Abstract
Fibroblast growth factors (FGFs) 15/19 and 21 belong to a subfamily of FGFs that function as hormones. Produced in response to specific nutritional cues, they act on overlapping sets of cell surface receptors composed of classic FGF receptors in complex with βKlotho, and regulate metabolism and related processes during periods of fluctuating energy availability. Pharmacologically, both FGF15/19 and FGF21 cause weight loss and improve both insulin sensitivity and lipid parameters, in rodent and primate models of metabolic disease. Recently, FGF21 was shown to have similar effects in obese patients with type 2 diabetes. Here, we discuss emerging concepts in FGF15/19 and FGF21 tissue specific actions and critically assess their putative role as candidate targets for treating metabolic disease.
Keywords: βKlotho, brown adipose tissue, hypothalamus, sympathetic nervous system, arginine vasopressin, corticotropin-releasing factor
Fibroblast growth factors: the basics
The fibroblast growth factors (FGFs; see Glossary) constitute a family of 22 proteins that regulate diverse biological processes such as growth, development, differentiation and wound repair (1). Most FGFs have a high affinity for heparan sulfate in the extracellular matrix and, thus, are restricted to functioning locally as autocrine or paracrine factors. By contrast, the endocrine FGFs, which include FGF15/19, FGF21 and FGF23, have little or no affinity for heparan sulfate and can therefore enter the circulation and function as hormones (2). (FGF15 and FGF19 are the mouse and human orthologs, respectively; we refer to them collectively as FGF15/19 unless referring to a specific ortholog.) FGF23 signals from the bone to the kidney to regulate phosphate levels (3). FGF15/19 and FGF21 regulate important aspects of metabolism, and as such, they represent potential pharmaceutical targets for the treatment of obesity, type 2 diabetes and dylipidemia (4). In this review, we will discuss the tissue-specific mechanisms of action of FGF15/19 and FGF21 with a focus on liver, adipose tissue and the nervous system.
Expression and physiological functions
FGF15/19 is expressed in ileal enterocytes of the small intestine, where it is induced by the nuclear receptor farnesoid X receptor (FXR), in response to the postprandial re-uptake of bile acids (Table 1) (5). After entering the portal venous circulation, FGF15/19 acts on the liver to repress bile acid synthesis and gluconeogenesis, and to promote glycogen and protein synthesis (4). FGF15/19 also stimulates gallbladder filling (6). Thus, FGF15/19 regulates diverse aspects of the postprandial response.
Table 1.
Summary of FGF15/19 and FGF21 actions in different biological contexts
| Hormone | Stimulus (transcriptional inducer) | Tissue source of FGF | Target Tissue | Effect | References |
|---|---|---|---|---|---|
|
| |||||
| FGF15/19 | Postprandial bile acids (FXR) | Small intestine | Liver | ↓ Bile acid synthesis | (5) |
| ↓ Gluconeogenesis | (56) | ||||
| ↑ Glycogen synthesis | (87) | ||||
| ↑ Protein synthesis | (87) | ||||
|
| |||||
| Pharmacology | Liver CNS BAT WAT |
Beneficial | |||
| ↓ Bile acid synthesis | (5) | ||||
| ↓ Gluconeogenesis | (56) | ||||
| ↑ Glycogen synthesis | (87) | ||||
| ↑ Thermogenesis and weight loss | (41, 42) | ||||
| ↑ Insulin sensitivity | (41, 42) | ||||
| ↓ Blood triglyceride levels | (41, 42) | ||||
| ↓ Blood cholesterol levels | (41, 42) | ||||
|
| |||||
| Adverse | |||||
| ↑ Liver cell growth, neoplasia | (60) | ||||
|
| |||||
| FGF21 | Starvation (PPARα,CREB-H) | Liver | CNS | ↑ Hepatic fatty acid oxidation, ketogenesis and gluconeogenesis | (9, 10, 13) |
| ↑ Growth hormone resistance | (15) | ||||
| ↓ Ovulation | (16) | ||||
| ↓ Wheel-running activity | (17) | ||||
|
| |||||
| Fasting/refeeding, overfeeding (PPARα, PPARγ) | Liver WAT |
BAT WAT |
↑ Glucose uptake and fatty acid storage | (8, 30) | |
|
| |||||
| Cold (ATF2) | BAT WAT |
BAT WAT |
↑ Thermogenesis | (31–33) | |
| ↑ Browning of WAT | (33) | ||||
|
| |||||
| Ketogenic, low amino acid/protein diets (PPARα, ATF4) | Liver | CNS BAT |
↑ Hepatic fatty acid oxidation and ketogenesis | (9, 22, 91) | |
| ↑ Thermogenesis and weigh↑ loss | (19, 22) | ||||
|
| |||||
| Mitochondrial dysfunction, pancreatitis (ATF4) | Skeletal muscle Pancreas | N/K | N/K | (35, 37, 92) | |
|
| |||||
| Pharmacology | CNS BAT WAT |
Beneficial | |||
| ↑ Thermogenesis and weigh↑ loss | (14, 39, 40) | ||||
| ↑ Browning of WAT | (33) | ||||
| ↑ Glucose uptake | (40, 49) | ||||
| ↑ Insulin sensitivity | (40, 93) | ||||
| ↓ Blood triglyceride levels | (39, 43) | ||||
| ↓ Blood cholesterol levels | (9, 10, 43) | ||||
| ↑ Lifespan | (46) | ||||
|
| |||||
| Adverse | |||||
| ↑ Bone loss | (47) | ||||
| ↑ Glucocorticoids | (17, 64) | ||||
N/K, not known.
Unlike FGF15/19, FGF21 is expressed in multiple tissues including liver, brown adipose tissue (BAT), white adipose tissue (WAT) and pancreas (7). However, under normal physiologic conditions, all circulating FGF21 appears to be derived from liver (8). The molecular basis for this selective secretion of FGF21 from liver is not yet known. In the liver, FGF21 is induced by prolonged fasting by the nuclear receptor, peroxisome proliferator-activated receptor alpha (PPARα) (9–11), and by cyclic AMP responsive element-binding protein H (CREB-H) (12). Gain-of-function experiments show that FGF21 on its own can elicit diverse aspects of this starvation response. Among these, FGF21 stimulates hepatic fatty acid oxidation, ketogenesis and gluconeogenesis, and suppresses lipogenesis, discussed in detail below (9, 10, 13, 14). It blocks ovulation in female mice and suppresses growth (15, 16). In circadian wheel-running experiments, FGF21 reduces overall activity (17). It also sensitizes mice to the hibernation-like state of torpor (10). In complementary loss-of-function studies, FGF21-knockout (KO) mice have impaired ketogenesis and gluconeogenesis, and become prematurely hypoglycemic in response to starvation (13, 18). They also have reduced glucose uptake in BAT in response to refeeding (8).
