5. Membranes were probed with infrared fluorescent secondary antibodies (IRDyes 800CW or 680LT, Licor Biosciences) and imaged on a Licor Odyssey scanner (Licor Biosciences). Images were quantified and data were normalized to α-actinin.
For most data sets, a one- or two-way analysis of variance (ANOVA) with Bonferroni’s post-hoc testing was used to determine statistical significance between groups. For premature ventricular complex (PVC) data, a generalized linear form of a univariate ANOVA was performed. A Pearson’s test was used to determine relationships between inflammation and electrophysiological properties and for co-localization analysis of immunohistochemistry images. A Fisher’s exact test was used to determine statistical significance of inducible arrhythmia between groups. A Student’s t-test was used to determine statistical significance of western blot protein levels. All data presented are mean±SD. p<0.05 was considered statistically significant.
Results
Action potential properties are altered in post-MI ApoE−/− hearts
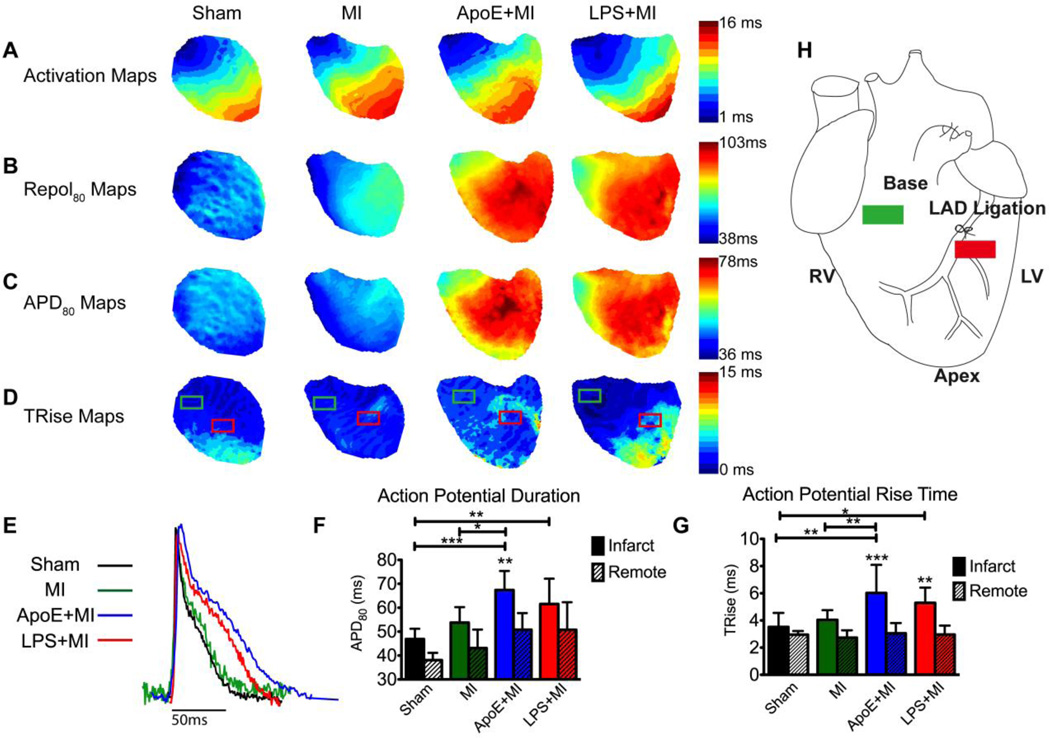
Optical mapping of transmembrane potential (Vm) was performed 5 days post-MI to assess electrophysiological remodeling and revealed marked changes in post-MI models of atherosclerosis and acute inflammation. ApoE+MI and LPS+MI hearts showed prolongation of activation, repolarization (Repol80), action potential duration (APD80), and action potential rise time (TRise) compared with WT+MI and Sham hearts (Figure 1). Optical APs from the infarct (Figure 1E) show clear differences in AP morphology; the ApoE+MI and LPS+MI APs are prolonged and display a more prominent plateau phase compared with WT+MI and Sham. Summary data for APD80 and TRise from both the infarct and remote regions (red and green boxes in Figure 1D, respectively) are shown in Figure 1F–1G. Importantly, electrophysiological parameters in ApoE+MI and LPS+MI hearts were nearly indistinguishable from one another, indicating similar post-MI electrophysiological remodeling in these two inflammatory models.
Figure 1.
Post-MI electrophysiology. (A–D) Maps of activation, repolarization (Repol80), action potential duration (APD80) and action potential (AP) rise time (TRise). Slowing of activation and prolongation of repolarization and APD are observed in the ApoE+MI and LPS+MI hearts. (E) Example optical APs from the infarct region (red box in D). (F) APD80 from remote and infarct regions (green and red boxes, respectively in D). (G) TRise from remote and infarct regions. (H) Schematic showing location of LAD ligation and regions of interest for electrophysiological and molecular imaging analysis. RV: right ventricle, LV: left ventricle, LAD: left anterior descending coronary artery. *p<0.05, **p<0.01, ***p<0.001.
