Abstract
Phosphatase and tensin homolog deleted from chromosome 10 (PTEN) has been implicated in the maintenance of cardiac homeostasis although the underlying mechanism(s) remains elusive. We generated a murine model of cardiomyocyte-specific knockout of PTEN to evaluate cardiac geometry and contractile function, as well as the effect of metformin on PTEN deficiency-induced cardiac anomalies, if any. Cardiac histology, autophagy and related signaling molecules were evaluated. Cardiomyocyte-specific PTEN deletion elicited cardiac hypertrophy and contractile anomalies (echocardiographic and cardiomyocyte contractile dysfunction) associated with compromised intracellular Ca2+ handling. PTEN deletion-induced cardiac hypertrophy and contractile anomalies were associated with dampened phosphorylation of PTEN-inducible kinase 1 (Pink1) and AMPK. Interestingly, administration of AMPK activator metformin (200 mg/kg/d, in drinking H2O for 4 weeks) rescued against PTEN deletion-induced geometric and functional defects as well as interrupted autophagy and autophagic flux in the heart. Moreover, metformin administration partially although significantly attenuated PTEN deletion-induced accumulation of superoxide. RNA interference against Pink1 in H9C2 myoblasts overtly increased intracellular ATP levels and suppressed AMPK phosphorylation, confirming the role of AMPK as a downstream target for PTEN-Pink1. Further scrutiny revealed that activation of AMPK and autophagy using metformin and rapamycin, respectively, rescued against PTEN deletion-induced mechanical anomalies with little additive effect. These data demonstrated that cardiomyocyte-specific deletion of PTEN leads to the loss of Pink1-AMPK signaling, development of cardiac hypertrophy and contractile defect. Activation of AMPK rescued against PTEN deletion-induced cardiac anomalies associated with restoration of autophagy and autophagic flux.
Keywords: PTEN, Pink1, AMPK, cardiac hypertrophy, contractile function
1. Introduction
Compromised insulin signaling and insulin resistance are well known to contribute to the increased prevalence of metabolic syndrome and cardiovascular diseases [1–3]. Not surprisingly, pharmacological strategy targeting insulin signaling cascade has become an attractive therapeutic remedy in the management of cardiovascular in particular heart diseases [2, 4]. Among various components in insulin signaling cascade, phosphatase and tensin homolog deleted from chromosome 10 (PTEN) may represent one of the novel signaling targets in the regulation of insulin sensitivity. It has been reported that PTEN negatively regulates insulin signaling by dephosphorylating phosphatidyl inositol triphosphate to ultimately terminate Akt activation [5]. Individuals possessing haploinsufficiency of PTEN display enhanced insulin sensitivity despite increased body mass [6]. PTEN deletion improves insulin signaling in skeletal muscles albeit prompting cardiomyocyte dysfunction and hypertrophy [7–9]. This is somewhat in line with the notion that overactivation of Akt leads to the development of cardiac hypertrophy and contractile dysfunction over time [9, 10].
Up-to-date, the signaling regulatory machineries in cardiac homeostasis remain elusive for PTEN. One possible pathway participating in PTEN signaling may be PTEN-inducible kinase 1 (Pink1). Pink1 is a kinase essential to cardiac homeostasis in physiological conditions, as deletion of which results in cardiac hypertrophy and dysfunction in a manner reminiscent to PTEN deficiency [11]. Pink1 knockout may trigger the pathological phenotype via mitochondrial dysfunction and oxidative stress [11]. As implicated by the nomenclature, expression of Pink1 is tightly correlated with the PTEN activity, where decreased PTEN results in loss of Pink1 [12]. The apparent connection and similarity in cardiac phenotype upon genetic deletion between these two signaling molecules depict an essential role of the PTEN-Pink1 pair in the maintenance of cardiac homeostasis under both physiological and pathophysiological conditions.
AMP-activated protein kinase (AMPK) is a critical energy fuel sensor. AMPK regulates metabolism via promoting glycolysis, glucose transport and fatty acid oxidation [13]. AMPK is activated following a rise in intracellular AMP/ATP ratio as well as through protein kinases such as LKB1 and CaMKKβ [14]. More recent finding suggests a pivotal role of AMPK in the regulation of cardiac autophagy, a cellular process essential to break down cellular proteins and organelles to be recycled for production of ATP and nutrients [15]. However, little information is available with regards to the role of AMPK in the PTEN-Pink1-associated regulation of cardiac geometry and function. To this end, the present study was designed to examine the possible link among PTEN, Pink1, AMPK and autophagy in the regulation of cardiac geometry and contractile function. A cardiomyocyte-specific knockout of PTEN murine model was generated recently in our laboratory displaying moderate cardiac dysfunction [9]. To evaluate the causal relationship between Pink1 and AMPK, Pink1 was knocked down in H9C2 cells prior to the assessment of AMPK phosphorylation. To better elucidate the role of AMPK in PTEN deletion-induced cardiac anomalies, if any, the AMPK activator metformin was administered in vivo to provide the proof-of-concept evidence for the role of AMPK in PTEN deletion-induced cardiac responses. Status of autophagy and autophagic flux were monitored using the autophagic protein makers LC3B isoforms and the autophagosome adaptor protein p62. Our data demonstrated that PTEN deletion may contribute to loss of Pink1-AMPK signaling and cardiac pathological changes in association with impaired autophagy and autophagic flux.
2. Materials and methods
2.1. Generation of cardiomyocyte-specific PTEN knockout mice and metformin treatment
All animal procedures carried out in this study were approved by the Animal Care and Use Committee at the University of Wyoming (Laramie, WY) and was in compliance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85- 23, revised 1996). Cardiomyocyte-specific PTEN(flox/flox)/α-myosin heavy chain (α-MHC) Cre mice, henceforth referred to as CM PTENKO, were generated as reported previously. The wild-type (WT) PTEN (−/−), heterozygous PTEN (+/−) and floxed PTEN (+/+) alleles were analyzed by PCR with tail DNA [9]. Only PTEN(flox/flox) littermates negative for α-MHC Cre were used as WT. Mice were housed in a 12:12 hour light-dark cycle with free access to food and drinking water. Metformin was administered in the drinking water (200 mg/kg/d, Sigma Cat# D150959) as previously described for one month [16].