FGF21 is also induced in liver by ketogenic, amino acid-deficient and low protein diets via PPARα and activating transcription factor 4 (ATF4)-dependent mechanisms (9, 19–21), suggesting that it plays a broad role in regulating energy homeostasis in response to nutritional stress. Accordingly, FGF21-KO mice fed a ketogenic diet gained more weight, developed hepatosteatosis and had impaired ketogenesis compared to wild-type mice (22). Interestingly, ketogenic diets do not induce FGF21 in humans (23–25). A plausible explanation for this discrepancy is that ketogenic diets used in rodent studies contain less protein than those used in humans (19). FGF21 is also elevated in serum of obese rodents and humans (24, 26–28), which may be an induced state of FGF21 resistance (28).
In adipose tissue, FGF21 is induced by the nuclear receptor PPARγ (29). In contrast to its regulation in liver, FGF21 in WAT is induced during the transition from the fasted to the fed state as part of a feed-forward regulatory loop that increases fatty acid storage (30). In BAT, FGF21 is induced by cold exposure and β3-adrenergic receptor stimulation by the stress-activated transcription factor, ATF2, and contributes to thermogenesis (31–33). In cold exposure experiments, FGF21-KO mice had lower body temperatures and elevated circulating levels of creatine kinase, consistent with increased shivering (33). The finding that FGF21 is downstream of agonists for both PPARα (in liver) and PPARγ (in adipose) suggests that some of the actions of the lipid-lowering fibrate and insulin-sensitizing thiazolidinedione drugs are due to FGF21. Indeed, FGF21-KO mice were refractory to the insulin-sensitizing actions of the thiazolidinedione, rosiglitazone (30).
In skeletal muscle, FGF21 is induced under conditions of mitochondrial dysfunction by ATF4 (34, 35). FGF21 is also expressed in the α- and β-cells of the endocrine pancreas (36) and in the exocrine pancreas, where its expression is induced by chemically-induced injury (37). While FGF21's physiologic function in pancreas remains to be determined, it increases insulin content and glucose-stimulated insulin secretion and inhibits glucagon secretion in isolated islets (38, 39), and it is protective in a mouse model of pancreatitis (37). Interestingly, PPARα, and ATF4 induce FGF21 in response to amino acid-deficient and low protein diets (9, 19–21). The induction of FGF21 by multiple nutrient-sensing transcription factors (e.g., PPARs, ATFs, and CREB-H) provides a mechanistic explanation for the tissue-specific expression of FGF21 under a range of nutritional contexts (Table 1).
Collectively these data highlight the complex metabolic actions of these hormones.
Pharmacologic effects
FGF15/19 and FGF21 have similar, powerful pharmacologic effects on metabolism (Figure 1). Transgenic overexpression or pharmacological administration of FGF19 or FGF21 to obese rodents increases energy expenditure without decreasing food intake, improves insulin sensitivity, reverses hepatic steatosis and lowers circulating and hepatic triglyceride and cholesterol concentrations (14, 39–42). FGF21 has similar pharmacologic effects in obese, insulin-resistant monkeys (43, 44). Thus, FGF15/19 and FGF21 are promising clinical candidates for treating metabolic disease. Recently, FGF21 was shown to improve metabolic parameters, including body weight and insulin and lipid levels, in obese humans with type 2 diabetes (45). Consistent with its ability to markedly lower insulin and insulin-like growth factor, long-term pharmacologic administration of FGF21 also leads to a profound extension of lifespan in mice (46). One possible adverse side effect of FGF21 is bone loss. In mice, FGF21 inhibits bone formation and stimulates bone resorption (47).
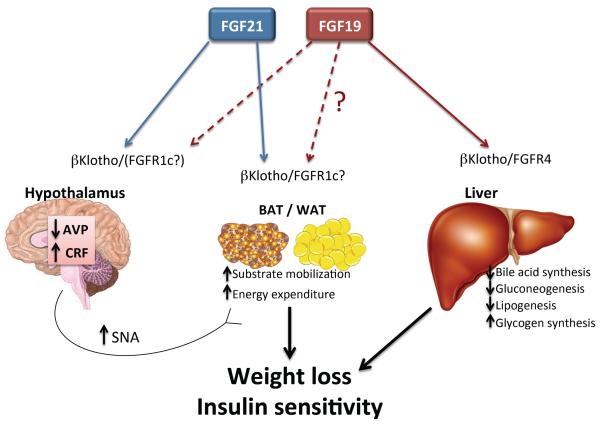
Figure 1. Schematic representation of the beneficial pharmacological actions of FGF19 and FGF21.
In obese animals, pharmacologic administration of FGF19 and FGF21 causes weight loss and increases insulin sensitivity. FGF21 increases thermogenic energy expenditure through its coordinate action on adipose tissue and the hypothalamus to mobilize glucose and lipids, and to stimulate sympathetic nerve activity via AVP and CRF. These effects of FGF21 require signalling through βKlotho and one of the FGFRs (likely FGFR1c). In this way, FGF21 provides both the fuel (oxidative substrate) and the fire (beta adrenergic signaling) to drive heat production in the obese state. FGF19 works directly on liver through βKlotho and FGFR4 to decrease bile acid synthesis and provide beneficial effects on cholestatic diseases. In addition, FGF19 suppress hepatic glucose and lipid production, but may also improve metabolic parameters by activating similar processes to FGF21 in adipose and the CNS.
FGF15/19 and FGF21 require βKlotho
FGF receptors (FGFRs) consist of an extracellular ligand-binding domain, a single transmembrane domain and an intracellular tyrosine kinase domain. There are four FGFRs (FGFR1-4), with two splice variants (b and c) existing for FGFR1-3 (1).
Signaling by the endocrine FGFs requires a co-receptor from the Klotho family of single transmembrane proteins. While FGF23 signaling requires αKlotho, both FGF15/19 and FGF21 require βKlotho to bind to their FGF receptors (48). This then activates extracellular signal-regulated kinases 1 and 2 (ERK1/2) and other downstream kinases. Studies with global βKlotho-KO mice show that βKlotho is essential for most, if not all, of the physiological and pharmacological functions of FGF15/19 and FGF21 (49–51). FGF15/19 binds to βKlotho in complex with FGFR1c, 2c, 3c and 4. FGF21 also binds to βKlotho in complex with FGFR1c, 2c or 3c but, notably, not FGFR4 (48). Knockout studies in mice suggest that the FGFR1c isoform is particularly important for the in vivo actions of FGF21 (52, 53). Interestingly, many of the metabolic actions of both FGF19 and FGF21, in particular the weight loss and insulin-sensitizing effects, are recapitulated by activating antibodies directed against either FGFR1c or the FGFR1c/βKlotho heteromer (53, 54).
While the FGFRs are broadly expressed across tissues, βKlotho has a more restricted expression pattern (7). This distribution along with the availability of tissue-specific βKlotho-KO mice (17, 49) has facilitated studies into the tissue-specific actions of FGF15/19 and FGF21.