ApoE−/− hearts have slow conduction and are susceptible to post-MI arrhythmias
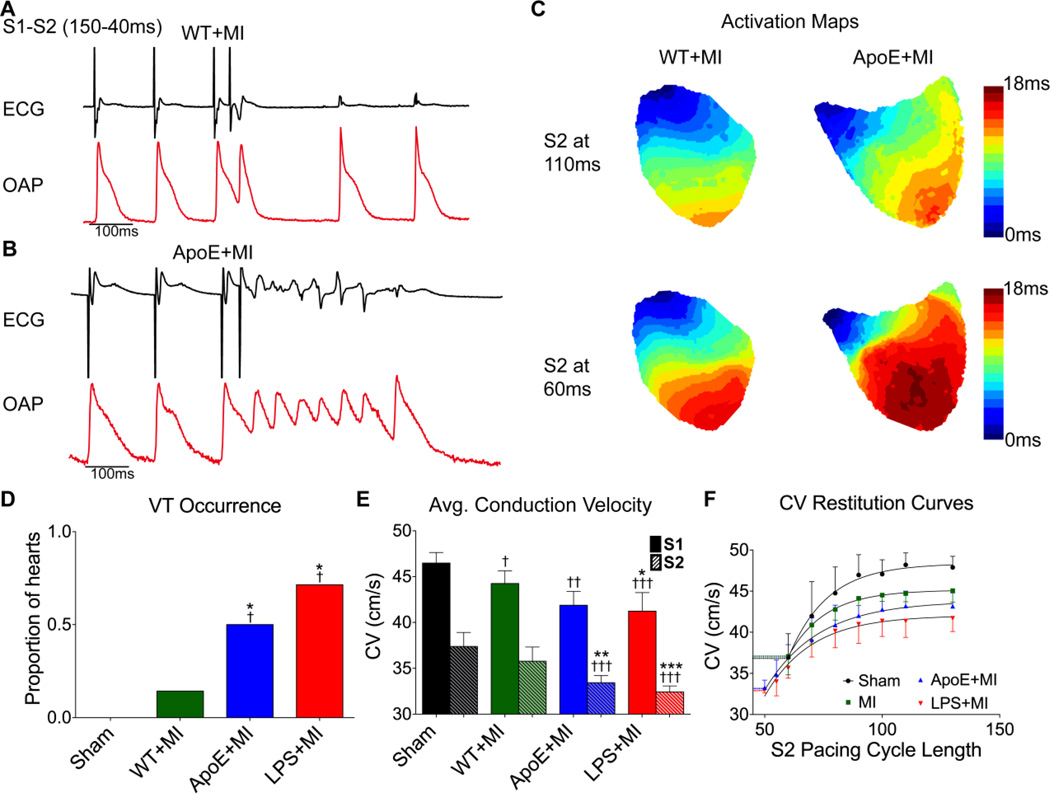
To investigate whether atherosclerosis increases the incidence of re-entrant arrhythmia following MI, an S1-S2 pacing protocol was performed (Figure 2A–2B). A single premature pacing stimulus (S2) led to only one incidence of arrhythmia (i.e., non-sustained ventricular tachycardia [VT]) in WT+MI hearts (1/8, Figure 2A, 2D) and VT was never induced in the Sham hearts (0/4). In contrast, ApoE+MI and LPS+MI hearts had significantly increased arrhythmia propensity (5/9 and 5/7 hearts, respectively, Figure 2B, 2D). An example ECG and optical action potential (OAP) at S2=40 ms for a WT+MI and ApoE+MI heart are shown in Figure 2A–2B and Supplemental Movies 1–2. In response to the same premature stimulus, the ApoE+MI heart exhibits a bout of non-sustained VT whereas the WT+MI heart does not. Since re-entrant arrhythmias can be precipitated by slow conduction, a detailed analysis of conduction velocity (CV) was performed (Figure 2C, 2E–2F). The ApoE+MI and LPS+MI hearts had significantly slower conduction at a pacing cycle length of 150 ms (S1 stimulus), which was further exacerbated at short coupling intervals (Figures 2E–2F).
Figure 2.