2.2. Echocardiographic assessment
Cardiac geometry and function were evaluated in anesthetized (ketamine 80 mg/kg and xylazine 12 mg/kg, i.p.) mice using the 2-D guided M-mode echocardiography (Phillips Sonos 5500) equipped with a 15-6 MHz linear transducer. Left ventricular (LV) anterior and posterior wall dimensions during diastole and systole were recorded from three consecutive cycles in M-mode using method adopted by the American Society of Echocardiography. Fractional shortening was calculated from LV end-diastolic (EDD) and end-systolic (ESD) diameters using the equation (EDD-ESD)/EDD. Heart rates were averaged over 10 cycles [17].
2.3. Isolation of murine cardiomyocytes
Cardiomyocytes were isolated as previously described [17]. Mice were anaesthetized with ketamine/xylazine, hearts were removed and perfused with oxygenated (5%CO2–95%O2) Krebs-Henseleit bicarbonate (KHB) buffer at 37°C containing (in mM) 118 NaCl, 4.7 KCl, 1.2 KH2PO4, 25 NaHCO3, 10 HEPES and 11.1 glucose. Hearts were then perfused with KHB buffer containing 10mg/ml Liberase Blendzyme (Roche Diagnostics, Indianapolis IN) for 15 minutes. Left ventricles were subsequently minced, dispersing the cardiomyocytes. Ca2+ was added back slowly to a final concentration of 1.25 mM. Only rod shaped myocytes without spontaneous contractions were used for analysis [18]. To assess the role of AMPK and autophagy in PTEN knockout-induced cardiomyocyte contractile dysfunction, cardiomyocytes from adult PTEN−/− mice were treated with metformin (50 µM) [19, 20], the mTOR inhibitor rapamycin (5 µM) to stimulate autophagy [21], or both, at 37°C for 4 hrs prior to the assessment of cardiomyocyte mechanical function.
2.4. Cardiomyocyte mechanics and intracellular Ca2+ handling
Cell mechanics and Ca2+ properties were assessed using an IonOptix softedge detection system. Myocytes were placed on a chamber mounted on an Olympus IX-70 microscope stage in a buffer containing (in mM) 131 NaCl, 4 KCl, 1 CaCl2, 1 MgCl2, 10 glucose and 10 HEPES. Myocytes were then stimulated at 0.5 Hz and the following indicies were evaluated: peak shortening (PS), time-to-PS (TPS), time-to-90% relengthening (TR90), and maximal velocities of shortening/relengthening (± dL/dt). For Ca2+ analysis, myocytes were loaded with fura-2/AM (0.5 µM) for 10 minutes. A duel excitation fluorescence photomultiplier system (IonOptix) was used to measure fluorescence intensity. Myocytes were placed on the microscope as described above for cell mechanics, and exposed to light emitted by a 75W mercury lamp passed through either a 360- or 380-nm filter. While stimulated at 0.5 Hz, fluorescence emissions were detected by a photomultiplier tube between 480 nm and 520 nm after initial illumination at 360 nm for 0.5s followed by 380 nm for the remaining time. Changes in intracellular Ca2+ were calculated by the ratio of the fluorescence intensity at two wavelengths while single exponential curve fitting was used for Ca2+ decay rate. These techniques have been previously described [17, 22].
2.5. Myocardial histology and superoxide detection
Procedure was conducted as described [23]. Hearts were excised from anesthetized animals, washed with PBS, cut to expose cross section of left ventricle and embedded in O.C.T. embedding compound (Tissue-Tek, Torrence, CA) stored at −80°C until analysis. Five µm tissue sections were cut, placed on slides and washed 1× with PBS. FITC-conjugated lectin was added to the slides and kept at 37°C in a humidified chamber in the dark for 2 hrs. Slides were washed 2× with PBS and mounted with Prolong Gold Antifade reagent. For superoxide measurement, 7-µm tissue sections were cut from heart sections. Slides were incubated with dihydroethidium (DHE, 3µM, Molecular Probes) for 60 minutes at room temperature [24]. After being washed with PBS, tissues were stained with DAPI (1 µg/ml, Sigma, D9564) for 5 minutes at room temperature. Next, sections were mounted with Prolong gold antifade (Life Technologies). Images were acquired using a Zeiss 710 laser scanning confocal microscope. Fluorescence intensity per nucleus was calculated using Image J [24].
2.6. Immunoblotting
Heart tissue was homogenized in RIPA (Millipore, Billerica MA) lysis buffer and centrifuged at 12,000 g for 20 min at 4°C. Samples containing equal amounts of protein were separated on a 10 or 12% SDS-polyacrylamide gel in a mini-gel apparatus (Mini-PROTEAN II, Bio-Rad). Proteins were then transferred to nitrocellulose membranes and subsequently blocked with 5% BSA in tris buffered saline (TBS)-tween 20. Membranes were incubated overnight at 4°C in primary antibodies: anti-PTEN (Cell Signaling, Cat#9552), anti-pAMPK (T172, Cell Signaling, Cat#2535), anti-AMPK (Cell Signaling, Cat#2532), anti-p62 (Enzo Life Sciences, Cat#GP62-C), anti-LC3B (Cell Signaling, Cat#3868S), anti-GAPDH (Cell Signaling, Cat#2118L), and anti-Pink1 (Abcam, Cat#ab23707). Blots were washed with TBS-Tween 20 and incubated with horseradish peroxidase (HRP) conjugated secondary antibody. Membranes were subsequently washed and exposed by enzymatic chemiluminescence in a GelDoc system (Bio-Rad) [25].
2.7. Cell culture and siRNA treatment
Rat neonatal H9C2 myoblast cells were grown in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin under a humidified atmosphere of 5% CO2 in air and maintained at low confluence. When cells reached 80% confluence, Pink1 targeted siRNA (100 nM, Qiagen, Cat#68943) was transfected using DharmaFECT (Dharmacon) transfection reagent in DMEM with 10% FBS for 72 hours [26].
2.8. ATP measurement
Intracellular ATP concentration was measured as previously described [27]. Briefly, fresh H9C2 cells were homogenized with the lysis buffer. ATP Determination Kit (Molecular Probes, A22066) was purchased to measure the intracellular ATP level. In each sample, protein concentration was measured to evaluate ATP concentration.