FGF15/19 and FGF21 actions on liver
Both βKlotho and FGFR4 are abundantly expressed in liver, where FGF15/19 suppresses bile acid synthesis and gluconeogenesis (5, 55–57). These effects can be recapitulated in primary cultures of hepatocytes (55, 57), demonstrating that FGF15/19 acts directly on the liver. FGF15/19 inhibits bile acid synthesis by repressing the transcription of the gene encoding cholesterol 7α-hydroxylase (CYP7A1), the enzyme that catalyzes the first and rate-limiting step in bile synthesis (55, 57). Elimination of either βKlotho or FGFR4 in mice (5, 50) or chemical inhibition of ERK1/2 in primary human hepatocytes (58) blocks this inhibition of CYP7A1 by FGF15/19. As mentioned above, FGF15/19 also has a number of effects in hepatocytes that are similar to insulin, including stimulation of protein and glycogen synthesis and suppression of gluconeogenic gene expression (56, 57). Notably, however, an important difference to insulin is that FGF15/19 inhibits rather than stimulates fatty acid synthesis (59). In addition to its beneficial effects on liver metabolism, FGF15/19 also stimulates hepatocyte proliferation through an FGFR4-dependent mechanism (60, 61). Chronic exposure of mice to FGF19 resulted in hepatocellular carcinomas, and this effect was lost in FGFR4-KO mice (60, 62).
As noted above, FGF21 has dramatic effects on liver metabolism that include the induction of fatty acid oxidation, ketogenesis and gluconeogenesis and the suppression of lipogenesis (9, 10, 13, 14, 40). FGF21 has coordinate effects on hepatic gene expression that are consistent with these metabolic effects. However, in contrast to FGF15/19, these in vivo effects were not recapitulated in either isolated, perfused livers or primary rodent hepatocytes treated with FGF21 (13, 63). Moreover, tissue-specific knock out of βKlotho in nervous system (64) or FGFR1 in adipose tissue (52) eliminated most of FGF21's effect on hepatic gene expression. Thus, many of the effects of FGF21 on liver appear to be indirect. Consistent with this possibility, there is virtually no FGFR1 expression in liver and little or no FGFR2 and FGFR3 (7). However, FGF21 injection in mice was shown to induce ERK1/2 phosphorylation and immediate early gene expression in liver (65), suggesting at least some direct effects may occur at pharmacologic concentrations. Further studies with liver-specific βKlotho-KO mice are needed to determine precisely how much of the effect of FGF21 on liver metabolism is direct.
FGF15/19 and FGF21 actions on adipose tissue
Both WAT and BAT express βKlotho and FGFR1c at high levels (7), indicating that they are likely to be target tissues for FGF15/19 and FGF21. A role for FGF21 in regulating metabolism was first suggested by in vitro experiments in which it increased glucose uptake in murine 3T3-L1 and primary human adipocytes (39). Additional studies showed that FGF21 induces ERK1/2 phosphorylation and genes involved in glucose uptake, lipogenesis, lipolysis and other aspects of lipid metabolism in white adipocytes in vitro and in vivo (14, 39, 63). Among the genes induced by FGF21 in white adipocytes was uncoupling protein 1 (Ucp1) (33), which is typically found in brown adipocytes, where it causes uncoupled respiration and thermogenesis. This so-called “browning” of white adipocytes occurs in subcutaneous WAT depots that are innervated by the sympathetic nervous system and likely contributes to FGF21's thermogenic effects (33). The expression and secretion of adiponectin, a hormone that regulates glucose and fatty acid homeostasis was also induced by FGF21 in white adipocytes both in vitro and in vivo (66, 67). Adiponectin is required for the full metabolic efficacy of FGF21 in vivo (66, 67).
As in white adipocytes, FGF21 stimulates glucose uptake and thermogenic gene expression in brown adipocytes both in vitro and in vivo (32, 33, 40, 49, 68). In terms of molecular mechanism, FGF21 increased the phosphorylation of the transcription factor cAMP response element-binding protein (CREB), which directly regulates Ucp1 gene transcription (54). FGF21 also increased the phosphorylation of a second transcription factor, STAT3, which regulates mitochondrial respiration (54). Taken together, these findings highlight an important role for FGF21 in promoting thermogenesis by acting directly on BAT.
Two different loss-of-function approaches have been used to evaluate the importance of FGF21 acting on adipose tissue in vivo. First, FGF21 has been tested in aP2-SREBP-1c transgenic mice, which lack WAT and have dysfunctional BAT. These lipodystrophic mice were refractory to FGF21's beneficial effects on body weight, plasma insulin and glucose tolerance (54, 69). Second, the consequence of eliminating either βKlotho or FGFR1 selectively in adipose tissue in mice was examined using the aP2-Cre transgene, which disrupts floxed (fl) gene alleles in both WAT and BAT. The acute effects of FGF21 on insulin sensitivity and glucose uptake in BAT were lost in diet-induced obese mice with adipose tissue-selective βKlotho (βKlothofl/fl;aP2-Cre) deletion (49). Similarly, the effects of FGF21 on body weight as well as plasma glucose, insulin, triglycerides and adiponectin were lost in diet-induced obese mice with FGFR1 selective deletion in adipose tissue (FGFR1fl/fl;aP2-Cre) (52). However, since the aP2-Cre transgene is also expressed in the nervous system (70), including sites where βKlotho is expressed (see below), additional studies are required with adipose-specific Cre drivers to clarify the relative importance of adipose tissue in the FGF21 response.
FGF19 administration also induces ERK1/2 phosphorylation in WAT (63). A recent study compared the pharmacologic effects of FGF19 and FGF21 in diet-induced obese FGFR1fl/fl;aP2-Cre mice (52). The weight loss effects of both FGF19 and FGF21 were compromised in these KO mice, suggesting a common mechanism. However, while the beneficial glycemic actions of FGF21 were lost in FGFR1fl/fl;aP2-Cre mice, those of FGF19 were not. These data highlight important differences in the target tissues and/or target receptor complexes of FGF15/19 and FGF21.
FGF15/19 and FGF21 actions on the nervous system
While all four FGFRs are broadly expressed throughout the nervous system, βKlotho has a much more restricted expression pattern (7). In the hypothalamus, βKlotho is expressed in the suprachiasmatic nucleus (SCN) (17), which regulates circadian rhythm, and in the paraventricular nucleus (PVN) (18), which is activated in response to various physiological stresses. βKlotho is also expressed in the area postrema and solitary nucleus in the hindbrain and in the nodose ganglia of the periphery (17). Together these nuclei comprise the dorsal-vagal complex, which serves as a major integrative center for the autonomic nervous system.