Reentrant arrhythmias and conduction velocity (CV). (A) An example of the S1–S2 pacing protocol showing no arrhythmia in a WT+MI heart. (B) The same S1–S2 protocol produces non-sustained ventricular tachycardia (VT) in an ApoE+MI heart. (C) Activation maps at two different S2 intervals (110 ms and 60 ms) showing a pronounced slowing of CV in the ApoE+MI heart at shorter S2 coupling intervals. (D) Proportion of hearts in which VT was induced. (E) Mean CV at S1 (150 ms) and S2 (at the effective refractory period [ERP]). (F) CV restitution curves for all groups showing blocked conduction at an S2 of approximately 60 ms for WT+MI and Sham and continued slow conduction at shorter S2 coupling intervals for ApoE+MI and LPS+MI hearts. ECG: electrocardiogram, OAP: optical action potential, VT: ventricular tachycardia. *p<0.05, **p<0.01, ***p<0.001 vs WT. †p<0.05, ††p<0.01, †††p<0.001 vs Sham.
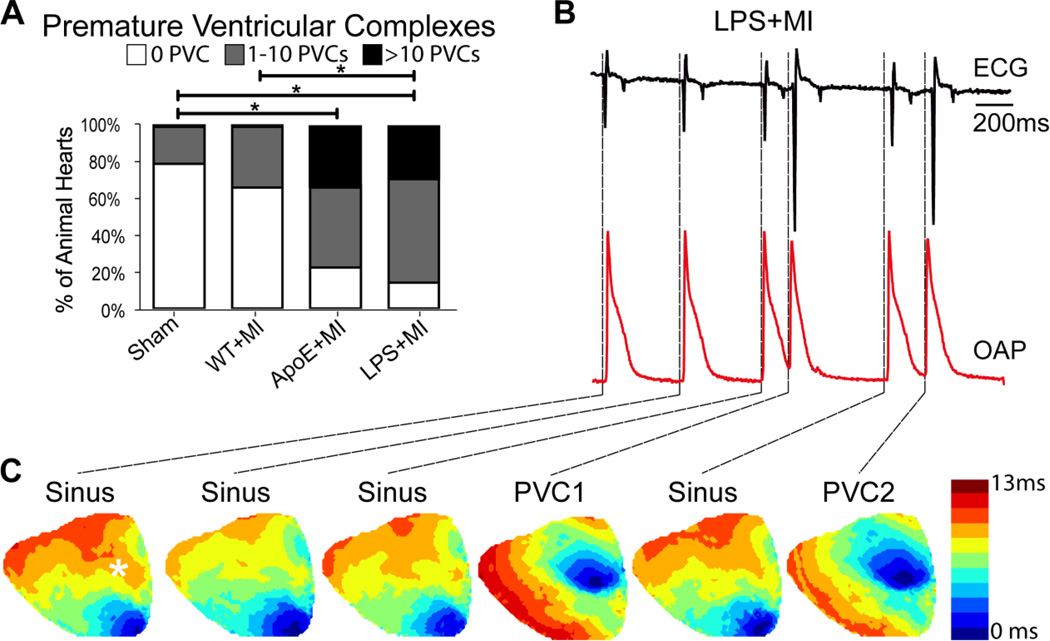
In addition to re-entrant arrhythmias, an increased incidence of PVCs in ApoE+MI and LPS+MI hearts was also observed (Figure 3A). The number of PVCs was quantified over a 20 min ECG recording period and several PVCs were optically recorded. An example of spontaneous PVCs in an LPS+MI heart is shown in Figure 3B. The PVCs are clearly distinguishable on the ECG by their irregular rhythm and large, broad QRS complexes. Activation maps (Figure 3C) revealed that PVCs arose from the basal infarct region (Supplemental Movie 3). There were no statistical differences in CV or arrhythmia propensity between the ApoE+MI and LPS+MI hearts, indicating a similar post-MI arrhythmogenic phenotype.
Figure 3.
PVCs in post-MI hearts. (A) Number of spontaneously occurring PVCs in 20 min. (B) Representative optical AP (OAP, red) and ECG (black) showing spontaneous PVCs in an LPS+MI heart. Location of the OAP is indicated with an asterisk in the first sinus rhythm activation map in C. (C) Activation maps of sinus rhythm and PVCs corresponding to the traces in B. The PVCs appear focal in origin and arise from the basal infarct region. *p<0.05.
ApoE−/− hearts have increased myocardial inflammatory activity
ProSense680 is a commercially available cathepsin-activatable fluorescent sensor and has previously been shown to report on inflammatory macrophage activity within the post-MI mouse heart.11 ProSense680 was visualized and quantified following optical mapping experiments. Figure 4A shows representative images of inflammatory activity across the anterior epicardial surface of the heart. ApoE+MI and LPS+MI hearts show a significant increase in ProSense680 fluorescence versus WT+MI and Sham (Figure 4C). Short-axis sections also show increased inflammation transmurally in ApoE+MI and LPS+MI hearts (Figure 4B). Importantly, independent of animal model, significant correlations were observed between the degree of local inflammatory activity (i.e., ProSense680 fluorescence in the infarct region, white box in Figure 4A) and APD80 and TRise from the same infarct location, as well as average epicardial CV (Figure 4D–F). Again, LPS+MI hearts recapitulated the ApoE+MI phenotype.
