Abstract
Cognitive reappraisal has been associated with increased activation in prefrontal cortex (PFC) and cingulate regions implicated in cognitive control and affect regulation. To date, neuroimaging studies of reappraisal have primarily used emotionally evocative scenes, and it remains unclear whether the same cognitive strategy applied to emotional facial expressions would involve similar or different neural underpinnings. The present study used fMRI to examine brain activation during cognitive reappraisal of negatively valenced facial expressions relative to passive viewing of negative and neutral facial expressions. Twenty-two healthy adults completed a cognitive reappraisal task comprised of three different conditions (Look-Neutral, Maintain-Negative, Reappraise-Negative). Results indicated that reappraisal was associated with a decrease in negative affect and engagement of PFC brain regions implicated in cognitive control and affect regulation (DLPFC, mPFC, and VLPFC). Furthermore, individual differences in habitual reappraisal use were associated with greater DLPFC and mPFC activation, while suppression use was associated with greater amygdala activation. The present study provides preliminary evidence that facial expressions are effective alternative ‘targets’ of prefrontal engagement during cognitive reappraisal. These findings are particularly relevant for future research probing the neural bases of emotion regulation in populations for whom aversive scenes may be less appropriate (e.g., children) and illnesses in which aberrant responses to social signals of threat and negative feedback are cardinal phenotypes.
Keywords: cognitive reappraisal, emotion, facial expressions, fMRI
Introduction
Emotion regulation refers to the processes involved in the evaluation and modification of emotional experience and expression (Gross, 1998). While several different types of emotion regulation strategies have been identified, reappraisal has been one of the most widely studied and better understood approaches to volitionally modulate affect (Ochsner & Gross, 2005). Reappraisal is a cognitive-linguistic approach that involves consciously reinterpreting or re-framing the meaning of a stimulus with the intention of modifying its initial emotion-eliciting characteristics and response (Gross, 2001). The ability to effectively reappraise negative emotion has been associated with better physical, psychological, and social outcomes (Gross, 2002; Gross & John, 2003) and is the foundation of most forms of cognitive therapy (Barlow, 2007). In contrast, difficulties with cognitive reappraisal have been associated with several psychopathological conditions (Kring & Werner, 2004) and suggested to be a core mechanism of mood and anxiety disorders (Gross, 2007).
Over the past decade imaging neuroscience research has begun to identify the neural substrates of cognitive reappraisal. These studies have consistently reported that reappraisal involves increased activation of specific areas within the prefrontal cortex (PFC) and anterior cingulate cortex (ACC) (Ochsner & Gross, 2008; see Ochsner et al., 2012 for review). Specifically, reappraisal has been posited to engage a network of regions associated with several important ‘cognitive’ functions, including allocation of attention and working memory implemented by dorsolateral PFC (DLPFC), interpretation of internal and external emotional states implemented by medial PFC (mPFC), response inhibition and selection of information from memory implemented by ventrolateral PFC (VLPFC), and performance monitoring implemented by dorsal ACC (Kim & Hamann, 2007; Ochsner et al., 2002; Phan et al., 2005; see Buhle et al., 2013 and Diekhof et al., 2011 for meta-analyses). Reappraisal has also been shown to decrease activation in limbic regions, such as the amygdala (Buhle et al., 2013; Diekhof et al., 2011)
Neuroimaging studies on emotional processing in general have used several different types of emotional stimuli. Initial studies on the neural correlates of emotional processing often used emotional facial expressions (Gur et al., 2002; Hariri et al., 2000, Phan et al., 2002), which were associated with increased activation in a number of prefrontal (ACC, MPFC), limbic (amygdala, insula) and visual regions (inferior occipital gyrus, fusiform gyrus) (Fusar-Poli et al., 2009). A parallel line of research has used complex emotionally evocative scenes taken from the International Affective Picture System (IAPS; Lang et al., 2008). A meta-analysis of studies examining the processing of emotionally-valenced (both positive and negative) scenes and facial expressions identified increased activation in several overlapping regions, including the amygdala, mPFC, inferior frontal cortex, inferior temporal cortex, and extrastriate occipital cortex (Sabatinelli et al., 2011).
Neuroimaging research on cognitive reappraisal noted above has primarily used aversive, negatively valenced scenes (e.g., IAPS images) to probe the neural correlates of reappraisal, and less often employed emotional faces as ‘target’ stimuli which may have different properties, utility, and advantages. For example, facial expressions can engage attention and cognitive processes without over-activating autonomic and somatic reactions indicative of intense emotional responding (Wangelin et al., 2012). In addition, facial expressions may be more suitable for child and adolescent populations relative to the more vivid, complex, and provocative content (e.g., violent scenes) often depicted in IAPS stimuli to evoke negative affect. Indeed, late childhood and early adolescence is a high risk period for the emergence of psychopathology (Costello et al., 2003, 2006), and future investigations may prefer to use more age-appropriate emotional stimuli (e.g., facial expressions). Lastly, emotional faces that convey threat and/or negative social feedback may have more ecological validity for certain forms of psychopathology (e.g., social phobia, schizophrenia) and future studies examining the neural bases of reappraisal in illnesses in which aberrant responses to aversive social signals are cardinal phenotypes. Thus, it is important to understand the neural correlates of cognitive reappraisal of facial expressions.
To our knowledge, only two studies examining the neural substrates of cognitive reappraisal have used facial expressions. McRae, Misra, and colleagues (2012) found that, relative to passive viewing, reappraisal of negative faces was associated with increased amygdala activation. Goldin and colleagues (2009) compared individuals with social anxiety disorder (SAD) and healthy controls on the neural correlates of cognitive reappraisal using social (‘harsh’ facial expressions) and physical (violent scenes) threat, and the authors reported that healthy control participants exhibited activation of ACC, DLPFC, mPFC, and VLPFC when reappraising harsh facial expressions (and to a greater degree in controls relative to SAD participants). However, there were important limitations to these studies. Specifically, McRae et al. used an ROI-approach and only examined neural activity in the amygdala, and Goldin et al. did not report results for the neural correlates of reappraisal in controls only and used neutral scenes (rather than neutral faces) as a comparison condition, precluding any definitive conclusions about cognitive reappraisal of facial expressions.