Neither FGF15/19 nor FGF21 are expressed in the adult brain (7), although FGF15/19 is expressed in the nervous system during development (71, 72). FGF21 crosses the blood-brain barrier by simple diffusion (73) and is present in human cerebrospinal fluid (74) and in the hypothalamus of fasted mice (18), where it induces ERK1/2 phosphorylation (75). Intracerebroventricular (i.c.v) injection of FGF21 increases energy expenditure and insulin sensitivity in diet-induced obese rats (76), and central administration of FGF21 is sufficient to promote hepatic gluconeogenesis in lean mice (18), demonstrating that FGF21 can act directly on the brain. Likewise, i.c.v. injection of FGF19 improved glucose tolerance and increased metabolic rate in ob/ob mice through an insulin-independent mechanism (41, 77). In rats, i.c.v. injection of FGF19 improved glucose tolerance, decreased food intake and caused weight loss (78). However, FGF19 crosses the blood-brain barrier less efficiently than FGF21 (79). Whether this difference in brain permeability has physiologic or pharmacologic consequences remains to be determined.
Recent work has shown that βKlotho expression in the nervous system is required for many of the chronic actions of FGF21 in lean mice, including its effects on ketogenesis, growth, circadian behavior and female reproduction (16, 17). Likewise, in diet-induced obese mice, the beneficial metabolic effects of FGF21 on body weight and insulin sensitivity were absent in mice lacking βKlotho in the nervous system as were its effects on metabolic gene expression in WAT, BAT and liver (64). These studies further showed that FGF21 acts on the nervous system to stimulate sympathetic nerve outflow to BAT, which induces fatty acid oxidation and thermogenesis. All of these effects require that FGF21 acts on the hypothalamus, since the phenotypes were maintained in mice lacking βKlotho in the dorsal-vagal complex but were lost in mice lacking βKlotho in both the hypothalamus and the dorsal-vagal complex (64). FGF21 may also stimulate sympathetic outflow to WAT to induce both the browning of white adipocytes and the lipolysis required for hepatic ketogenesis. This would explain why these effects of FGF21 are lost in mice lacking βKlotho in the nervous system (17, 64).
In the hypothalamus, FGF21 affects the expression of two key neuropeptides: arginine vasopressin (AVP) and corticotropin-releasing factor (CRF) (16–18, 64). In lean mice, chronic administration of FGF21 dampens Avp expression in the SCN (16). Conversely, Avp mRNA levels were elevated in the hypothalamus of fasted, nervous system-specific βKlotho-KO mice. In female mice, reduced AVP levels cause lower levels of luteinizing hormone, which is secreted from the pituitary to induce ovulation. Accordingly, FGF21 administration blocked the estrous cycle. This anovulatory effect of FGF21 was overcome by i.c.v. administration of AVP (16).
In addition to suppressing AVP, FGF21 administration induces Crf expression in the hypothalamus (17, 18, 64). CRF in turn increases adrenocorticotropic hormone secretion from the pituitary, which stimulates adrenal glucocorticoid secretion and hepatic gluconeogenesis. Accordingly, FGF21 administration increased circulating corticosterone levels and gluconeogenesis in mice (13, 17). Conversely, the induction of Crf and gluconeogenesis by starvation was attenuated in FGF21-KO mice (13, 18). CRF also activates sympathetic nerve activity to BAT (80). Notably, FGF21's induction of sympathetic nerve activity to BAT was completely blocked by a CRF receptor antagonist (64). Thus, induction of CRF represents a common mechanism whereby FGF21 induces glucocorticoids/gluconeogenesis and thermogenesis/weight loss. CRF also inhibits growth and female reproduction (81, 82), and thus may contribute to FGF21's effects on these processes as well.
It remains to be determined precisely where in the hypothalamus FGF21 acts to induce Crf expression. βKlotho and CRF are most highly expressed in the SCN and PVN, respectively. Since SCN-mediated regulation of CRF is the basis for daily fluctuations in glucocorticoid concentrations (83), FGF21 may regulate CRF expression in the PVN indirectly by acting on the SCN. However, a recent report showed that βKlotho is also expressed in CRF neurons in the PVN, where FGF21 induces Crf gene expression by activating the transcription factor CREB (18), much like FGF21 induces Ucp1 in BAT (54). Thus, FGF21 could induce Crf in the PVN through either direct or indirect actions.
Concluding remarks and future perspectives
Since their cloning at the turn of the millennium, the characterization of FGF15/19 and FGF21 has been replete with interesting twists and turns, from the finding that both can act as hormones to the discovery of their striking and diverse biological actions (Table 1). Although tremendous progress has been made in understanding the physiologic and pharmacologic actions of these two hormones, much remains to be learned regarding their mechanisms of action. For example, while this review is focused on liver, adipose tissue and brain (summarized in Figure 1), βKlotho and the FGF receptors are expressed in other key metabolic tissues such as endocrine and exocrine pancreas (37, 38), where its contribution to the whole-body actions of FGF15/19 and FGF21 remains largely unexplored. In addition, while the requirement of βKlotho is well established, further studies using tissue-specific knockouts of the various FGFR genes are needed to establish their definitive involvement in mediating many of the effects of FGF15/19 and FGF21.
FGF21 mediates many of its pharmacologic effects by acting directly on the nervous system and adipose tissue. One outstanding question is why knocking out either βKlotho in the nervous system or FGFR1 in adipose tissue has a similar disruptive effect on FGF21 action. One possibility is that FGF21 acts on adipose tissue and brain simultaneously to provide both the fuel and the fire to drive weight loss. In this regard, efficient BAT-mediated thermogenesis requires both the mobilization of oxidative substrate and the induction of sympathetic outflow (84). FGF21 regulates both processes in BAT and presumably browned WAT, which likely accounts for its efficacy in causing weight loss in the context of obesity. The finding that FGF21-mediated induction of thermogenesis requires a state of energy excess (64) explains why FGF21 does not increase energy expenditure in lean mice. While at least some humans have metabolically-active BAT and the capacity to brown WAT (85), it remains to be determined if FGF21 acts through the same mechanism in people. It also remains to be seen whether FGF21 activates sympathetic outflow to tissues other than BAT such as the heart and vasculature. If so, FGF21 may have effects on heart rate and blood pressure.
As noted above, activating antibodies against either FGFR1c or the FGFR1c/βKlotho heteromer can recapitulate many of the pharmacologic effects of both FGF19 and FGF21 (53, 54). Given that the SCN and PVN are protected by the blood-brain barrier, which is generally considered impervious to antibodies, how can these findings be reconciled with a requirement for FGF21 to act on the nervous system? We suggest two possibilities. First, assuming that there is cooperativity between FGFR1c/βKlotho complexes in the nervous system and adipose tissue, efficacious activation of receptors in the adipose tissue alone may elicit a response in the context of some minimal degree of endogenous FGF21 activity in the brain. A second, trivial explanation is suggested by evidence that at least some antibodies, including one against FGFR1 (86), can cross the blood-brain barrier, albeit inefficiently. Thus, even low levels of high-affinity antibodies might be sufficient to elicit the response.