Figure 4.
Ex-vivo fluorescence reflectance imaging of macrophage protease activity (ProSense680 fluorescence). (A) Prosense680 intensity on the anterior epicardial surface of the heart showing increased fluorescence in the infarct region of ApoE+MI and LPS+MI hearts (images identically scaled). (B) Representative 2 mm short-axis images showing transmural protease activity. Slice locations correspond to dashed white lines in A. (C) Mean fluorescence intensity from the infarct region (white box in A). (D–F) Correlations between inflammation in the infarct region (ProSense fluorescence) and corresponding electrophysiological parameters from the same region when data from all groups are pooled (color of data point indicates group). *p<0.05, ***p<0.001.
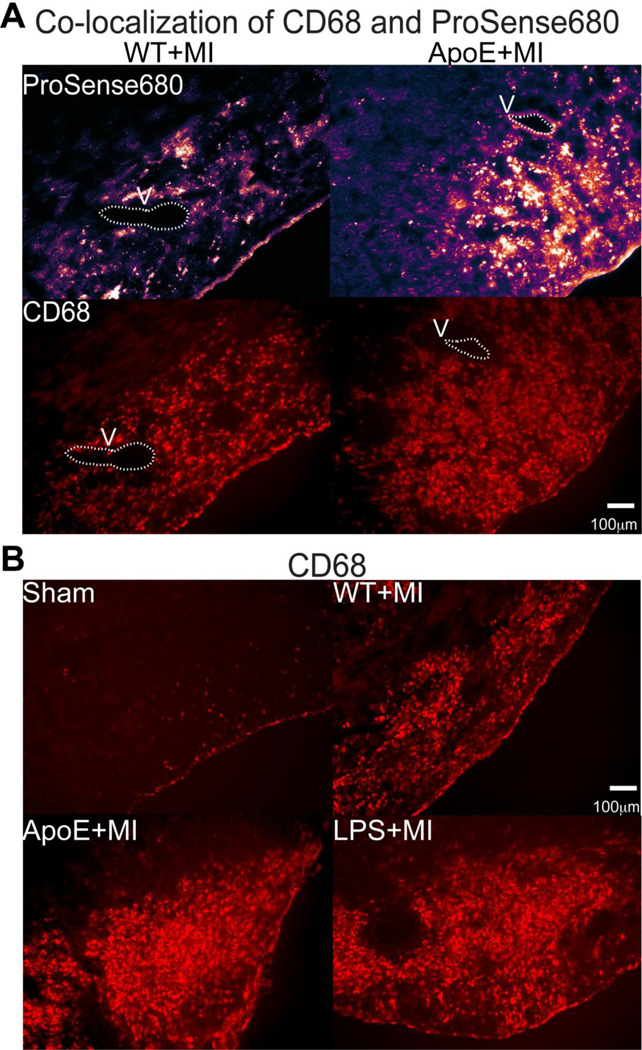
We then verified that the ProSense680 signal corresponded to macrophage infiltration with immunohistochemistry of the macrophage marker CD68. Unstained frozen tissue sections were first imaged with a Cy5.5 filter to visualize residual ProSense680 fluorescence. Those same tissue sections were then immunolabeled for CD68. An example of CD68 and ProSense680 co-localization is shown in Figure 5A where ProSense680-positive areas are also CD68 positive. Consistent with the whole heart and short axis sections (Figure 4A–B), a clear increase in CD68 positive cells was observed in the ApoE+MI and LPS+MI hearts compared with WT+MI and Sham, indicating increased myocardial macrophage infiltration (Figure 5B).
Figure 5.
Fluorescence microscopy of residual ProSense680 fluorescence and the macrophage marker CD68. (A) Residual ProSense680 fluorescence (top, purple) from the infarct region of a WT+MI (left) and ApoE+MI (right) heart. These same tissue sections were then labeled for CD68 (red, bottom) to assess co-localization of ProSense680 and macrophages. V: vessel used for anatomical landmark. (B) CD68 immunofluorescence of infarct regions show increased macrophage infiltration in ApoE+MI and LPS+MI hearts.