The present study used functional magnetic resonance imaging (fMRI) and examined the neural substrates of cognitive reappraisal to negatively valenced facial expressions. Twenty-two healthy adults completed a cognitive reappraisal task of facial expressions, adapted from a prior task that employed evocative scenes (Ochsner et al., 2002; Phan et al., 2005; Urry et al., 2006) and self-report affect was measured after every block of trials. Based on prior research, we hypothesized that, similar to negative scenes, there would be decreased negative affect and increased activation in prefrontal regions implicated in cognitive control (ACC, DLPFC, mPFC, and VLPFC) during reappraisal of negative facial expressions. Several investigations have reported decreased amygdala activation during cognitive reappraisal of negative scenes (Ochsner et al., 2002; Urry et al., 2002). Thus, it is likely that reappraisal of negative facial expressions will also be accompanied by a decrease in amygdala activation. However, the only other study to specifically examine emotion regulation of negative facial expressions found increased amygdala activation during cognitive reappraisal (McRae, Misra, et al., 2012). Therefore, it is also possible that reappraisal of negative facial expressions will be associated with an increase in amygdala activation. Given these conflicting results, we did not make specific hypotheses regarding amygdala activation during reappraisal of negative facial expressions, but the present study may provide further support for either of these perspectives.
Finally, the present study also examined the association between individual differences in habitual emotion regulation strategy use and brain activation during the cognitive reappraisal of negative facial expressions. As previously mentioned, reappraisal is one of the most widely studied approaches to volitionally modulate affect (Ochsner & Gross, 2005); however, there are other strategies available. For instance, expressive suppression is another form of emotion regulation that is associated with poor physical and psychosocial outcomes (Butler et al., 2003; Forkmann et al., 2014). To examine individual differences in typical emotion regulation strategy use, participants completed the Emotion Regulation Questionnaire (ERQ; Gross & John, 2003), which provides separate indices of the tendency to use cognitive reappraisal and expressive suppression when regulating emotions. We hypothesized that greater use of reappraisal (and not suppression) would be associated with increased activation in prefrontal cognitive control regions (ACC, DLPFC, mPFC, and VLPFC) during reappraisal of negative facial expressions
Methods
Participants
The sample included 22 right-handed adults (50% female, 81.8% Caucasian) between the ages of 18 and 55 (M = 25.2, SD = 5.8) recruited via community advertisements. Participants were interviewed by a licensed clinician using the Structured Clinical Interview for DSM-IV (First et al., 2002) and examined by a board-certified psychiatrist. Exclusion criteria included current use of psychoactive medications, a history of any Axis I diagnosis, or history of major medical or neurological illnesses. No participant tested positive for alcohol or illegal substances as screened by breathalyzer and urine drug screen at the time of scanning. All participants provided written informed consent as approved by the local Institutional Review Board.
Measures
The Emotion Regulation Questionnaire (ERQ; Gross & John, 2003) is a 10-item self-report measure of the habitual use of cognitive reappraisal and expressive suppression. Each item is rated on a 7-point Likert scale range from 1 (Strongly disagree) to 7 (Strongly agree), with higher scores indicating a greater tendency to use the particular emotional regulation strategy. The ERQ produces a six-item Reappraisal factor (M = 30.24 SD = 6.66, Cronbach's α= .82) and a four-item Suppression factor (M = 12.67, SD = 4.07, Cronbach's α = .79) that are orthogonal, r(21) = .02, ns.
Cognitive Reappraisal Task and Stimuli
Participants completed a face-variant of a well-established cognitive reappraisal task that employed evocative scenes (Ochsner et al., 2002; Phan et al., 2005; Urry et al., 2006). The task employed active, voluntary regulation of negative emotion using cognitive reappraisal (Ochsner & Gross, 2005), and involved three conditions of interest: Look-Neutral (Look-Neut), Maintain-Negative (Maintain-Neg), and Reappraise-Negative (Reappraise-Neg). Neutral facial expressions were shown during the Look-Neut condition and participants were instructed to simply look at the neutral face. Negative facial expressions were shown during the Maintain-Neg and Reappraise-Neg conditions. During the Maintain-Neg condition, participants were instructed to attend to, be aware of, and experience naturally (without trying to change or alter) the emotional state elicited by the negative facial expression. During the Reappraise-Neg condition, participants were instructed to use cognitive reappraisal (reinterpret, reframe) and voluntarily decrease the intensity of any evoked negative emotion (Gross, 1999; Lazarus, 1991). Prior to the scan, participants were provided with extensive instruction on reappraisal, and participant-generated strategies were reviewed and practiced with faces not used in the actual fMRI experiment to confirm understanding of task instructions.
For reappraisal training, two well-validated examples were provided to facilitate understanding of the strategy: 1) conceptualizing the depicted facial expression in a less negative or more positive way (e.g., anger facial expression could be interpreted as getting pumped up for a sporting event); and 2) objectifying the content of the facial expression (e.g., interpreted as a model practicing an expression). These types of reappraisal strategies have been found to be successful in the volitional regulation of the negative emotions and neural response evoked by aversive stimuli (Ochsner et al., 2002; Phan et al., 2005). We provided these examples for illustrative purposes, but also explained to participants that no single type of reinterpretation strategy would likely be applicable to every stimulus. Thus, participants were instructed to use the most effective reframing strategy they could think of for each facial expression. Participants were also instructed to not look away from the stimuli, close their eyes, or think about other things as a means of reducing their emotional response.