2.9. Statistical analysis
Data are presented as Mean ± SEM. Statistical significance was determined by 2-tailed Student’s t-test in cases of two groups, and one way ANOVA followed by Tukey’s post hoc analysis in cases of 3 groups. Significance for each analysis was judged as p < 0.05.
3. Results
3.1. PTEN deletion results in cardiac hypertrophy and dysfunction
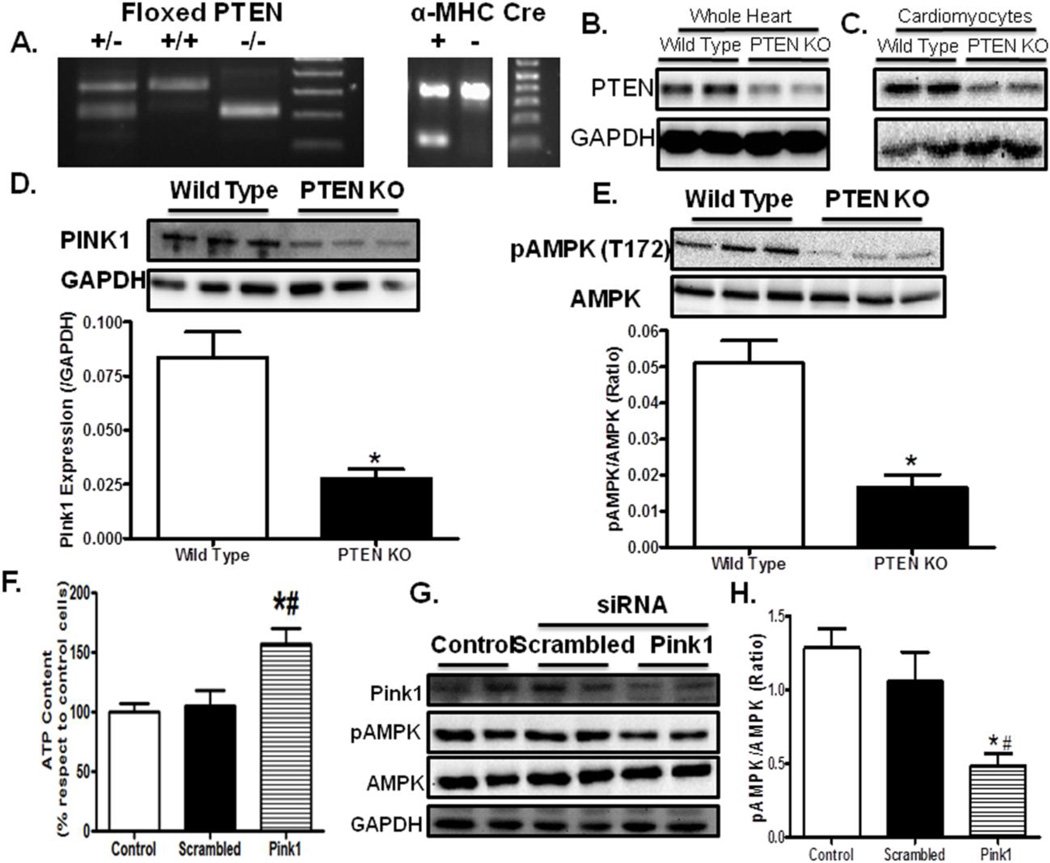
Crossing mice expressing α-MHC Cre with PTEN(flox/flox) led to ~75% reduction of PTEN protein expression in whole hearts and ~85% reduction in isolated cardiomyocytes (Fig. 1A–C), with the subtle difference in the PTEN abundance attributed to non-cardiomyocyte components present in whole heart homogenates (e.g., fibroblasts, endothelial cells). Lectin staining and cross sectional area analysis demonstrated overt cardiac hypertrophy following PTEN deletion (Fig. 2A, B). This is confirmed by a significant rise in the heart-to-body weight ratio in PTEN KO mice (Fig. 2C). Cardiac function assessed using echocardiography revealed subtle although significant decline in fractional shortening following PTEN deletion (Fig. 2D, E). These data collectively support a pathological phenotype in hearts following cardiomyocyte-specific PTEN knockout.
Fig. 1.
Cardiomyocyte-specific PTEN knockout affects myocardial PINK1 and AMPK. A: Representative PCR gels containing floxed PTEN and the α-myosin heavy chain (α-MHC) Cre transgene; B: Immunoblot depicting PTEN expression in PTEN KO murine whole hearts; C: Immunoblot showing PTEN expression in cardiomyocytes isolated from PTEN KO murine hearts; D: Pink 1 expression; E: AMPK phosphorylation; F: Intracellular ATP levels in H9C2 cells treated with scrambled or Pink1 siRNA; G: Representative gel blots of Pink1 and AMPK (total and phosphorylated) in control, scrambled siRNA and Pink1 siRNA groups; and H: AMPK phosphorylation (pAMPK-to-AMPK ratio) in control, scrambled siRNA and Pink1 siRNA cell groups. Mean ± SEM, n = 3 (panel D–E) and 8 (panel F, H), *p < 0.05 vs. Wild Type or Control group, #p < 0.05 vs. scrambled RNA group.
Fig. 2.
Effect of AMPK activation with metformin (200 mg/kg/d, drinking water for 4 weeks) on PTEN knockout-induced cardiac geometric and functional anomalies. A: Representative lectin staining of myocardial sections in wild type, PTEN knockout and PTEN knockout plus metformin mouse groups. B: Pooled data depicting cross sectional area from lectin staining; C: Heart weight (HW) to body weight (BW) ratio; D: Representative echocardiographic images in wild type, PTEN knockout and PTEN knockout with metformin treatment mice; E: Fractional shortening. Mean ± SEM n= 4–7 mice per group.