Macrophage infiltration is associated with Cx43 degradation
Because arrhythmia propensity and CV can be altered by gap junction coupling, we assessed expression and distribution of Cx43 and the relationship between Cx43 and macrophage infiltration. Tissue sections were co-labeled with Cx43 and CD68 antibodies. Remote from the infarct, normal Cx43 distribution and relatively few macrophages were observed (Figure 6A). Interestingly, in the Sham animals, a small area of Cx43 degradation was observed at the mock ligation site and this area exactly corresponds to the area of macrophage infiltration (Figure 6B). Upon closer examination, clear Cx43 internalization and degradation is observed in the myocytes bordering CD68-positive macrophages (Figure 6B, Inset). Thus, even without ischemia, inflammatory macrophage activity is associated with Cx43 degradation. Accordingly, in the ApoE+MI hearts, where abundant macrophage infiltration is observed, large areas of Cx43 loss and degradation are visible (Figure 6C). To further assess the distribution of Cx43, co-labeling of Cx43 and the adherens junctional protein, N-Cad was performed (Supplemental Figure 2). Remote from the infarct, strong co-localization of Cx43 and N-Cad was observed (Supplemental Figure 2A). In the peri-infarct regions, however, significant non-junctional Cx43 expression is evident and is more pronounced in the ApoE+MI compared to the WT+MI hearts (Supplemental Figure 2B–D). Western blot confirmed an approximate 2-fold decrease in total Cx43 protein levels in ApoE+MI compared to WT+MI hearts (Figure 6D). Voltage-gated Na+ channels may also impact conduction, however no significant difference in expression of Nav1.5 was found between ApoE+MI and WT+MI hearts (Supplemental Figure 3).
Figure 6.
Fluorescence microscopy of macrophage infiltration and corresponding Cx43 expression. (A) Uninjured myocardium remote from the infarct shows uniform staining of Cx43 (green) and few macrophages (CD68, red) in an ApoE+MI heart. (B) The region of mock ligation in a sham heart shows Cx43 degradation at the site of injury, which corresponds to increased macrophage infiltration (CD68). Inset reveals Cx43 internalization and degradation in myocytes within and neighboring CD68 positive regions. (C) The infarct region of an ApoE+MI heart shows marked reduction in Cx43 expression corresponding to an increase in macrophage infiltration. Inset reveals degradation and internalization of Cx43. (D) Western blots (each sample run in duplicate) and corresponding quantification showing decreased expression of Cx43 and increased expression of IL-1β in ApoE+MI versus WT+MI. HEK: Hek293 cell lysates, which do not express Cx43 or IL-1β were used as a negative control, Mark: molecular weight marker. *p<0.05, **p<0.01.
Inflammatory macrophages secrete IL-1β, which is known to degrade Cx43 in both brain24 and heart.18 An approximate 3-fold increase in IL-1β expression was found in the ApoE+MI compared to WT+MI hearts (Figure 6D). Collectively, these data suggest a role for macrophage-secreted cytokines in post-MI Cx43 degradation and Cx43-mediated slowing of conduction in inflamed hearts following MI.
Discussion
Here we show, for the first time, that an altered inflammatory response impacts post-MI electrophysiological remodeling and arrhythmia susceptibility. Specifically, we found that at 5 days post-MI, ApoE+MI hearts had significantly prolonged APD80, slowed conduction, and an increased incidence of both focal and re-entrant ventricular arrhythmias compared to WT+MI. Importantly, administration of LPS to WT mice with MI fully recapitulated the ApoE+MI phenotype, serving as a positive control for inflammation and indicating that heightened inflammation contributes to post-MI arrhythmias in the ApoE+MI hearts independent of other factors, such as hypercholesterolemia.19 ApoE+MI and LPS+MI hearts had similar levels of heightened myocardial inflammation, quantified by molecular imaging of ProSense680 and confirmed with immunohistochemical staining of the macrophage marker CD68. Importantly, CD68-positive cells were associated with areas of Cx43 degradation, even in the absence of ischemia (Sham hearts), suggesting a possible role for macrophage-secreted cytokines and proteases in post-MI gap junction degradation. Decreased expression of Cx43 in ApoE+MI hearts was confirmed by western blot and was associated with a 3-fold increase in the inflammatory cytokine, IL-1β, which has been shown to degrade Cx43 in the epicardial border zone.18
Inflammatory-mediated Cx43 degradation
At 5-days post-MI, the pro-inflammatory (M1) macrophage is the predominant inflammatory cell present in the infarct.11 M1 macrophages produce high levels of IL-1β, which has been previously implicated in Cx43 degradation both in the brain24 and post-MI heart,18,25 leading to cell-cell uncoupling both in vitro and in vivo following MI. Accordingly, we found a 3-fold increase in IL-1β and a 2-fold decrease in Cx43 expression in the ApoE+MI compared to WT+MI hearts. Although not specifically investigated here, M1 macrophages in the infarct also produce MMPs, including MMP-7, which has been implicated in Cx43 degradation and conduction slowing post-MI.17 Thus, macrophage-secreted IL-1β and MMP-7 may synergistically decrease Cx43 expression post-MI and may explain why Cx43 degradation was primarily observed in areas surrounding CD68-positive macrophages (Figure 6). Interestingly, Cx43 degradation was also found in Sham hearts where Cx43 loss was observed adjacent to CD68-positive macrophages even in the absence of ischemia (Figure 6C). Thus, even the mild inflammatory response induced by Sham surgery led to macrophage recruitment and local Cx43 degradation. Collectively, these findings suggest the inflammatory macrophage contributes to post-MI Cx43 degradation.