The reappraisal task was completed across three runs. During each run, participants viewed two 20-s blocks of each condition (Look-Neut, Maintain-Neg, Reappraise-Neg) interspersed with 20-s baseline blocks consisting of an image of a white fixation cross on a black background to allow the fMRI signal to return to baseline. During the baseline blocks, participants were asked to stop maintaining or reappraising and attend to the fixation cross. Each block consisted of four images, presented for 5-s each -consecutively, without an interstimulus interval. Prior to each block of images, the instruction to ‘Look’, ‘Maintain’, or ‘Reappraise’ appeared in white text at the center of a black screen for a duration of 5-s. Immediately following each Look-Neut, Maintain-Neg, and Reappraise-Neg block, a black screen with a rating scale appeared for 5-s asking participants to rate “How negative do you feel?” on a 5-point scale (1 = not at all, 5 = extremely) via button response. The order of Look-Neut, Maintain-Neg and Reappraise-Neg blocks was presented in a fixed order across the three scans (Scan 1 – Look-Neut, Maintain-Neg, Reappraise-Neg, Maintain-Neg, Look-Neut, Reappraise-Neg; Scan 2 – Maintain-Neg, Reappraise-Neg, Look-Neut, Reappraise-Neg, Maintain-Neg, Look-Neut; Scan 3 – Reappraise-Neg, Look-Neut, Maintain-Neg, Look-Neut, Reappraise-Neg, Maintain-Neg).
Emotional facial expressions were 72 unique images selected from the NimStim Set of Facial Expressions (http://www.macbrain.org/resources.htm), which consists of naturally posed photographs of professional actors, displaying angry, fearful/afraid, and neutral expressions.1 Stimuli were classified into negative (n = 48) and neutral categories (n = 24), and negative stimuli were equally divided between fear (n = 24) and anger expressions (n = 24). Neutral, negative-fear, and negative-anger stimuli were balanced on gender, ethnicity, and mouth-open vs. mouth-closed expressions. In addition, negative stimuli used in the Maintain-Neg vs. Reappraise-Neg conditions were balanced for the number of fear vs. anger expressions, gender, ethnicity, and mouth-open vs. mouth-closed expressions.
fMRI Data Acquisition
Functional imaging was performed with blood-oxygen-level-dependent (BOLD) sensitive whole-brain fMRI on a 3.0 Tesla GE Signa System (General Electric; Milwaukee, WI) using a standard head coil. Images were acquired with 30 axial, 5 mm thick slices using a standard T2*-sensitive gradient echo reverse-spiral sequence (2-s TR; 25-ms TE; 64 × 64 matrix; 24 cm FOV; 77° flip angle; 3.75 × 3.75 × 5 mm final voxel size) optimized for minimal signal loss in susceptibility-prone areas (amygdala, orbitofrontal cortex). A high-resolution, T1-weighted anatomical scan was also acquired in the same axial orientation (9-ms TR, 1.8-ms TE; 256 × 256 matrix; 256 mm FOV; 15° flip angle; 124 1.2 mm thick slices).
fMRI Data Processing and Analyses
One participant was excluded from analyses due to excessive head movement (> 3 mm), leaving a final N = 21. Conventional preprocessing steps were completed using Statistical Parametric Mapping software (SPM8; Wellcome Trust Centre for Neuroimaging, London, http://www.fil.ion.ucl.ac.uk/spm). Images were temporally corrected for differences in slice time collection, spatially realigned to the first scan, normalized to the Montreal Neurological Institute (MNI) template, and smoothed with a 8 mm isotropic Gaussian kernel.
The general linear model was applied to the time series, convolved with the canonical hemodynamic response function and with a 128-s high-pass filter. Condition effects were modeled with box-car regressors representing the occurrence of each block type, and effects were estimated at each voxel and for each participant. The 5-s instruction and negative affect rating were not included in the model. In addition, the six movement parameters obtained during realignment were included in the model as regressors to account for motion-related effects in BOLD. Contrasts of interest (Maintain-Neg vs. Look-Neut and Reappraise-Neg vs. Maintain-Neg) were generated for each participant; these individual statistical parametric maps were then analyzed at the second level in a random-effects statistical model. Consistent with prior fMRI studies of cognitive reappraisal employing whole-brain analyses (see Diekhof et al., 2011 for meta-analysis) and to strike suitable balance between Type I and Type II errors (Lieberman & Cunningham, 2009), significance was set at p < .005 (uncorrected) with cluster extent threshold greater than 20 contiguous resampled voxels (volume > 160 mm3) for activations falling within a priori regions of interest (ACC, DLPFC, mPFC, and VLPFC). For completeness and to obviate bias and generate new hypotheses for subsequent studies, we report all activations that survived this statistical threshold. The software xjView (http://www.alivelearn.net/xjview) was used to identify anatomical labels for whole-brain findings based on the WFU Pickatlas (Maldjian et al., 2003).
Given our strong a priori hypotheses about the role of the ACC, DLPFC, mPFC, and VLPFC, cluster-based significance thresholding was performed via simulation using the 3dClustSim utility (10,000 iterations) (Analysis of Functional NeuroImages, 2005). Given smoothness estimates of this data (8.5 × 9.9 × 7.4 mm3) across the ACC/DLPFC/mPFC/VLPFC ROI mask with volume of 469 cm3, a family-wise error correction at α < .05 was realized with a voxel threshold of p < .005 and minimum cluster size of 121 voxels (968 mm3).
To clarify magnitude and direction of findings in regions of interest and conduct correlational analyses with subjective ratings of negative affect and individual differences in emotion regulation tendencies, BOLD signal responses (parameter estimates, β-weights in arbitrary units [au] of activation) averaged across all ‘activated’ voxels were extracted from each participant using spherical (10 mm diameter) volumes of interest centering on peak group activations from a priori regions of interest. Separate parameter estimates were extracted for Maintain-Neg vs. Look-Neut and Reappraise-Neg vs. Look-Neut contrasts.