3.2. Decreased PTEN/Pink1 increased intracellular ATP levels and decreased pAMPK
To examine possible signaling mechanism of PTEN knockout-induced cardiac anomalies, two important signaling regulators of cardiac function, Pink1 and AMPK, were evaluated. PTEN deficient mice displayed decreased levels of Pink1 and AMPK phosphorylation in the heart (Fig. 1D, E). To further evaluate the cause-effect relationship between Pink1 and AMPK, siRNA directed against Pink1 was utilized in H9C2 cells. Seventy-two hours of Pink1 siRNA treatment led to a significant removal of the Pink1 protein in H9C2 cells. Interestingly, Pink1 knockdown drastically increased intracellular ATP levels but suppressed AMPK phosphorylation, suggesting a causative relationship between Pink1 and AMPK (Fig. 1F, G).
3.3. PTEN deletion-induced whole heart dysfunction and hypertrophy is AMPK dependent
To test the role of suppressed AMPK phosphorylation in PTEN knockout-induced cardiac dysfunction, The AMPK activator metformin was administered (200 mg/kg/d in drinking water for 4 weeks) in PTEN deficient mice prior to assessment of cardiac morphology and function. Cross sectional area analysis revealed an increased cardiomyocyte size in PTEN knockout, the effect of which was partially attenuated by metformin treatment. Consistently, the enhanced heart weight in PTEN knockout mice was also abrogated by metformin. Echocardiographic analysis depicted that PTEN knockout significantly suppressed fractional shortening, the effect of which was mitigated by metformin (Fig. 2).
3.4. Cardiomyocyte mechanical dysfunction is reversed by AMPK activation
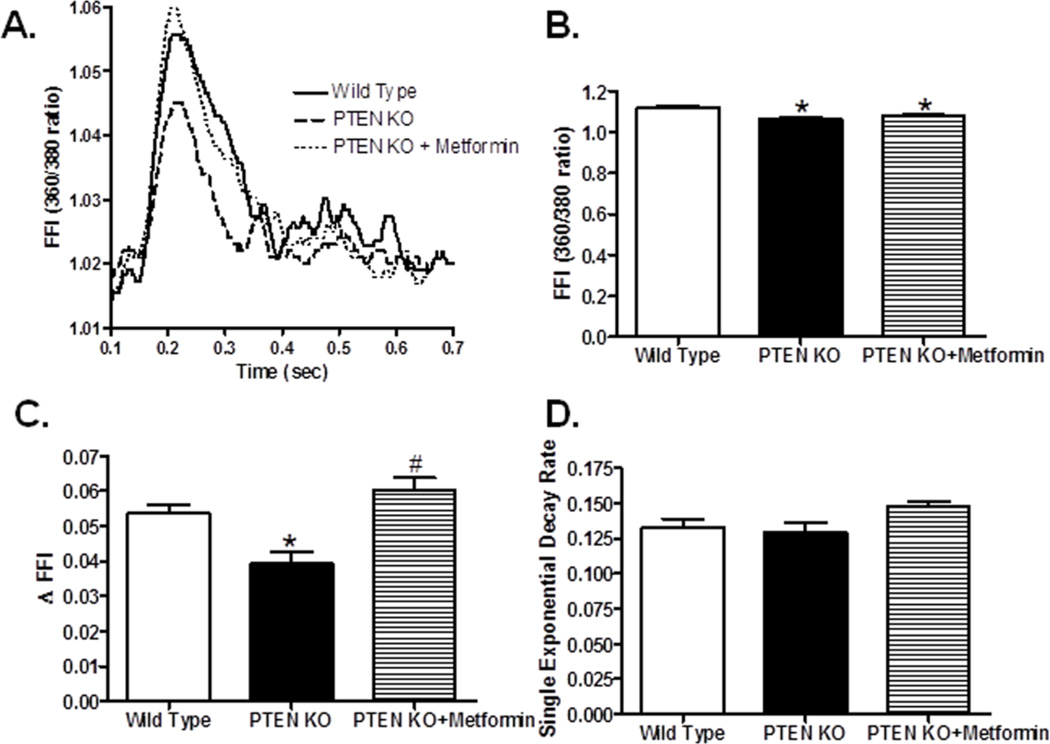
To examine the role of individual cardiomyocytes in PTEN deletion- and/or metformin-induced cardiac anomalies, cardiomyocyte contractile function and intracellular Ca2+ handling were evaluated. Our results depicted that PTEN deletion induced overt cardiomyocyte contractile dysfunction as manifested by depressed peak shortening, maximal velocity of shortening/relengthening, as well as prolong TPS and TR90, all of which were nullified by metformin (Fig. 3). Assessment of intracellular Ca2+ transients revealed that PTEN deletion significantly depressed baseline and electrically-stimulated rise in intracellular Ca2+ (baseline FFI and ΔFFI, respectively) without affecting the intracellular Ca2+ decay rate. Metformin effectively reversed PTEN deletion-induced drop in ΔFFI without affecting baseline FFI and intracellular Ca2+ decay (Fig. 4).
Fig. 3.
Effect of AMPK activation with metformin (200 mg/kg/d, drinking water for 4 weeks) on PTEN knockout-induced cardiomyocyte contractile anomalies. A: Representative cell shortening traces from wild type, PTEN knockout and PTEN knockout with metformin treatment groups; B: Peak shortening (normalized to resting cell length); C: Maximum velocity of shortening (+ dL/dt); D: Maximum velocity of relengthening (− dL/dt); E:Time-to-90% relengthening (TR90) ; and F: Time-to-PS (TPS). Mean ± SEM, n= 139–200 cells from 3 mice per group, *p < 0.05 vs. Wild Type, #p < 0.05 vs. PTEN KO group.
Fig. 4.
Effect of AMPK activation with metformin (200 mg/kg/d, drinking water for 4 weeks) on PTEN knockout-induced intracellular Ca2+ handling in cardiomyocytes. A: Representative intracellular Ca2+ transient traces from wild type, PTEN knockout and PTEN knockout with metformin treatment groups; B: Resting fura-2 fluorescence intensity (FFI); C: Electronically-stimulated rise in FFI (ΔFFI); and D: Intracellular Ca2+ decay rate. Mean ± SEM, n = 58–60 cells from 3 mice per group, *p < 0.05 vs. Wild Type, #p < 0.05 vs. PTEN KO group.