Mechanisms of post-MI arrhythmias in ApoE−/− and LPS-treated hearts
ApoE+MI and LPS+MI hearts exhibited increased incidence of non-sustained VT in response to a single premature stimulus compared to WT+MI and Sham hearts (Figure 2). This increase in re-entrant arrhythmias may stem from both a prolonged APD in the infarct region (Figure 1) and slowed conduction (Figure 2E–2F) in ApoE+MI and LPS+MI hearts. Both conditions set the stage for strong gradients of repolarization, unidirectional conduction block, and re-entrant arrhythmia. ApoE+MI and LPS+MI hearts also exhibited significantly more focal arrhythmias compared to WT+MI and Sham hearts (Figure 3). PVCs captured on optical recordings were spontaneous focal excitations originating from the infarct region (Figure 3C). The exact mechanism underlying these PVCs is unknown; however, abnormal Ca2+ handling may contribute to focal excitation. Interestingly, the pro-inflammatory cytokine IL-1β potentiates spontaneous Ca2+ release and Ca2+ waves in isolated cardiac myocytes.26 Thus, IL-1β may have additional pro-arrhythmic effects. In addition to conduction slowing, decreased gap junction coupling may also facilitate the escape of PVCs by reducing the source-sink mismatch.23 Therefore, Cx43 degradation in inflamed hearts likely contributes to the increased susceptibility of ApoE+MI and LPS+MI hearts to both re-entrant and focal arrhythmias.
Integrated molecular and functional imaging of inflammation and arrhythmia
For the first time, we combined high-speed, high-resolution optical mapping of Vm with molecular imaging of myocardial inflammation to determine the relationship between inflammatory activity and electrophysiological remodeling. This approach has several advantages, including the ability to visualize and quantify inflammatory protease activity via ProSense680 fluorescence. This approach provides more functional information on inflammatory activity than simply confirming the presence or absence of a particular inflammatory cell type, as with traditional histology. Furthermore, using this novel combination of imaging techniques, we were able to precisely correlate electrophysiological properties with myocardial inflammation in the same intact heart at high spatial resolution (Figure 4). This approach revealed strong relationships between the degree of local myocardial inflammation and the severity of post-MI electrophysiological remodeling (Figure 4D–4F), likely impossible with traditional post-mapping destructive histology or more global measures of inflammatory activity such as circulating inflammatory biomarkers.
Study Limitations
Decreased gap junction coupling may unmask underlying APD heterogeneity and may, in part, explain the prolonged APD observed in ApoE+MI and LPS+MI hearts. However, other pro-inflammatory factors may also contribute to the prolonged APD in inflamed hearts and these mechanisms remain an area of future investigation. Inflammation contributes to the transformation of resident fibroblasts into myofibroblasts, which also produce inflammatory cytokines and can contribute to post-MI electrophysiological remodeling. However, the precise source of elevated IL-1β and the specific contributions of myofibroblasts were not investigated in the present study. In order to avoid timing of the estrous cycle and reduce the overall number of animals required for the study, only male mice were investigated. Sex differences in post-MI inflammation and resulting arrhythmogenesis remain an area for future investigation. Western blot and immunohistochemistry report only on protein expression and do not allow for the specific investigation of Cx43 function or direct assessment of cell-cell coupling.
Conclusions
Using a novel imaging approach, we found that elevated post-MI inflammation, as occurs in the setting of underlying atherosclerosis, significantly contributes to adverse electrophysiological remodeling and increased post-MI arrhythmia risk. These findings have important implications for the understanding, prevention, and treatment of post-MI arrhythmias in the human population in which advanced coronary artery disease and chronic inflammation are often present. Further detailed investigations into additional mechanisms by which inflammation mediates electrophysiological remodeling may unveil novel therapeutic targets for the prevention and treatment of post-MI arrhythmias.
Supplementary Material
Clinical Perspectives.
Here we show, for the first time, that underlying atherosclerosis significantly increases electrophysiological remodeling and arrhythmia following MI. Our results indicate that elevated systemic inflammation, as occurs in humans with atherosclerosis, may be a key contributor to post-MI arrhythmias. This important finding may lead to improvements in patient care because often, basic studies on the mechanisms of post-MI arrhythmias and early pre-clinical testing of therapeutic agents are performed in otherwise healthy animals with surgically induced MI. Thus, our study highlights the importance of investigation of arrhythmia mechanisms and anti-arrhythmic strategies in a clinically relevant MI animal model with underlying atherosclerosis. Moreover, further investigations into the detailed mechanisms by which inflammation contributes to post-MI arrhythmias may unveil novel therapeutic targets and new avenues for anti-arrhythmic treatment.