While FGF15/19 shares many of FGF21's pharmacologic effects, less is known about its target tissues. Because FGF15/19 acts on the FGFR4/βKlotho complex, it has strong effects in liver that are not shared by FGF21. In addition to its requisite role in bile acid homeostasis, FGF15/19 suppresses hepatic gluconeogenesis and induces glycogen synthesis (56, 57, 87), which could contribute to its beneficial glycemic profile. However, the finding that FGF19 derivatives with little or no FGFR4 activity retain their glycemic activity in ob/ob mice (88, 89) suggests that FGF19 exerts a major part of its metabolic effect through direct actions on tissues other than liver.
In closing, FGF15/19 and FGF21 are both exciting candidates for treating metabolic disease, and derivatives of both are currently in clinical trials. In addition, FGF15/19 may have utility in treating bile acid-related diseases such as primary biliary cirrhosis and bile acid diarrhea. However, both hormones have potential side effects, including liver mitogenicity for FGF15/19 (60) and bone loss for FGF21 (47). Whether these side effects are manifest in patients remains to be seen. Notably, it may be possible to engineer derivatives that minimize or lack these side effects. Already, FGF15/19 derivatives have been reported that lack the liver mitogenicity while retaining its beneficial metabolic or bile acid effects (89, 90). Clearly, future studies into understanding the mechanisms of action of FGF15/19 and FGF21 are the key to unlocking their full pharmaceutical potential.
FGF15/19 and FGF21 have beneficial effects on body weight and insulin sensitivity.
FGF15/19 acts on the liver to regulate bile acid homeostasis.
FGF21 acts on adipose tissue and the brain to exert many of its metabolic effects.
FGF21 activates the sympathetic nervous system.
Outstanding Questions Box.
Do FGF15/19 and FGF21 exert their beneficial effects on body weight, insulin sensitivity and dyslipidemia through the same mechanism?
Do activating antibodies against either FGFR1c or the FGFR1c/βKlotho heteromer exert their metabolic effects through the same pathways as FGF15/19 and FGF21?
Why does elimination of the FGFR1c/βKlotho receptor complex in either adipose tissue or brain disrupt FGF21's weight loss and insulin sensitizing actions?
How much of the effect of FGF15/19 and FGF21 on hepatic metabolism is mediated directly on the liver?
Does FGF21 activate sympathetic outflow to tissues other than BAT such as WAT, adrenal, heart and the vasculature?
What are the functions of βKlotho in the endocrine and exocrine pancreas?
Acknowledgments
This work was supported by National Institutes of Health (grant R01DK067158 to S.A.K. and D.J.M.), the Robert A. Welch Foundation (grant I-1558 to S.A.K. and grant I-1275 to D.J.M.), and the Howard Hughes Medical Institute (D.J.M.).
Glossary Box
- Arginine vasopressin (AVP)
Neuropeptide that regulates many physiologic processes, including water retention and vasoconstriction. Among its actions, AVP secreted from the SCN inhibits the hypothalamic-pituitary-adrenal axis and glucocorticoid secretion
- Brown adipose tissue (BAT)
Type of adipose tissue that is specialized to dissipate the mitochondrial proton gradient as heat
- Corticotropin-releasing factor (CRF)
Neuropeptide that regulates many stress-related processes. Among its actions, CRF secreted from the PVN activates the hypothalamic-pituitary-adrenal axis to stimulate glucocorticoid secretion
- Cytochrome P450 7A1 (CYP7A1)
Enzyme that catalyzes the first and rate-limiting step in the classic bile acid synthetic pathway
- Extracellular signal-regulated kinases 1 and 2 (ERK1/2)
Intracellular protein kinases activated by diverse extracellular stimuli, including FGFs
- Fibroblast growth factors (FGFs)
Family of secreted proteins that regulate diverse biological processes, including growth, development, differentiation and metabolism
- FGF receptors (FGFRs)
Transmembrane proteins with an intracellular tyrosine kinase domain that is activated by the binding of an FGF to an extracellular domain. In mammals, there are four genes encoding FGFRs
- Intracerebroventricular (i.c.v.)
Direct introduction of drugs or chemicals into the ventricles of the brain
- Paraventricular nucleus (PVN)
Region of the hypothalamus in brain activated by various stress conditions. It secretes the neuropeptide CRF
- Suprachiasmatic nucleus (SCN)
Region of hypothalamus in brain that controls circadian behavior
- Uncoupling protein-1 (UCP1)
Protein present on inner mitochondrial membrane that disrupts the respiratory proton gradient, resulting in the generation of heat. Present in BAT and induced by cold and other stress condition in WAT
- White adipose tissue (WAT)
Type of adipose tissue that is specialized to synthesize and store fatty acids as triglyceride
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8:235–253. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goetz R, Beenken A, Ibrahimi OA, Kalinina J, Olsen SK, Eliseenkova AV, Mohammadi M. Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol Cell Biol. 2007;27:3417–3428. doi: 10.1128/MCB.02249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu MC, Shiizaki K, Kuro-o M, Moe OW. Fibroblast growth factor 23 and Klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annual review of physiology. 2013;75:503–533. doi: 10.1146/annurev-physiol-030212-183727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Potthoff MJ, Kliewer SA, Mangelsdorf DJ. Endocrine fibroblast growth factors 15/19 and 21: from feast to famine. Genes Dev. 2012;26:312–324. doi: 10.1101/gad.184788.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Kliewer SA. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Choi M, Moschetta A, Bookout AL, Peng L, Umetani M, Holmstrom SR, Kliewer SA. Identification of a hormonal basis for gallbladder filling. Nat Med. 2006;12:1253–1255. doi: 10.1038/nm1501. [DOI] [PubMed] [Google Scholar]