3.5. Impaired AMPK activation impacts autophagy and increases superoxide accumulation in PTEN deficient mice
To explore the mechanism of action behind metformin-offered beneficial effect against PTEN deletion, we went on to examine autophagy, an essential cellular event driven by AMPK with a known role in cardiac pathologies including ischemia, hypertrophy and heart failure [15]. Cardiomyocyte-specific knockout of PTEN overtly suppressed phosphorylation of AMPK and autophagy as evidenced by decreased LC3BII expression and LC3-II/I ratio and elevated accumulation of the autophagosome cargo protein p62, the effects of which were abrogated or significantly attenuated by metformin (Fig. 5A–F). Furthermore, PTEN knockout dramatically increased the phosphorylation of ULK1 at Ser757 in the heats, the effect of which was alleviated by metformin treatment (Fig. 5A, G). Given that reactive oxygen species accumulation is common in heart failure and was recently reported in PINK1 deficiency [11], we evaluated the levels of superoxide, serving both as a potent inducer and a degradation target for autophagy [28–30]. Our results revealed a drastic rise in superoxide levels in myocardium from PTEN deficient mice, the effect of which was partially although significantly attenuated by metformin (Fig. 5H, I). These results favored disrupted autophagy in particular autophagic flux following PTEN deletion possibly associated with impaired removal of reactive oxygen species. Activation of AMPK using metformin is capable of alleviating PTEN deletion-induced defect in autophagy and superoxide accumulation.
Fig. 5.
Effect of AMPK activation with metformin (200 mg/kg/d, drinking water for 4 weeks) on PTEN knockout-induced changes in autophagy and ROS generation. A: Representative gel blots depicting levels of total and phosphorylated AMPK, p62, LC3B, and phosphorylated ULK1 in wild type, PTEN knockout and PTEN knockout with metformin treatment groups; B: pAMPK-to-AMPK ratio; C: p62 expression; D: LC3B-I expression; E: LC3B-II expression; F: LC3B-II to LC3B-I ratio; G: pULK1-to-ULK1 ratio; H: Representative images from WT, PTEN knockout mice treated with or without metformin stained with DHE and DAPI. Oxidized DHE intercalates into DNA and the nuclei appear in red; and I: Quantification of the DHE-positive nuclei. Mean ± SEM, n= 4–7 (panel B–F) and 5–10 mice (panel H) per group, *p < 0.05 vs. Wild Type group; #p < 0.05 vs. PTEN KO group.
3.6. Cardiomyocyte mechanical dysfunction is reversed by rapamycin
To better elucidate the cause-effect relationship between AMPK and autophagy in PTEN knockout-induced cardiac contractile dysfunction, freshly isolated cardiomyocytes from PTEN−/− mice were incubated with the AMPK activator metformin, the autophagy inducer rapamycin, or both, for 4 hrs prior to assessment of cardiomyocyte contractile function. Our results shown in Fig. 6 demonstrated that metformin and rapamycin significantly attenuated PTEN knockout-induced cardiomyocyte mechanical anomalies, with little additive effect between the two.
Fig. 6.
Effect of metformin (Met, 50 µM) and rapamycin (Rapa, 5 µM), or both, on PTEN knockout-induced cardiomyocyte contractile anomalies. Cardiomyocytes from adult PTEN−/− mice were treated with metformin (50 µM), mTOR inhibitor (autophagy inducer) rapamycin (5 µM), or both, at 37°C for 4 hrs prior to assessment of cardiomyocyte mechanical function. A: Resting cell length; B: Peak shortening (normalized to resting cell length); C: Maximum velocity of shortening (+ dL/dt); D: Maximum velocity of relengthening (− dL/dt); E: Time-to-90% relengthening (TR90); and F: Time-to-PS (TPS). Mean ± SEM, n= 100 cells from 3 mice per group, *p < 0.05 vs. Wild Type, #p < 0.05 vs. PTEN KO group.
4. Discussion
Findings from this study depicted a novel possible sequential pathway connecting PTEN, Pink1, AMPK and autophagy in the regulation of cardiac geometry and function. Our data revealed overt cardiac hypertrophy and contractile dysfunction following cardiomyocyte-specific knockout of PTEN. PTEN knockout-induced cardiac hypertrophy is consistent with the earlier finding from the skeletal muscle PTEN genetic model and is not entirely unexpected due to the negative effect of PTEN on Akt signaling [31]. Conflicting data have been observed with regards to the outcome of Akt overactivation on cardiac homeostasis. Overexpression of Akt has been reported to elicit little [32] or hypercontractile [33] effect on the basal cardiac function. Earlier evidence has demonstrated the involvement of the overactive G-protein coupled receptor PI3Kγ signaling and reduced cyclic AMP production in PTEN deficient hearts [7].
Pink1 has recently emerged as an excellent candidate for a PI3K/Akt alternative pathway involved in the governance of cardiac function in the PTEN deficient hearts. Pink1 was initially recognized for its unique role in Parkinson’s disease and other neurological disorders [34]. It was suggested that lack of Pink1 directly leads to impaired mitochondrial function and generation of ROS [35]. More importantly, recent studies indicate that PINK1 possesses an indispensable role in the maintenance of cardiac homeostasis [11]. Concomitant heart dysfunction and hypertrophy along with elevated ROS generation were revealed in Pink1 deficient mice [11]. Furthermore, decreased levels of PINK1 were found in patients with end-stage heart failure, suggesting that PINK1 reduction might contribute to the development of heart failure [11]. Data from our study suggested significantly decreased Pink1 levels in PTEN knockout mice, implicating a possible role of lost Pink1 signaling in PTEN deletion. This finding is consistent with an earlier report in haploinsufficient PTEN hearts where decreased Pink1 was seen [36]. Perhaps the most intriguing data from our study was decreased AMPK phosphorylation following PTEN deletion. This finding prompted the speculation that AMPK phosphorylation may occur as a downstream target of Pink1, which was consolidated using the Pink1 siRNA knockdown in H9C2 cells where Pink1 knockdown was sufficient to suppress AMPK phosphorylation. A direct link between Pink1 and AMPK phosphorylation has not been reported previously.