Acknowledgments
Sources of funding:
This work was funded by NIH T32 GM099608 (N.M.D.; A.W.H.; D.M.B.), Howard Hughes Medical Institute (N.M.D.), China Scholarship Council and Natural Science Foundation of Shandong Province ZR2010HQ031 (J.W.), NIH P01 HL080101 (D.M.B.), the San Antonio Cardiovascular Proteomics Center (M.L.L., funded from NHLBI HHSN 268201000036C N01-HV-00244), NIH R01 HL075360 (M.L.L.), the UC Davis Clinical and Translational Science Center (C.M.R., funded from UL1 RR024146), NIH P30 HL101280 (C.M.R.; D.M.B.), and NIH R01 HL111600 (C.M.R.).
Abbreviations
- AP
action potential
- APD80
action potential duration at 80% repolarization
- ApoE−/−
apolipoprotein E deficient
- CD68
cluster of differentiation 68 - macrophage marker
- CV
conduction velocity
- Cx43
connexin43
- ECG
electrocardiogram
- ERP
effective refractory period
- FRI
fluorescence reflectance imaging
- IL-1β
interleukin-1β
- I.P.
intraperitoneal
- LAD
left anterior descending coronary artery
- LPS
lipopolysaccharide
- LV
left ventricle
- MI
myocardial infarction
- MMP
matrix metalloproteinase
- Nav1.5
voltage gated sodium channel
- N-Cad
N-cadherin
- OAP
optical action potential
- PCL
pacing cycle length
- PVC
premature ventricular complex
- Repol80
time at 80% repolarization
- S1
drive train pacing stimulus
- S2
premature pacing stimulus
- TRise
action potential rise time
- Vm
transmembrane potential
- VT
ventricular tachycardia
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflicts of interest.
Disclosures
None.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129 doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mills WR, Mal N, Forudi F, Popovic ZB, Penn MS, Laurita KR. Optical mapping of late myocardial infarction in rats. Am J Physiol Heart Circ Physiol. 2006;290:306. doi: 10.1152/ajpheart.00437.2005. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen DT, Ding C, Wilson E, Marcus GM, Olgin JE. Pirfenidone mitigates left ventricular fibrosis and dysfunction after myocardial infarction and reduces arrhythmias. Heart Rhythm. 2010;7:1438–1445. doi: 10.1016/j.hrthm.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 4.Pu J, Robinson RB, Boyden PA. Abnormalities in Ca(i)handling in myocytes that survive in the infarcted heart are not just due to alterations in repolarization. J Mol Cell Cardiol. 2000;32:1509–1523. doi: 10.1006/jmcc.2000.1184. [DOI] [PubMed] [Google Scholar]
- 5.Yao J-AA, Hussain W, Patel P, Peters NS, Boyden PA, Wit AL. Remodeling of gap junctional channel function in epicardial border zone of healing canine infarcts. Circ Res. 2003;92:437–443. doi: 10.1161/01.RES.0000059301.81035.06. [DOI] [PubMed] [Google Scholar]
- 6.Beardslee MA, Lerner DL, Tadros PN, Laing JG, Beyer EC, Yamada KA, Kléber AG, Schuessler RB, Saffitz JE. Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circ Res. 2000;87:656–662. doi: 10.1161/01.res.87.8.656. [DOI] [PubMed] [Google Scholar]
- 7.de Groot JR, Wilms-Schopman FJ, Opthof T, Remme CA, Coronel R. Late ventricular arrhythmias during acute regional ischemia in the isolated blood perfused pig heart. Role of electrical cellular coupling. Cardiovasc Res. 2001;50:362–372. doi: 10.1016/s0008-6363(01)00222-x. [DOI] [PubMed] [Google Scholar]
- 8.Ripplinger CM, Lou Q, Li W, Hadley J, Efimov IR. Panoramic imaging reveals basic mechanisms of induction and termination of ventricular tachycardia in rabbit heart with chronic infarction: implications for low-voltage cardioversion. Heart Rhythm. 2008;6:87–97. doi: 10.1016/j.hrthm.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Libby P. Inflammation in atherosclerosis. Nature. 2001;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 10.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2006;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panizzi P, Swirski FK, Figueiredo J-LL, Waterman P, Sosnovik DE, Aikawa E, Libby P, Pittet M, Weissleder R, Nahrendorf M. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J Am Coll Cardiol. 2010;55:1629–1638. doi: 10.1016/j.jacc.2009.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo J-LL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsujioka H, Imanishi T, Ikejima H, et al. Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J Am Coll Cardiol. 2009;54:130–138. doi: 10.1016/j.jacc.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 14.Maekawa Y, Anzai T, Yoshikawa T, Asakura Y, Takahashi T, Ishikawa S, Mitamura H, Ogawa S. Prognostic significance of peripheral monocytosis after reperfused acute myocardial infarction:a possible role for left ventricular remodeling. J Am Coll Cardiol. 2002;39:241–246. doi: 10.1016/s0735-1097(01)01721-1. [DOI] [PubMed] [Google Scholar]