- 7.Fon Tacer K, Bookout AL, Ding X, Kurosu H, John GB, Wang L, Kliewer SA. Research resource: Comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol Endocrinol. 2010;24:2050–2064. doi: 10.1210/me.2010-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markan KR, Naber MC, Ameka MK, Anderegg MD, Mangelsdorf DJ, Kliewer SA, Potthoff MJ. Circulating FGF21 is Liver Derived and Enhances Glucose Uptake During Refeeding and Overfeeding. Diabetes. 2014 doi: 10.2337/db14-0595. 10.2337/db14-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Kliewer SA. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Lundasen T, Hunt MC, Nilsson LM, Sanyal S, Angelin B, Alexson SE, Rudling M. PPARalpha is a key regulator of hepatic FGF21. Biochem Biophys Res Commun. 2007;360:437–440. doi: 10.1016/j.bbrc.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Giannikopoulos P, Duncan SA, Wang J, Johansen CT, Brown JD, Lee AH. The transcription factor cyclic AMP-responsive element-binding protein H regulates triglyceride metabolism. Nature Med. 2011;17:812–815. doi: 10.1038/nm.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, Burgess SC. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci U S A. 2009;106:10853–10858. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Kharitonenkov A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149:6018–6027. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- 15.Inagaki T, Lin VY, Goetz R, Mohammadi M, Mangelsdorf DJ, Kliewer SA. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab. 2008;8:77–83. doi: 10.1016/j.cmet.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Owen BM, Bookout AL, Ding X, Lin VY, Atkin SD, Gautron L, Mangelsdorf DJ. FGF21 contributes to neuroendocrine control of female reproduction. Nature Med. 2013;19:1153–1156. doi: 10.1038/nm.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bookout AL, de Groot MH, Owen BM, Lee S, Gautron L, Lawrence HL, Kliewer SA. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nature Med. 2013;19:1147–1152. doi: 10.1038/nm.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang Q, Zhong L, Zhang J, Wang Y, Bornstein SR, Triggle CR, Xu A. FGF21 Maintains Glucose Homeostasis by Mediating the Cross Talk Between Liver and Brain During Prolonged Fasting. Diabetes. 2014 doi: 10.2337/db14-0541. 10.2337/db14-0541. [DOI] [PubMed] [Google Scholar]
- 19.Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC, Morrison CD. FGF21 is an endocrine signal of protein restriction. J Clin Invest. 2014 doi: 10.1172/JCI74915. 10.1172/JCI74915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Sousa-Coelho AL, Relat J, Hondares E, Perez-Marti A, Ribas F, Villarroya F, Haro D. FGF21 mediates the lipid metabolism response to amino acid starvation. J Lipid Res. 2013;54:1786–1797. doi: 10.1194/jlr.M033415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Sousa-Coelho AL, Marrero PF, Haro D. Activating transcription factor 4-dependent induction of FGF21 during amino acid deprivation. Biochem J. 2012;443:165–171. doi: 10.1042/BJ20111748. [DOI] [PubMed] [Google Scholar]
- 22.Badman MK, Koester A, Flier JS, Kharitonenkov A, Maratos-Flier E. Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endocrinology. 2009;150:4931–4940. doi: 10.1210/en.2009-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christodoulides C, Dyson P, Sprecher D, Tsintzas K, Karpe F. Circulating fibroblast growth factor 21 is induced by peroxisome proliferator-activated receptor agonists but not ketosis in man. J Clin Endocrinol Metab. 2009;94:3594–3601. doi: 10.1210/jc.2009-0111. [DOI] [PubMed] [Google Scholar]
- 24.Dushay J, Chui PC, Gopalakrishnan GS, Varela-Rey M, Crawley M, Fisher FM, Maratos-Flier E. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology. 2010;139:456–463. doi: 10.1053/j.gastro.2010.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galman C, Lundasen T, Kharitonenkov A, Bina HA, Eriksson M, Hafstrom I, Rudling M. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARalpha activation in man. Cell Metab. 2008;8:169–174. doi: 10.1016/j.cmet.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 26.Mraz M, Bartlova M, Lacinova Z, Michalsky D, Kasalicky M, Haluzikova D, Haluzik M. Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor-21 in patients with type 2 diabetes and obesity. Clin Endocrinol (Oxf) 2009;71:369–375. doi: 10.1111/j.1365-2265.2008.03502.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Yeung DC, Karpisek M, Stejskal D, Zhou ZG, Liu F, Xu A. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008;57:1246–1253. doi: 10.2337/db07-1476. [DOI] [PubMed] [Google Scholar]
- 28.Fisher FM, Chui PC, Antonellis PJ, Bina HA, Kharitonenkov A, Flier JS, Maratos-Flier E. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes. 2010;59:2781–2789. doi: 10.2337/db10-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muise ES, Azzolina B, Kuo DW, El-Sherbeini M, Tan Y, Yuan X, Wong KK. Adipose fibroblast growth factor 21 is up-regulated by peroxisome proliferator-activated receptor gamma and altered metabolic states. Mol Pharmacol. 2008;74:403–412. doi: 10.1124/mol.108.044826. [DOI] [PubMed] [Google Scholar]
- 30.Dutchak PA, Katafuchi T, Bookout AL, Choi JH, Yu RT, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor-21 regulates PPARgamma activity and the antidiabetic actions of thiazolidinediones. Cell. 2012;148:556–567. doi: 10.1016/j.cell.2011.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chartoumpekis DV, Habeos IG, Ziros PG, Psyrogiannis AI, Kyriazopoulou VE, Papavassiliou AG. Brown adipose tissue responds to cold and adrenergic stimulation by induction of FGF21. Mol Med. 2011;17:736–740. doi: 10.2119/molmed.2011.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hondares E, Iglesias R, Giralt A, Gonzalez FJ, Giralt M, Mampel T, Villarroya F. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J Biol Chem. 2011;286:12983–12990. doi: 10.1074/jbc.M110.215889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Spiegelman BM. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tyynismaa H, Carroll CJ, Raimundo N, Ahola-Erkkila S, Wenz T, Ruhanen H, Suomalainen A. Mitochondrial myopathy induces a starvation-like response. Hum Mol Genet. 2010;19:3948–3958. doi: 10.1093/hmg/ddq310. [DOI] [PubMed] [Google Scholar]
- 35.Kim KH, Jeong YT, Oh H, Kim SH, Cho JM, Kim YN, Lee MS. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nature Med. 2013;19:83–92. doi: 10.1038/nm.3014. [DOI] [PubMed] [Google Scholar]
- 36.Omar BA, Andersen B, Hald J, Raun K, Nishimura E, Ahren B. Fibroblast growth factor 21 (FGF21) and glucagon-like peptide 1 contribute to diabetes resistance in glucagon receptor-deficient mice. Diabetes. 2014;63:101–110. doi: 10.2337/db13-0710. [DOI] [PubMed] [Google Scholar]
- 37.Johnson CL, Weston JY, Chadi SA, Fazio EN, Huff MW, Kharitonenkov A, Pin CL. Fibroblast growth factor 21 reduces the severity of cerulein-induced pancreatitis in mice. Gastroenterology. 2009;137:1795–1804. doi: 10.1053/j.gastro.2009.07.064. [DOI] [PubMed] [Google Scholar]
- 38.Wente W, Efanov AM, Brenner M, Kharitonenkov A, Koster A, Sandusky GE, Gromada J. Fibroblast growth factor-21 improves pancreatic beta-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes. 2006;55:2470–2478. doi: 10.2337/db05-1435. [DOI] [PubMed] [Google Scholar]