There are several different ways to calculate change between conditions (Nelson, Shankman, Olino, & Klein, 2011). For the correlational analyses, we elected to use residual scores rather than subtraction-based difference scores, which are less effective at isolating variance unique to a particular condition, primarily because the resulting difference score remains correlated with both initial values (Cronbach, & Furby, 1970; DuBois, 1957). Residuals reflect the difference between an individual's observed average response to Reappraise-Neg (vs. Look-Neut) and the average that would be predicted from Maintain-Neg (vs. Look-Neut). The residuals will be independent from the average response to Maintain-Neg but correlated with the average response to Reappraise-Neg. Thus, linear regressions were conducted for each a priori ROI and subjective ratings of negative affect, predicting Reappraise-Neg from Maintain-Neg. Standardized residuals representing variance unique to Reappraise-Neg after controlling for the response to Maintain-Neg were saved for each participant and from each regression and submitted to correlational analyses using Statistical Package for the Social Sciences (SPSS; Version 22).
Results
Behavioral Ratings
A repeated-measures analysis of variance (ANOVA) indicated that negative affect ratings differed between conditions, F(1, 20) = 21.44, p < .001, ηp2 = .52, such that negative affect was greater during Maintain-Neg (M = 2.34, SD = 0.97) relative to Look-Neut (M = 1.33, SD = 0.41), F(1, 20) = 24.02, p < .001, ηp2 = .55, and Reappraise-Neg (M = 1.43, SD = 0.51), F(1, 20) = 27.09, p < .001, ηp2 = .58, while Reappraise-Neg and Look-Neut did not differ, F(1, 20) = 0.78, ns. These results indicate that participants were able to decrease their negative affect during the reappraisal of negative facial expressions to a comparable level as the passive viewing of neutral facial expressions.
Neuroimaging Results
Viewing of negative facial expressions
A direct contrast between Maintain-Neg and Look-Neut was conducted to identify which regions were uniquely activated for viewing negative relative to neutral facial expressions (see Table 1). Results indicated that for Maintain-Neg facial expressions there was greater activation in right VLPFC, left occipital lobe, and right cerebellum.
Table 1.
Whole-Brain Analysis of the Viewing and Reappraisal of Negative Facial Expressions
| MNI Coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Contrast | Region | Hemisphere | Voxels | x | y | z | Z Value |
| M > L | |||||||
| Cerebellum | R | 140 | 20 | −72 | −34 | 4.06 | |
| Occipital Lobe | L | 29 | −42 | −88 | −8 | 3.14 | |
| VLPFC | R | 35 | 54 | 28 | −8 | 3.12 | |
| R > M | |||||||
| DLPFC | L | 1939 | −42 | 2 | 46 | 4.56 | |
| −62 | 6 | 26 | 3.79 | ||||
| MPFC | L | 688 | −4 | 26 | 52 | 3.17 | |
| Amygdala | L | 670 | −16 | −8 | −18 | 4.55 | |
| R | 52 | 26 | 4 | −18 | 3.69 | ||
| Temporal Pole | L | 110 | −40 | 10 | −30 | 4.10 | |
| VLPFC | R | 1648 | 66 | 12 | 12 | 3.98 | |
| 44 | 28 | 34 | 3.43 | ||||
| 56 | 30 | 6 | 3.09 | ||||
| L | 145 | −50 | 32 | −6 | 3.68 | ||
| Occipital Lobe | R | 344 | 38 | −82 | 22 | 3.75 | |
| Fusiform Gyrus | L | 172 | −46 | −48 | −20 | 3.68 | |
| Cuneus | L | 685 | −8 | −78 | 12 | 3.45 | |
Note. Bolded regions indicate a priori areas of interest. All findings were significant at p < .005 (uncorrected) with cluster extent threshold greater than 20 contiguous voxels. All prefrontal clusters for R > M also survived 3dClustSim family wise error correction at α < .05 (voxel threshold of p < .005 and a cluster size of at least 121 voxels). Coordinates with no reported voxel size indicate sub peaks within the larger cluster. DLPFC = dorsolateral prefrontal cortex; L = Look-Neut; M = Maintain-Neg; MNI = Montreal Neurological Institute, MPFC = medial prefrontal cortex; R = Reappraise-Neg; VLPFC = ventrolateral prefrontal cortex.
Reappraisal of negative facial expressions
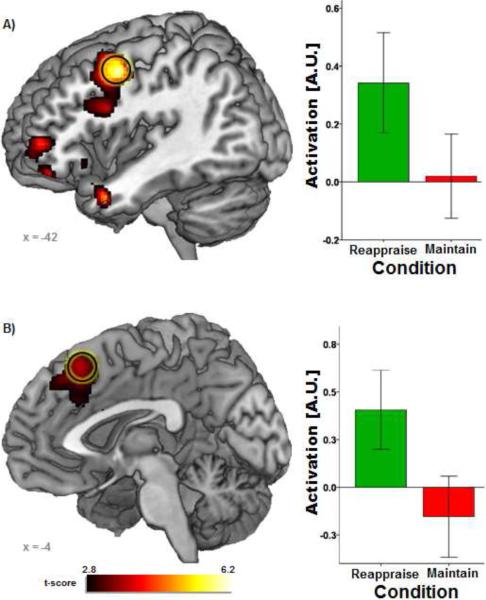
A direct contrast between Reappraise-Neg and Maintain-Neg was conducted to identify regions that were activated during cognitive reappraisal of negative facial expressions (see Table 1). Reappraise-Neg (relative to Maintain-Neut) elicited greater activation in several a priori areas of interest, including left DLPFC, left mPFC, and bilateral VLPFC. Furthermore, Reappraise-Neg was also associated with greater bilateral amygdala activation.2
Change in amygdala activation and prefrontal cognitive control regions during reappraisal
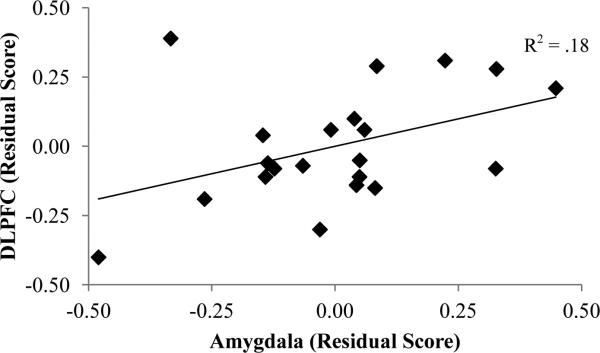
Correlational analyses were conducted to examine the association between reappraisal-based changes in amygdala activation and a priori prefrontal cognitive control regions (DLPFC, mPFC, and VLPFC). Partial correlations were conducted (controlling for age and sex)3 between these regions using residual scores (Reappraise-Neg independent of Maintain-Neg) calculated from the extracted parameter estimates. There was a positive association between amygdala activation and neural response in left DLPFC, pr(21) = .52, p < .05 (see Figure 2), such that increased amygdala activation during reappraisal was accompanied by increased activation in left DLPFC.