- 15.Andreassen AK, Nordøy I, Simonsen S, Ueland T, Müller F, Frøland SS, Gullestad L, Aukrust P. Levels of circulating adhesion molecules in congestive heart failure and after heart transplantation. Am J Cardiol. 1998;81:604–608. doi: 10.1016/s0002-9149(97)00972-7. [DOI] [PubMed] [Google Scholar]
- 16.Aukrust P, Ueland T, Müller F, Andreassen AK, Nordøy I, Aas H, Kjekshus J, Simonsen S, Frøland SS, Gullestad L. Elevated circulating levels of C-C chemokines in patients with congestive heart failure. Circulation. 1998;97:1136–1143. doi: 10.1161/01.cir.97.12.1136. [DOI] [PubMed] [Google Scholar]
- 17.Lindsey ML, Escobar GP, Mukherjee R, Goshorn DK, Sheats NJ, Bruce JA, Mains IM, Hendrick JK, Hewett KW, Gourdie RG, Matrisian LM, Spinale FG. Matrix metalloproteinase-7 affects connexin-43 levels, electrical conduction, and survival after myocardial infarction. Circulation. 2006;113:2919–2928. doi: 10.1161/CIRCULATIONAHA.106.612960. [DOI] [PubMed] [Google Scholar]
- 18.Baum JR, Long B, Cabo C, Duffy HS. Myofibroblasts cause heterogeneous Cx43 reduction and are unlikely to be coupled to myocytes in the healing canine infarct. Am J Physiol Heart Circ Physiol. 2012;302:800. doi: 10.1152/ajpheart.00498.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y-BB, Wu C-CC, Lu L-SS, Su M-JJ, Lin C-WW, Lin S-FF, Chen LS, Fishbein MC, Chen P-SS, Lee Y-TT. Sympathetic nerve sprouting, electrical remodeling, and increased vulnerability to ventricular fibrillation in hypercholesterolemic rabbits. Circ Res. 2003;92:1145–1152. doi: 10.1161/01.RES.0000072999.51484.92. [DOI] [PubMed] [Google Scholar]
- 20.Tarnavski O, McMullen JR, Schinke M, Nie Q, Kong S, Izumo S. Mouse cardiac surgery: comprehensive techniques for the generation of mouse models of human diseases and their application for genomic studies. Physiol Genomics. 2004;16:349–360. doi: 10.1152/physiolgenomics.00041.2003. [DOI] [PubMed] [Google Scholar]
- 21.Caglič D, Globisch A, Kindermann M, Lim N-HH, Jeske V, Juretschke H-PP, Bartnik E, Weithmann KU, Nagase H, Turk B, Wendt KU. Functional in vivo imaging of cysteine cathepsin activity in murine model of inflammation. Bioorg Med Chem. 2011;19:1055–1061. doi: 10.1016/j.bmc.2010.10.028. [DOI] [PubMed] [Google Scholar]
- 22.Nahrendorf M, Sosnovik DE, Waterman P, Swirski FK, Pande AN, Aikawa E, Figueiredo J-LL, Pittet MJ, Weissleder R. Dual channel optical tomographic imaging of leukocyte recruitment and protease activity in the healing myocardial infarct. Circ Res. 2007;100:1218–1225. doi: 10.1161/01.RES.0000265064.46075.31. [DOI] [PubMed] [Google Scholar]
- 23.Myles RC, Wang L, Kang C, Bers DM, Ripplinger CM. Local β-adrenergic stimulation overcomes source-sink mismatch to generate focal arrhythmia. Circ Res. 2012;110:1454–1464. doi: 10.1161/CIRCRESAHA.111.262345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duffy HS, John GR, Lee SC, Brosnan CF, Spray DC. Reciprocal regulation of the junctional proteins claudin-1 and connexin43 by interleukin-1beta in primary human fetal astrocytes. J Neurosci. 2000;20 doi: 10.1523/JNEUROSCI.20-23-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baum JR, Dolmatova E, Tan A, Duffy HS. Omega 3 fatty acid inhibition of inflammatory cytokine-mediated Connexin43 regulation in the heart. Front Physiol. 2011;3:272. doi: 10.3389/fphys.2012.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duncan DJ, Yang Z, Hopkins PM, Steele DS, Harrison SM. TNF-alpha and IL-1beta increase Ca2+ leak from the sarcoplasmic reticulum and susceptibility to arrhythmia in rat ventricular myocytes. Cell Calcium. 2010;47:378–386. doi: 10.1016/j.ceca.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.