- 39.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Shanafelt AB. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Veniant MM. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58:250–259. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fu L, John LM, Adams SH, Yu XX, Tomlinson E, Renz M, Stewart TA. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology. 2004;145:2594–2603. doi: 10.1210/en.2003-1671. [DOI] [PubMed] [Google Scholar]
- 42.Tomlinson E, Fu L, John L, Hultgren B, Huang X, Renz M, Stewart TA. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology. 2002;143:1741–1747. doi: 10.1210/endo.143.5.8850. [DOI] [PubMed] [Google Scholar]
- 43.Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, Etgen GJ. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007;148:774–781. doi: 10.1210/en.2006-1168. [DOI] [PubMed] [Google Scholar]
- 44.Veniant MM, Komorowski R, Chen P, Stanislaus S, Winters K, Hager T, Xu J. Long-acting FGF21 has enhanced efficacy in diet-induced obese mice and in obese rhesus monkeys. Endocrinology. 2012;153:4192–4203. doi: 10.1210/en.2012-1211. [DOI] [PubMed] [Google Scholar]
- 45.Gaich G, Chien JY, Fu H, Glass LC, Deeg MA, Holland WL, Moller DE. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 2013;18:333–340. doi: 10.1016/j.cmet.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Xie Y, Berglund ED, Coate KC, He TT, Katafuchi T, Mangelsdorf DJ. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. Elife. 2012;1:e00065. doi: 10.7554/eLife.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei W, Dutchak PA, Wang X, Ding X, Wang X, Bookout AL, Wan Y. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of PPAR-gamma. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1200797109. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuro-o M. Klotho and betaKlotho. Adv Exp Med Biol. 2012;728:25–40. doi: 10.1007/978-1-4614-0887-1_2. [DOI] [PubMed] [Google Scholar]
- 49.Ding X, Boney-Montoya J, Owen BM, Bookout AL, Coate KC, Mangelsdorf DJ, Kliewer SA. betaKlotho is required for fibroblast growth factor 21 effects on growth and metabolism. Cell Metab. 2012;16:387–393. doi: 10.1016/j.cmet.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ito S, Fujimori T, Furuya A, Satoh J, Nabeshima Y. Impaired negative feedback suppression of bile acid synthesis in mice lacking betaKlotho. J Clin Invest. 2005;115:2202–2208. doi: 10.1172/JCI23076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adams AC, Cheng CC, Coskun T, Kharitonenkov A. FGF21 requires betaklotho to act in vivo. PLoS One. 2012;7:e49977. doi: 10.1371/journal.pone.0049977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adams AC, Yang C, Coskun T, Cheng CC, Gimeno RE, Luo Y, Kharitonenkov A. The breadth of FGF21's metabolic actions are governed by FGFR1 in adipose tissue. Molecular metabolism. 2012;2:31–37. doi: 10.1016/j.molmet.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Foltz IN, Hu S, King C, Wu X, Yang C, Wang W, Li Y. Treating diabetes and obesity with an FGF21-mimetic antibody activating the betaKlotho/FGFR1c receptor complex. Sci Transl Med. 2012;4:162ra153. doi: 10.1126/scitranslmed.3004690. [DOI] [PubMed] [Google Scholar]
- 54.Wu AL, Kolumam G, Stawicki S, Chen Y, Li J, Zavala-Solorio J, Sonoda J. Amelioration of type 2 diabetes by antibody-mediated activation of fibroblast growth factor receptor 1. Sci Transl Med. 2011;3:113ra126. doi: 10.1126/scitranslmed.3002669. [DOI] [PubMed] [Google Scholar]
- 55.Holt JA, Luo G, Billin AN, Bisi J, McNeill YY, Kozarsky KF, Jones SA. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev. 2003;17:1581–1591. doi: 10.1101/gad.1083503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Potthoff MJ, Boney-Montoya J, Choi M, He T, Sunny NE, Satapati S, Kliewer SA. FGF15/19 Regulates Hepatic Glucose Metabolism by Inhibiting the CREB-PGC-1alpha Pathway. Cell Metab. 2011;13:729–738. doi: 10.1016/j.cmet.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shin DJ, Osborne TF. FGF15/FGFR4 integrates growth factor signaling with hepatic bile acid metabolism and insulin action. J Biol Chem. 2009;284:11110–11120. doi: 10.1074/jbc.M808747200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song KH, Li T, Owsley E, Strom S, Chiang JY. Bile acids activate fibroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7alpha-hydroxylase gene expression. Hepatology. 2009;49:297–305. doi: 10.1002/hep.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bhatnagar S, Damron HA, Hillgartner FB. Fibroblast growth factor-19, a novel factor that inhibits hepatic fatty acid synthesis. J Biol Chem. 2009;284:10023–10033. doi: 10.1074/jbc.M808818200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nicholes K, Guillet S, Tomlinson E, Hillan K, Wright B, Frantz GD, French DM. A mouse model of hepatocellular carcinoma: ectopic expression of fibroblast growth factor 19 in skeletal muscle of transgenic mice. Am J Pathol. 2002;160:2295–2307. doi: 10.1016/S0002-9440(10)61177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu X, Ge H, Lemon B, Vonderfecht S, Weiszmann J, Hecht R, Li Y. FGF19-induced hepatocyte proliferation is mediated through FGFR4 activation. J Biol Chem. 2010;285:5165–5170. doi: 10.1074/jbc.M109.068783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.French DM, Lin BC, Wang M, Adams C, Shek T, Hotzel K, Desnoyers LR. Targeting FGFR4 inhibits hepatocellular carcinoma in preclinical mouse models. PLoS One. 2012;7:e36713. doi: 10.1371/journal.pone.0036713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kurosu H, Choi M, Ogawa Y, Dickson AS, Goetz R, Eliseenkova AV, Kuroo M. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem. 2007;282:26687–26695. doi: 10.1074/jbc.M704165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Owen BM, Ding X, Morgan DA, Coate KC, Bookout AL, Rahmouni K, Mangelsdorf DJ. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab. 2014 doi: 10.1016/j.cmet.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fisher FM, Estall JL, Adams AC, Antonellis PJ, Bina HA, Flier JS, Maratos-Flier E. Integrated Regulation of Hepatic Metabolism by Fibroblast Growth Factor 21 (FGF21) in Vivo. Endocrinology. 2011;152:2996–3004. doi: 10.1210/en.2011-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Holland WL, Adams AC, Brozinick JT, Bui HH, Miyauchi Y, Kusminski CM, Scherer PE. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 2013;17:790–797. doi: 10.1016/j.cmet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin Z, Tian H, Lam KS, Lin S, Hoo RC, Konishi M, Li X. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 2013;17:779–789. doi: 10.1016/j.cmet.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 68.Hondares E, Rosell M, Gonzalez FJ, Giralt M, Iglesias R, Villarroya F. Hepatic FGF21 expression is induced at birth via PPARalpha in response to milk intake and contributes to thermogenic activation of neonatal brown fat. Cell Metab. 2010;11:206–212. doi: 10.1016/j.cmet.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Veniant MM, Hale C, Helmering J, Chen MM, Stanislaus S, Busby J, Lloyd DJ. FGF21 Promotes Metabolic Homeostasis via White Adipose and Leptin in Mice. PLoS One. 2012;7:e40164. doi: 10.1371/journal.pone.0040164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martens K, Bottelbergs A, Baes M. Ectopic recombination in the central and peripheral nervous system by aP2/FABP4-Cre mice: implications for metabolism research. FEBS letters. 2010;584:1054–1058. doi: 10.1016/j.febslet.2010.01.061. [DOI] [PubMed] [Google Scholar]