Figure 2.
Scatterplot depicting the association between DLPFC and amygdala activation during Reappraise-Neg. Residual scores were calculated by regressing Reappraise-Neg on Maintain-Neg. DLPFC = dorsolateral prefrontal cortex.
Change in negative affect and brain activation during reappraisal
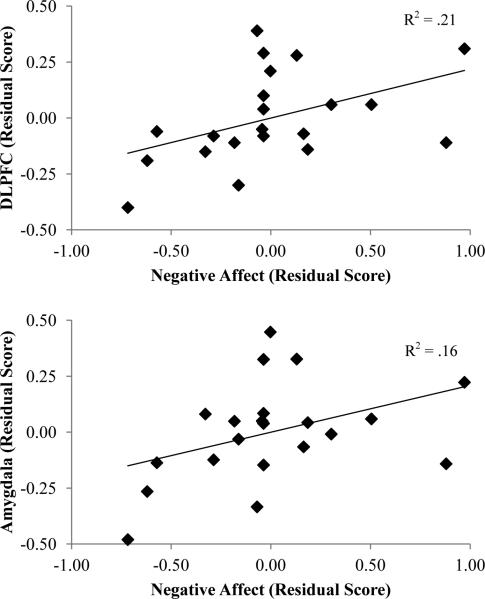
Partial correlations were also conducted to examine the association between change in negative affect and brain activation during reappraisal in a priori cognitive control (DLPFC, mPFC, and VLPFC) and limbic regions (amygdala). Residual scores were used for the change in negative affect and brain activation. There was a positive association between negative affect and brain activation in left DLPFC, pr(21) = .47, p < .05, and bilateral amygdala, pr(21) = .61, p < .01 (see Figure 3). Thus, decreased negative affect during reappraisal was associated with decreased activation in these specific cognitive control and limbic regions.
Figure 3.
Scatterplots depicting the association between DLPFC (top) and amygdala activation (bottom) and negative affect during Reappraise-Neg. Residual scores were calculated by regressing Reappraise-Neg on Maintain- Neg. DLPFC = dorsolateral prefrontal cortex.
Individual differences in emotion regulation and brain activation during reappraisal
Finally, we examined whether individual differences in the habitual tendency to use reappraisal or expressive suppression were associated with brain activation during reappraisal in a priori cognitive control (DLPFC, mPFC, and VLPFC) and limbic regions (amygdala). Residual scores were used for change in brain activation. There was a positive association between ERQ-Reappraisal and brain activation in left DLPFC, pr(21) = .46, p < .05, and mPFC, pr(21) = .46, p < .05. In addition, ERQ-Suppression was positively associated with brain activation in bilateral amygdala, pr(21) = .49, p < .05. These results indicate that the more participants reported using reappraisal, the greater their left DLPFC and MPFC activation, whereas the more they reported using suppression, the greater their bilateral amygdala activation during reappraisal of negative facial expressions.
Discussion
The present study found that cognitive reappraisal of negatively valenced, aversive faces was associated with a decrease in negative affect and engaged several prefrontal brain regions. Specifically, relative to viewing negative faces, reappraisal of negative faces was associated with increased activation in several cognitive control regions, including left DLPFC, bilateral VLPFC, and mPFC. These results are consistent with previous neuroimaging studies of reappraisal-based cognitive strategies using emotional scenes (Diekhof et al., 2011; Phan et al., 2005) and replicate the finding of increased DLPFC and mPFC activation in one of the few studies that has examined the neural substrates of reappraisal using facial expressions (Goldin et al., 2009). Interestingly, reappraisal was not associated with ACC activation. However, research has been mixed regarding ACC involvement during cognitive reappraisal (Ochsner & Gross, 2008), and some evidence suggests that ACC activation may be more related to emotional suppression than reappraisal (Goldin et al., 2008).
Cognitive reappraisal is a multifaceted process encompassing a number of different cognitive functions. The present study suggests that DLPFC, mPFC, and VLPFC regions might constitute a common, shared PFC module used to implement cognitive reappraisal regardless of stimulus type. Specifically, cognitive reappraisal of negative stimuli appears to involve selective attention and working memory implemented by DLPFC, response selection and inhibition implemented by VLPFC, and semantic and reflective processes relevant to modifying stimulus meaning implemented by mPFC (Ochsner & Gross, 2008; see Ochsner et al., 2012). Furthermore, a recent meta-analysis of neuroimaging studies on cognitive reappraisal of emotion indicated that reappraisal consistently activated DLPFC, mPFC, and VLPFC regions and deactivated amygdala (Buhle et al., 2013)4 and further supports these regions as comprising a common reappraisal neural network. However, it is important to note that several of these regions have been implicated in other emotion regulation strategies (e.g., distancing; Koenigsberg et al., 2010) and may not necessarily be unique to cognitive reappraisal.