AMPK has been identified as a target in the management of multiple heart diseases [37]. For example, targeting insufficient AMPK phosphorylation is known to protect against ischemia reperfusion-induced injury [38, 39]. Likewise, impaired AMPK activation is prevalent in diabetic heart while reactivation with metformin recovers function in diabetes through preserving autophagy [16, 40]. In our hands, stimulating AMPK with metformin restored myocardial dysfunction following PTEN knockout. Our study further demonstrated preserved intracellular Ca2+ handling against PTEN knockout following metformin treatment. Recently Oliveira and colleagues showed that AMPK phosphorylation of troponin I increases myofilament sensitivity to Ca2+ [41]. Thus impaired AMPK activity may underscore myocardial dysfunction in isolated cardiomyocytes under PTEN deficiency.
Our result revealed overt cardiac hypertrophy with cardiomyocyte-specific deletion of PTEN, consistent with the findings from AMPK deficiency. In our hands, AMPK activation using metformin effectively alleviated the PTEN deletion-induced cardiac hypertrophy (heart weight and cross sectional area), consistent with the anti-hypertrophic property of AMPK. It has been shown that AMPK activation using AICAR reversed pressure overload-induced cardiac hypertrophy [42]. Along the same line, metformin also inhibits hypertrophic protein synthesis in neonatal rat cardiomyocytes [43]. It may be speculated that the enhanced Akt phosphorylation (as a result of PTEN knockout) in conjunction with lost AMPK activation contribute to cardiac hypertrophy in PTEN deficiency. AMPK is known to serve as a check point against Akt in cardiac hypertrophy.
Autophagy has been shown to play an essential role in the regulation of cardiac function [44]. Autophagy is protective under stress conditions whereas excess autophagy impairs cell function. Activation of AMPK inhibits mTORC1 activity by phosphorylating TSC2 and Raptor to turn on autophagy [25, 45–47]. Alternatively, AMPK may facilitate autophagosome initiation by promoting ULK1 through phosphorylation at Ser317, Ser777, Ser467, Ser555, Ser574 and Ser637 sites [48, 49]. Once phosphorylated, ULK1 phosphorylates autophagy initiation complex Atg13 and FIP200 [50]. On the other hand, phosphorylation of ULK1 at Ser757 has been found to suppress autophagy [9]. Data from our study revealed impaired AMPK activation and autophagy in PTEN deficiency mice accompanied with cardiac hypertrophy and impaired contractility, suggesting a possible role of AMPK in PTEN deficiency-induced cardiac anomalies. This received support from the fact that AMPK activation using metformin rescued PTEN deficiency-induced cardiac hypertrophy and contractile dysfunction. The beneficial effect of metformin is closely accompanied with restored autophagy and autophagic flux as evidenced by LC3B isozyme conversion and p62. Our finding suggested that Pink1 may be the upstream regulator for AMPK, en route to autophagy governance. Pink1 has been shown to promote mitophagy [51, 52] although its precise role in cardiac autophagy is unknown. Our study further revealed that metformin elicited its beneficial effect on cardiac geometry, function, autophagy, and superoxide accumulation in PTEN deficient murine hearts. During stress, autophagy may step up to remove abnormal protein aggregates, oxidized proteins, damaged mitochondria and other cellular components, ultimately preserving cell physiological function [30]. Our data indicated that metformin restores PTEN knockout-induced cardiac anomalies and autophagy, partially through alleviating superoxide accumulation. Last but not least, the fact that metformin and rapamycin each rescued against PTEN deletion-induced cardiomyocyte contractile dysfunction with little additive effect indicates a likely sequential correlation between AMPK activation and autophagy.
In conclusion, our study provided the evidence that cardiomyocyte-specific PTEN knockout elicited cardiac hypertrophy and contractile dysfunction associated with interrupted autophagy and autophagic flux. Our data further depicted a role of Pink1 and AMPK in PTEN knockout-induced cardiac anomalies. Activation of AMPK using metformin rescued against PTEN deletion-induced changes in myocardial geometry, function and autophagy, supporting a role of AMPK in PTEN deletion-induced cardiac anomalies. Although it is somewhat premature to consolidate for a unique role of Pink1-AMPK in the cardiomyocyte-specific PTEN deletioninduced hypertrophic cardiomyopathy, our results should shed some lights towards a better understanding for a role of PTEN in the maintenance of myocardial geometry and function.
Research highlights.
We examined the effect of cardiac PTEN deletion on heart function;
PTEN deletion leads to cardiac geometric and contractile defect;
The detrimental effect of PTEN deletion was related to loss of Pink1-AMPK signaling;
ACKNOWLEDGEMENT
This work was supported in part by NIH/NCRR 5P20RR016474 and NIH/NIGMS 8P20GM103432
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COMPETING INTERESTS: The authors have declared that no competing interests exist.