- 71.Gimeno L, Brulet P, Martinez S. Study of Fgf15 gene expression in developing mouse brain. Gene Expr Patterns. 2003;3:473–481. doi: 10.1016/s1567-133x(03)00059-0. [DOI] [PubMed] [Google Scholar]
- 72.Nishimura T, Utsunomiya Y, Hoshikawa M, Ohuchi H, Itoh N. Structure and expression of a novel human FGF, FGF-19, expressed in the fetal brain. Biochim Biophys Acta. 1999;1444:148–151. doi: 10.1016/s0167-4781(98)00255-3. [DOI] [PubMed] [Google Scholar]
- 73.Hsuchou H, Pan W, Kastin AJ. The fasting polypeptide FGF21 can enter brain from blood. Peptides. 2007;28:2382–2386. doi: 10.1016/j.peptides.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tan BK, Hallschmid M, Adya R, Kern W, Lehnert H, Randeva HS. Fibroblast growth factor 21 (FGF21) in human cerebrospinal fluid: relationship with plasma FGF21 and body adiposity. Diabetes. 2011;60:2758–2762. doi: 10.2337/db11-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang C, Jin C, Li X, Wang F, McKeehan WL, Luo Y. Differential specificity of endocrine FGF19 and FGF21 to FGFR1 and FGFR4 in complex with KLB. PLoS One. 2012;7:e33870. doi: 10.1371/journal.pone.0033870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sarruf DA, Thaler JP, Morton GJ, German J, Fischer JD, Ogimoto K, Schwartz MW. Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes. 2010;59:1817–1824. doi: 10.2337/db09-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Morton GJ, Matsen ME, Bracy DP, Meek TH, Nguyen HT, Stefanovski D, Schwartz MW. FGF19 action in the brain induces insulin-independent glucose lowering. J Clin Invest. 2013;123:4799–4808. doi: 10.1172/JCI70710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ryan KK, Kohli R, Gutierrez-Aguilar R, Gaitonde SG, Woods SC, Seeley RJ. Fibroblast growth factor-19 action in the brain reduces food intake and body weight and improves glucose tolerance in male rats. Endocrinology. 2013;154:9–15. doi: 10.1210/en.2012-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hsuchou H, Pan W, Kastin AJ. Fibroblast growth factor 19 entry into brain. Fluids Barriers CNS. 2013;10:32. doi: 10.1186/2045-8118-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arase K, York DA, Shimizu H, Shargill N, Bray GA. Effects of corticotropin-releasing factor on food intake and brown adipose tissue thermogenesis in rats. Am J Physiol. 1988;255:E255–259. doi: 10.1152/ajpendo.1988.255.3.E255. [DOI] [PubMed] [Google Scholar]
- 81.Kalantaridou SN, Makrigiannakis A, Zoumakis E, Chrousos GP. Stress and the female reproductive system. J Reprod Immunol. 2004;62:61–68. doi: 10.1016/j.jri.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 82.Rivier C, Vale W. Corticotropin-releasing factor (CRF) acts centrally to inhibit growth hormone secretion in the rat. Endocrinology. 1984;114:2409–2411. doi: 10.1210/endo-114-6-2409. [DOI] [PubMed] [Google Scholar]
- 83.Kalsbeek A, Fliers E, Hofman MA, Swaab DF, Buijs RM. Vasopressin and the output of the hypothalamic biological clock. J Neuroendocrinol. 2010;22:362–372. doi: 10.1111/j.1365-2826.2010.01956.x. [DOI] [PubMed] [Google Scholar]
- 84.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 85.Nedergaard J, Bengtsson T, Cannon B. Three years with adult human brown adipose tissue. Annals of the New York Academy of Sciences. 2010;1212:E20–36. doi: 10.1111/j.1749-6632.2010.05905.x. [DOI] [PubMed] [Google Scholar]
- 86.Sun HD, Malabunga M, Tonra JR, DiRenzo R, Carrick FE, Zheng H, Kussie P. Monoclonal antibody antagonists of hypothalamic FGFR1 cause potent but reversible hypophagia and weight loss in rodents and monkeys. American journal of physiology. Endocrinology and metabolism. 2007;292:E964–976. doi: 10.1152/ajpendo.00089.2006. [DOI] [PubMed] [Google Scholar]
- 87.Kir S, Beddow SA, Samuel VT, Miller P, Previs SF, Suino-Powell K, Mangelsdorf DJ. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science. 2011;331:1621–1624. doi: 10.1126/science.1198363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu AL, Coulter S, Liddle C, Wong A, Eastham-Anderson J, French DM, Sonoda J. FGF19 regulates cell proliferation, glucose and bile acid metabolism via FGFR4-dependent and independent pathways. PLoS One. 2011;6:e17868. doi: 10.1371/journal.pone.0017868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu X, Ge H, Lemon B, Vonderfecht S, Baribault H, Weiszmann J, Li Y. Separating mitogenic and metabolic activities of fibroblast growth factor 19 (FGF19) Proc Natl Acad Sci U S A. 2010;107:14158–14163. doi: 10.1073/pnas.1009427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou M, Wang X, Phung V, Lindhout DA, Mondal K, Hsu JY, Ling L. Separating Tumorigenicity from Bile Acid Regulatory Activity for Endocrine Hormone FGF19. Cancer research. 2014;74:3306–3316. doi: 10.1158/0008-5472.CAN-14-0208. [DOI] [PubMed] [Google Scholar]
- 91.Badman MK, Kennedy AR, Adams AC, Pissios P, Maratos-Flier E. A Very Low Carbohydrate Ketogenic Diet Improves Glucose Tolerance in ob/ob Mice Independent of Weight Loss. Am J Physiol Endocrinol Metab. 2009;297:E1197–E1204. doi: 10.1152/ajpendo.00357.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tyynismaa H, Raivio T, Hakkarainen A, Ortega-Alonso A, Lundbom N, Kaprio J, Pietilainen KH. Liver fat but not other adiposity measures influence circulating FGF21 levels in healthy young adult twins. J Clin Endocrinol Metab. 2011;96:E351–355. doi: 10.1210/jc.2010-1326. [DOI] [PubMed] [Google Scholar]
- 93.Berglund ED, Li CY, Bina HA, Lynes SE, Michael MD, Shanafelt AB, Wasserman DH. Fibroblast growth factor 21 controls glycemia via regulation of hepatic glucose flux and insulin sensitivity. Endocrinology. 2009;150:4084–4093. doi: 10.1210/en.2009-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]