There was an association between change in self-reported negative affect and neural response during reappraise relative to maintain conditions. Specifically, decreased negative affect was associated with decreased activation in left DLPFC and bilateral amygdala. The association between negative affect and amygdala activation is consistent with previous research (Phan et al., 2005), and supports the notion that cognitive reappraisal of negative affect can attenuate both neural response during evaluation and experience of negative affect. However, the association between negative affect and DLPFC activation contradicts previous research demonstrating an inverse relationship and supporting the effectiveness of emotion regulation (Phan et al., 2005; Wager et al., 2008). It is possible though that as negative affect decreased, the need to utilize regulatory abilities implemented by cognitive control regions decreased as well. Of note, these correlations were between-subjects, and future research using alternative techniques, such as psychophysiological interaction, is needed to better understand the dynamic relationship between changes in negative affect and neural response during reappraisal within-subjects.
Changes in neural response during reappraisal were also associated with changes in amygdala activation. Interestingly, there was a positive association between change in bilateral amygdala activation and left DLPFC. These results are consistent with the amygdala signaling the need to reappraise and being accompanied by increased engagement of prefrontal regions implemented in cognitive control. There was also increased amygdala activation during reappraisal relative to viewing negative faces. There is a common theoretical view that reappraisal should decrease brain activation in regions associated with emotion representation (e.g., amygdala). However, this literature has almost exclusively relied on the use of evocative emotional scenes, and it is less clear whether reappraisal of facial expressions will also reduce amygdala activation. Consistent with our results, the one previous study to examine facial expressions found that reappraisal of negative faces (relative to look) actually increased amygdala response (McRae, Misra, et al., 2012). The authors suggest that facial expressions are more likely to elicit bottom-up emotion generation, which involves more inherent emotional perceptual properties of a stimulus (e.g., an open mouth). The process of identifying these properties, creating a context, and transforming them into a linguistic representation (i.e., the appraisal) may have contributed to increased amygdala activation. In contrast, emotional scenes often present a contextual foundation and allow the individual to more readily engage in reappraisal. Nonetheless, several of studies using emotional scenes have found no change in amygdala activation during reappraisal (Johnstone et al., 2007; Phan et al., 2005; Urry et al., 2006).
There were also methodological limitations that may have contributed to increased amygdala activation during reappraisal. For example, the present study used a block design that combined both initial appraisal and subsequent reappraisal of negative stimuli, and increased amygdala activation during reappraisal could have been due to increased attention and evaluation required to reinterpret negative stimuli. In other words, the neural response measured during reappraisal trials always contained the initial appraisal and evaluation of the negative stimulus, but may not have always included successful reappraisal. This hypothesis is further supported by the finding of greater activation in the left fusiform gyrus during the reappraise relative to maintain condition, and suggests that participants may have initially viewed more negative/threatening aspects of the stimuli (e.g., eyes) in order to effectively modulate their emotional state.
Interestingly, there was no difference in amygdala activation during the viewing of negative relative to neutral facial expressions. Indeed, previous studies have reported increased amygdala activation during the processing of negative relative to neutral facial expressions (Williams et al., 2004). However, several studies have indicated that the amygdala is not uniquely associated with emotional facial expressions (Winston et al., 2003; Yang et al., 2002). Furthermore, Fitzgerald et al. (2006) found that fearful, angry, disgusted, sad, happy, and neutral facial expressions all elicited increased amygdala activation relative to color photographs of portable radios, and none of the emotional facial expressions elicited greater amygdala activation relative to neutral facial expressions. The authors suggest that the amygdala may have a broader role in emotional salience or relevance detection during the perception and processing of faces and is better defined as a general-purpose processor of all forms of salient social information.
Individual differences in the habitual use of different emotion regulation strategies were associated with prefrontal and limbic activation during cognitive reappraisal of negative facial expressions. Reappraisal use was positively associated with brain activation in left DLPFC and mPFC, whereas suppression use was positively associated with brain activation in bilateral amygdala. These results are consistent with previous research indicating individual differences in reappraisal use predicts prefrontal engagement during reappraisal (Drabant et al., 2009), while expressive suppression increases amygdala activation (Goldin et al., 2008). Furthermore, these results may at least partially explain the finding of increased amygdala activation during reappraisal of negative facial expressions—that is, individuals who tend to suppress their emotional reaction may have inadvertently done so when attempting to engage in cognitive reappraisal.
The present study has several important research implications. The results suggest that emotional faces are effective alternative ‘targets’ of prefrontal engagement during cognitive reappraisal in functional imaging studies on the neural substrates of reappraisal. Indeed, there are many different types of emotion-eliciting stimuli (e.g., movie clips, electric shocks, imagery), and it remains unclear whether the neural substrates for reappraisal of those (and other) stimuli are comparable. Moreover, emotionally evocative images and aversive experiences may be less appropriate in certain vulnerable populations (e.g., children) in the context of fMRI studies. Facial expressions may be less likely to produce residual negative affect after completion of a cognitive reappraisal task. Finally, faces may have more ecological validity for certain forms of psychopathology (e.g., social phobia, schizophrenia) and future studies examining the neural bases of reappraisal in in illnesses in which aberrant responses to social signals of threat and negative feedback are cardinal phenotypes.
It is important to note that despite the potential advantages of using facial expressions to examine cognitive reappraisal, there are some precautions to consider. Most importantly, the present study and prior research (McRae, Misra, et al., 2012) indicates that reappraisal of negative facial expressions may be accompanied by increased amygdala activation, and the nature of the association between cognitive control regions, limbic activation, and negative affect may differ from that typically found using emotional scenes. In addition, the present study did not directly compare prefrontal engagement during reappraisal of negative facial expressions to that during reappraisal of emotional scenes. Therefore, it is unclear whether both types of stimuli elicit comparable prefrontal and limbic activation during cognitive reappraisal.