REFERENCES
- 1.Aroor AR, Mandavia CH, Sowers JR. Insulin resistance and heart failure: molecular mechanisms. Heart Fail Clin. 2012;8:609–617. doi: 10.1016/j.hfc.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abel ED, O'Shea KM, Ramasamy R. Insulin resistance: metabolic mechanisms and consequences in the heart. Arterioscler Thromb Vasc Biol. 2012;32:2068–2076. doi: 10.1161/ATVBAHA.111.241984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ren J, Pulakat L, Whaley-Connell A, Sowers JR. Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J Mol Med (Berl) 2010;88:993–1001. doi: 10.1007/s00109-010-0663-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jay MA, Ren J. Peroxisome proliferator-activated receptor (PPAR) in metabolic syndrome and type 2 diabetes mellitus. Curr Diabetes Rev. 2007;3:33–39. doi: 10.2174/157339907779802067. [DOI] [PubMed] [Google Scholar]
- 5.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 6.Pal A, Barber TM, Van de Bunt M, Rudge SA, Zhang Q, Lachlan KL, Cooper NS, Linden H, Levy JC, Wakelam MJ, Walker L, Karpe F, Gloyn AL. PTEN mutations as a cause of constitutive insulin sensitivity and obesity. N Engl J Med. 2012;367:1002–1011. doi: 10.1056/NEJMoa1113966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crackower MA, Oudit GY, Kozieradzki I, Sarao R, Sun H, Sasaki T, Hirsch E, Suzuki A, Shioi T, Irie-Sasaki J, Sah R, Cheng HY, Rybin VO, Lembo G, Fratta L, Oliveira-dos-Santos AJ, Benovic JL, Kahn CR, Izumo S, Steinberg SF, Wymann MP, Backx PH, Penninger JM. Regulation of myocardial contractility and cell size by distinct PI3K-PTEN signaling pathways. Cell. 2002;110:737–749. doi: 10.1016/s0092-8674(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 8.Oudit GY, Kassiri Z, Zhou J, Liu QC, Liu PP, Backx PH, Dawood F, Crackower MA, Scholey JW, Penninger JM. Loss of PTEN attenuates the development of pathological hypertrophy and heart failure in response to biomechanical stress. Cardiovasc Res. 2008;78:505–514. doi: 10.1093/cvr/cvn041. [DOI] [PubMed] [Google Scholar]
- 9.Xu X, Roe ND, Weiser-Evans MC, Ren J. Inhibition of mammalian target of rapamycin with rapamycin reverses hypertrophic cardiomyopathy in mice with cardiomyocyte-specific knockout of PTEN. Hypertension. 2014;63:729–739. doi: 10.1161/HYPERTENSIONAHA.113.02526. [DOI] [PubMed] [Google Scholar]
- 10.Matsui T, Nagoshi T, Hong EG, Luptak I, Hartil K, Li L, Gorovits N, Charron MJ, Kim JK, Tian R, Rosenzweig A. Effects of chronic Akt activation on glucose uptake in the heart. Am J Physiol Endocrinol Metab. 2006;290:E789–E797. doi: 10.1152/ajpendo.00564.2004. [DOI] [PubMed] [Google Scholar]
- 11.Billia F, Hauck L, Konecny F, Rao V, Shen J, Mak TW. PTEN-inducible kinase 1 (PINK1)/Park6 is indispensable for normal heart function. Proc Natl Acad Sci U S A. 2011;108:9572–9577. doi: 10.1073/pnas.1106291108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Unoki M, Nakamura Y. Growth-suppressive effects of BPOZ and EGR2, two genes involved in the PTEN signaling pathway. Oncogene. 2001;20:4457–4465. doi: 10.1038/sj.onc.1204608. [DOI] [PubMed] [Google Scholar]
- 13.Zaha VG, Young LH. AMP-Activated Protein Kinase Regulation and Biological Actions in the Heart. Circ Res. 2012;111:800–814. doi: 10.1161/CIRCRESAHA.111.255505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carling D, Sanders MJ, Woods A. The regulation of AMP-activated protein kinase by upstream kinases. Int J Obes (Lond) 2008;32(Suppl 4):S55–S59. doi: 10.1038/ijo.2008.124. [DOI] [PubMed] [Google Scholar]
- 15.Mughal W, Dhingra R, Kirshenbaum LA. Striking a Balance: Autophagy, Apoptosis, and Necrosis in a Normal and Failing Heart. Curr Hypertens Rep. 2012 doi: 10.1007/s11906-012-0304-5. [DOI] [PubMed] [Google Scholar]
- 16.Xie Z, Lau K, Eby B, Lozano P, He C, Pennington B, Li H, Rathi S, Dong Y, Tian R, Kem D, Zou MH. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes. 2011;60:1770–1778. doi: 10.2337/db10-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roe ND, Ren J. Akt2 knockout mitigates chronic iNOS inhibition-induced cardiomyocyte atrophy and contractile dysfunction despite persistent insulin resistance. Toxicol Lett. 2011;207:222–231. doi: 10.1016/j.toxlet.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu X, Pacheco BD, Leng L, Bucala R, Ren J. Macrophage migration inhibitory factor plays a permissive role in the maintenance of cardiac contractile function under starvation through regulation of autophagy. Cardiovasc Res. 2013;99:412–421. doi: 10.1093/cvr/cvt116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhamra GS, Hausenloy DJ, Davidson SM, Carr RD, Paiva M, Wynne AM, Mocanu MM, Yellon DM. Metformin protects the ischemic heart by the Akt-mediated inhibition of mitochondrial permeability transition pore opening. Basic Res Cardiol. 2008;103:274–284. doi: 10.1007/s00395-007-0691-y. [DOI] [PubMed] [Google Scholar]
- 20.Ren J, Dominguez LJ, Sowers JR, Davidoff AJ. Metformin but not glyburide prevents high glucose-induced abnormalities in relaxation and intracellular Ca2+ transients in adult rat ventricular myocytes. Diabetes. 1999;48:2059–2065. doi: 10.2337/diabetes.48.10.2059. [DOI] [PubMed] [Google Scholar]
- 21.Wang Q, Yang L, Hua Y, Nair S, Xu X, Ren J. AMP-Activated Protein Kinase Deficiency Rescues Paraquat-Induced Cardiac Contractile Dysfunction Through an Autophagy-Dependent Mechanism. Toxicol Sci. 2014 doi: 10.1093/toxsci/kfu158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roe ND, Thomas DP, Ren J. Inhibition of NADPH oxidase alleviates experimental diabetes-induced myocardial contractile dysfunction. Diabetes Obes Metab. 2011;13:465–473. doi: 10.1111/j.1463-1326.2011.01369.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang D, Patel VV, Ricciotti E, Zhou R, Levin MD, Gao E, Yu Z, Ferrari VA, Lu MM, Xu J, Zhang H, Hui Y, Cheng Y, Petrenko N, Yu Y, FitzGerald GA. Cardiomyocyte cyclooxygenase-2 influences cardiac rhythm and function. Proc Natl Acad Sci U S A. 2009;106:7548–7552. doi: 10.1073/pnas.0805806106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu X, Bucala R, Ren J. Macrophage migration inhibitory factor deficiency augments doxorubicin-induced cardiomyopathy. J Am Heart Assoc. 2013;2:e000439. doi: 10.1161/JAHA.113.000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu X, Hueckstaedt LK, Ren J. Deficiency of insulin-like growth factor 1 attenuates aging-induced changes in hepatic function: role of autophagy. J Hepatol. 2013;59:308–317. doi: 10.1016/j.jhep.2013.03.037. [DOI] [PubMed] [Google Scholar]