The present study had several limitations that warrant consideration. First, the sample size was relatively small (N = 21) and that may have limited our ability to detect other regions implicated in cognitive reappraisal of negative faces (e.g., ACC). Second, participants were allowed to use two different reappraisal strategies (e.g., ‘reinterpretation’ and ‘objectification’), and it is unclear whether these approaches are associated with similar or different neural substrates. Third, there were no behavioral (e.g., eye tracking) or self-report measures of reappraisal fidelity during the task, and it is possible that participants may have used other regulation strategies (e.g., suppression). Fourth, behavioral ratings were made retrospectively at the end of each block and may have been influenced by recall biases or demand characteristics. Lastly, we did not collect peripheral psychophysiological measures (e.g., skin conductance, heart rate) of emotion expression and regulation.
In conclusion, the present study found that cognitive reappraisal of negatively valenced, aversive faces was associated with a decrease in negative affect and engaged a network of prefrontal brain regions. Reappraisal was associated with increased neural response in regions implicated in cognitive control (left DLPFC, bilateral VLPFC, and mPFC) which serve as core processes implemented during cognitive reappraisal. Overall, the present study provides preliminary evidence suggesting that facial photographs are effective targets for cognitive reappraisal and have utility in functional neuroimaging studies on the neural substrates of cognitive regulation. These findings prompt their use in young children and clinical populations characterized by aberrant processing and response to social signals that convey threat and/or negative feedback.
Highlights.
Examined brain activation during reappraisal of negative facial expressions
Reappraisal reduced negative affect, engaged prefrontal cognitive control regions
Habitual reappraisal use correlated with prefrontal engagement during reappraisal
Faces effective alternative ‘targets’ of prefrontal engagement during reappraisal
Figure 1.
Voxel-wise statistical t-map on a canonical brain displaying differences in neural responses for Reappraise-Neg vs. Maintain-Neg. All voxels were significant at p < .005 (uncorrected) with cluster extent threshold greater than 20 contiguous voxels. Bar graphs illustrate extracted parameter estimates from the left DLPFC (top) and mPFC (bottom) for Reappraise-Neg vs. Look-Neut and Maintain-Neg vs. Look-Neut contrasts. AU = arbitrary units; DLPFC = dorsolateral prefrontal cortex; L = Look-Neut; M = Maintain-Neg; mPFC = medial prefrontal cortex; R = Reappraise-Neg.
Acknowledgements
This work was supported by grants from the National Institutes of Health, National Institute of Mental Health (MH076198 to K.L.P. and MH093679 to H.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Facial expressions consisted of stimuli from the NimStim Set of Facial Expressions and consisted of neutral, fear, and anger expressions from actors 1, 2, 3, 6, 7, 8, 9, 10, 11, 13, 16, 17, 22, 23, 24, 25, 27, 33, 36, 38, 39, 41, 42, and 45.
We also compared the neural correlates of cognitive reappraisal of anger and fear emotional expressions. Results indicated there were no differences between anger and fear stimuli in any a priori prefrontal or limbic region.
Age and sex were included as covariates given their association with emotion regulation abilities (Domes et al., 2010; McRae et al., 2008; Urry & Gross, 2010).
Buhle et al. (2013) only included amygdala voxels for which reappraisal < baseline and did not include voxels for which reappraisal > baseline. Thus, based on this approach increased amygdala activation during reappraisal cannot be definitively ruled out.
References
- Amir N, Klumpp H, Elias J, Bedwell J, Yanasak N, Miller L. Increased activation of the anterior cingulate cortex during processing of disgust faces in individuals with social phobia. Biol. Psychiatry. 2005;57:975–981. doi: 10.1016/j.biopsych.2005.01.044. [DOI] [PubMed] [Google Scholar]
- Analysis of Functional NeuroImages. AFNI program: 3DClustSim. 2005 Retrieved from http://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html.
- Barlow DH. Clinical Handbook of Psychological Disorders: A Step-by-Step Treatment Manual. 4th Ed. Guilford Press; New York, NY.: 2008. [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J. Neurosci. 2001;21RC165(1-6) doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton J, Taylor S, Sudheimer K, Liberzon I. Facial expressions and complex IAPS pictures: Common and differential networks. Neuroimage. 2006;31:906–919. doi: 10.1016/j.neuroimage.2005.12.050. [DOI] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Ochsner KH. Cognitive reappraisal of emotion: A meta-analyses of human neuroimaging studies. Cerebral Cortex. 2013 doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler EA, Egloff B, Wilhelm FH, Smith NC, Erickson EA, Gross JJ. The social consequences of expressive suppression. Emotion. 2003;3:48–67. doi: 10.1037/1528-3542.3.1.48. [DOI] [PubMed] [Google Scholar]
- Cronbach LJ, Furby L. How we should measure “change”: Or should we? Psychol.Bull. 1970;74:68–80. [Google Scholar]
- Costello EJ, Foley DL, Angold A. 10-year research update review: The epidemiology of child and adolescent psychiatric disorders: II. Developmental epidemiology. J. Am. Acad. Child Adolesc. Psychiatry. 2006;45:8–25. doi: 10.1097/01.chi.0000184929.41423.c0. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch. Gen. Psychiatry. 2003;60:837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Geier K, Falkai P, Gruber O. Fear is only as deep as the mind allows: A coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage. 2011;58:275–285. doi: 10.1016/j.neuroimage.2011.05.073. [DOI] [PubMed] [Google Scholar]
- Domes G, Schulze L, Böttger M, Grossman A, Hauenstein K, Herpertz SC. The neural correlates of sex differences in emotional reactivity and emotion regulation. Hum. Brain Mapp. 2010;31:758–769. doi: 10.1002/hbm.20903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabant EM, McRae K, Manuck SB, Hariri AR, Gross JJ. Individual differences in typical reappraisal use predict amygdala and prefrontal responses. Biol. Psychiatry. 2009;65:367–373. doi: 10.1016/j.biopsych.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois PH. Multivariate Correlational Analysis. Harper; New York: 1957. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research. State Psychiatric Institute; New York: 2002. Structured Clinical Interview for DSM IV-TR Axis I Disorders, Research Version, Non-Patient Edition (SCID-I/NP). [Google Scholar]
- Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KL. Beyond threat: Amygdala reactivity across multiple expressions of facial affect. Neuroimage. 2006;30:1441–1448. doi: 10.1016/j.neuroimage.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Forkman T, Scherer A, Böcker M, Pawelzik M, Gauggel S, Glaesmer H. The relation of cognitive reappraisal and expressive suppression to suicidal ideation and suicidal desire. Suicide. Life Threat. 2014 doi: 10.1111/sltb.12076. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, Politi P. Functional atlas of emotional faces processing: A voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J. Psychiatry Neurosci. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ. Neural bases of social anxiety disorder emotional reactivity and cognitive regulation during social and physical threat. Arch. Gen. Psychiatry. 2009;66:170–180. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biol. Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. The emerging field of emotion regulation: An integrative review. Rev. Gen. Psychol. 1998;2:271–299. [Google Scholar]
- Gross JJ. Emotion regulation in adulthood: Timing is everything. Curr. Dir. Psychol. Sci. 2001;10:214–219. [Google Scholar]
- Gross JJ. Emotion regulation: Affective, cognitive, and social consequences. Psychophysiology. 2002;39:281–291. doi: 10.1017/s0048577201393198. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Handbook of Emotion Regulation. Guilford Press; New York, NY.: 2007. [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. J. Pers. Soc. Psychol. 2003;85:348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Gur RC, Schroeder L, Turner T, McGrath C, Chan RM, Turetsky BI, Gur RE. Brain activation during facial emotion processing. Neuroimage. 2002;16:651–662. doi: 10.1006/nimg.2002.1097. [DOI] [PubMed] [Google Scholar]
- Hariri A, Bookheimer S, Mazziotta J. Modulating emotional responses: Effects of a neocortical network on the limbic system. Neuroreport. 2000;11:43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J. Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. J. Cogn. Neurosci. 2007;19:776–798. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- Koenigsberg HW, Fan J, Ochsner KN, Liu X, Guise K, Pizzarello S, Siever LJ. Neural correlates of using distancing to regulate emotional responses to social situations. Neuropsychologia. 2010;48:1813–1822. doi: 10.1016/j.neuropsychologia.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM, Werner KH. The Regulation of Emotion. Lawrence Erlbaum Associates Publishers; Mahwah, NJ, Mahwah, NJ, US: 2004. Emotion regulation and psychopathology. pp. 359–385. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-8. University of Florida; Gainesville, FL.: 2008. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual. [Google Scholar]
- Lazarus RS. Emotion and Adaptation. Oxford University Press; New York, NY.: 1991. [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and type II error concerns in fMRI research: Re-balancing the scale. Soc. Cogn. Affect. Neurosci. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McRae K, Gross JJ, Weber J, Robertson ER, Sokol-Hessner P, Ray RD, Ochsner KN. The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Soc. Cogn. Affect. Neurosci. 2012;7:11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Misra S, Prasad AK, Pereira SC, Gross JJ. Bottom-up and top-down emotion generation: implications for emotion regulation. Soc. Cogn. Affect. Neurosci. 2012;7:253–262. doi: 10.1093/scan/nsq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Ochsner KN, Mauss IB, Gabrieli JJD, Gross JJ. Gender differences in emotion regulation: An fMRI study of cognitive reappraisal. Group Process. Interg. 2008;11:143–162. doi: 10.1177/1368430207088035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, Shankman SA, Olino M, Klein DN. Defining reactivity: how several methodological decisions can affect conclusions about emotional reactivity in psychopathology. Cogn Emot. 2011;25:1439–1459. doi: 10.1080/02699931.2010.551185. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Curr. Dir. Psychol. Sci. 2008;17:153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn. Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Ann. NY Acad. Sci. 2012;1251:E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K, Bunge S, Gross J, Gabrieli J. Rethinking feelings: An fMRI study of the cognitive regulation of emotion. J. Cogn. Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald D, Nathan P, Moore G, Uhde T, Tancer M. Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biol. Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor S, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Fortune EE, Li Q, Siddiqui A, Krafft C, Oliver WT, Jeffries J. Emotion perception: Meta-analyses of face and natural scene processing. Neuroimage. 2011;54:2524–2533. doi: 10.1016/j.neuroimage.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Schaefer S, Jackson D, Davidson R, Aguirre G, Kimberg D, Thompson-Schill S. Modulation of amygdalar activity by the conscious regulation of negative emotion. J. Cogn. Neurosci. 2002;14:913–921. doi: 10.1162/089892902760191135. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Nelson C. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Res. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, Gross JJ. Emotion regulation in older age. Curr. Dir. Psychol. Sci. 2010;19:352–357. [Google Scholar]
- Urry H, van Reekum C, Johnstone T, Kalin N, Thurow M, Schaefer H, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J. Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontalsubcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangelin BC, Bradley MM, Kastner A, Lang PJ. Affective engagement for facial expressions and emotional scenes: The influence of social anxiety. Biol. Psychol. 2012;91:103–110. doi: 10.1016/j.biopsycho.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MA, Morris AP, McGlone F, Abbott DF, Mattingley JB. Amygdala responses to fearful and happy facial expressions under conditions of binocular suppression. J. Neurosci. 2004;24:2898–2904. doi: 10.1523/JNEUROSCI.4977-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston JS, O'Doherty J, Dolan RJ. Common and distinct neural responses during direct and incidental processing of multiple facial emotions. NeuroImage. 2003;20:84–97. doi: 10.1016/s1053-8119(03)00303-3. [DOI] [PubMed] [Google Scholar]
- Yang TT, Menon V, Eliez S, Blasey C, White CD, Ried AJ, Reiss AL. Amygdalar activation associated with positive and negative facial expressions. NeuroReport. 2002;13:1737–1741. doi: 10.1097/00001756-200210070-00009. [DOI] [PubMed] [Google Scholar]