- 26.Xu X, Hua Y, Nair S, Zhang Y, Ren J. Akt2 knockout preserves cardiac function in highfat diet-induced obesity by rescuing cardiac autophagosome maturation. J Mol Cell Biol. 2013;5:61–63. doi: 10.1093/jmcb/mjs055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morlino G, Barreiro O, Baixauli F, Robles-Valero J, Gonzalez-Granado JM, Villa- Bellosta R, Cuenca J, Sanchez-Sorzano CO, Veiga E, Martin-Cofreces NB, Sanchez-Madrid F. Miro-1 links mitochondria and microtubule Dynein motors to control lymphocyte migration and polarity. Mol Cell Biol. 2014;34:1412–1426. doi: 10.1128/MCB.01177-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scherz-Shouval R, Elazar Z. Regulation of autophagy by ROS: physiology and pathology. Trends Biochem Sci. 2011;36:30–38. doi: 10.1016/j.tibs.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Azad MB, Gibson SB. Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ. 2009;16:1040–1052. doi: 10.1038/cdd.2009.49. [DOI] [PubMed] [Google Scholar]
- 30.Kaushik S, Cuervo AM. Autophagy as a cell-repair mechanism: activation of chaperone-mediated autophagy during oxidative stress. Mol Aspects Med. 2006;27:444–454. doi: 10.1016/j.mam.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 32.Matsui T, Li L, Wu JC, Cook SA, Nagoshi T, Picard MH, Liao R, Rosenzweig A. Phenotypic spectrum caused by transgenic overexpression of activated Akt in the heart. J Biol Chem. 2002;277:22896–22901. doi: 10.1074/jbc.M200347200. [DOI] [PubMed] [Google Scholar]
- 33.Condorelli G, Drusco A, Stassi G, Bellacosa A, Roncarati R, Iaccarino G, Russo MA, Gu Y, Dalton N, Chung C, Latronico MV, Napoli C, Sadoshima J, Croce CM, Ross J., Jr Akt induces enhanced myocardial contractility and cell size in vivo in transgenic mice. Proc Natl Acad Sci U S A. 2002;99:12333–12338. doi: 10.1073/pnas.172376399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, Gonzalez-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 35.Anichtchik O, Diekmann H, Fleming A, Roach A, Goldsmith P, Rubinsztein DC. Loss of PINK1 function affects development and results in neurodegeneration in zebrafish. J Neurosci. 2008;28:8199–8207. doi: 10.1523/JNEUROSCI.0979-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siddall HK, Warrell CE, Davidson SM, Mocanu MM, Yellon DM. Mitochondrial PINK1--a novel cardioprotective kinase? Cardiovasc Drugs Ther. 2008;22:507–508. doi: 10.1007/s10557-008-6136-5. [DOI] [PubMed] [Google Scholar]
- 37.Beauloye C, Bertrand L, Horman S, Hue L. AMPK activation, a preventive therapeutic target in the transition from cardiac injury to heart failure. Cardiovasc Res. 2011;90:224–233. doi: 10.1093/cvr/cvr034. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Yang L, Rezaie AR, Li J. Activated protein C protects against myocardial ischemic/reperfusion injury through AMP-activated protein kinase signaling. J Thromb Haemost. 2011;9:1308–1317. doi: 10.1111/j.1538-7836.2011.04331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma H, Wang J, Thomas DP, Tong C, Leng L, Wang W, Merk M, Zierow S, Bernhagen J, Ren J, Bucala R, Li J. Impaired macrophage migration inhibitory factor-AMP-activated protein kinase activation and ischemic recovery in the senescent heart. Circulation. 2010;122:282–292. doi: 10.1161/CIRCULATIONAHA.110.953208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie Z, He C, Zou MH. AMP-activated protein kinase modulates cardiac autophagy in diabetic cardiomyopathy. Autophagy. 2011;7:1254–1255. doi: 10.4161/auto.7.10.16740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oliveira SM, Zhang YH, Solis RS, Isackson H, Bellahcene M, Yavari A, Pinter K, Davies JK, Ge Y, Ashrafian H, Walker JW, Carling D, Watkins H, Casadei B, Redwood C. AMP-activated protein kinase phosphorylates cardiac troponin I and alters contractility of murine ventricular myocytes. Circ Res. 2012;110:1192–1201. doi: 10.1161/CIRCRESAHA.111.259952. [DOI] [PubMed] [Google Scholar]
- 42.Li HL, Yin R, Chen D, Liu D, Wang D, Yang Q, Dong YG. Long-term activation of adenosine monophosphate-activated protein kinase attenuates pressure-overload-induced cardiac hypertrophy. J Cell Biochem. 2007;100:1086–1099. doi: 10.1002/jcb.21197. [DOI] [PubMed] [Google Scholar]
- 43.Chan AY, Soltys CL, Young ME, Proud CG, Dyck JR. Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J Biol Chem. 2004;279:32771–32779. doi: 10.1074/jbc.M403528200. [DOI] [PubMed] [Google Scholar]
- 44.Xu X, Ren J. Unmasking the janus faces of autophagy in obesity-associated insulin resistance and cardiac dysfunction. Clin Exp Pharmacol Physiol. 2012;39:200–208. doi: 10.1111/j.1440-1681.2011.05638.x. [DOI] [PubMed] [Google Scholar]
- 45.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 46.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu X, Hua Y, Nair S, Bucala R, Ren J. Macrophage migration inhibitory factor deletion exacerbates pressure overload-induced cardiac hypertrophy through mitigating autophagy. Hypertension. 2014;63:490–499. doi: 10.1161/HYPERTENSIONAHA.113.02219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, Shaw RJ. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mizushima N. The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol. 2010;22:132–139. doi: 10.1016/j.ceb.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 51.Meley D, Bauvy C, Houben-Weerts JH, Dubbelhuis PF, Helmond MT, Codogno P, Meijer AJ. AMP-activated protein kinase and the regulation of autophagic proteolysis. J Biol Chem. 2006;281:34870–34879. doi: 10.1074/jbc.M605488200. [DOI] [PubMed] [Google Scholar]
- 52.Kane LA, Youle RJ. PINK1 and Parkin flag Miro to direct mitochondrial traffic. Cell. 2011;147:721–723. doi: 10.1016/j.cell.2011.10.028. [DOI] [PubMed] [Google Scholar]