Structured Abstract
Purpose
To determine which types of pediatric retinal degeneration are associated with inflammatory cells in the anterior vitreous (AV).
Design
Retrospective, observational study in humans.
Methods
Retrospective chart review was performed for pediatric patients with suspected retinal degeneration presenting to a single examiner from 2008–2013. Age, visual acuity (VA), slit lamp examination of AV (SLAV), clinical and molecular genetic diagnoses were documented. Anterior vitreous cells were graded clinically with SLAV from rare cells (1–4) to 1+ (5–9), 2+ (10–30), or 3+ (more than 30). Cells were also counted in magnified slit beam photographs masked to molecular diagnosis when obtainable.
Main outcome measures
Cell counts in SLAV, best corrected VA, molecular and clinical diagnoses.
Results
One hundred and five charts were evaluated, 68 of which (64.8%) included SLAV data. Numerous (1+ or greater) cells were present in 22/68 (32.4%) patients, whereas 4/68 (5.9%) had rare cells and 42/68 (61.8%) had no cells. The average age between patients with cells, no-cells, and rare cells did not differ significantly (p=0.25). VA averaged 20/124 in patients with cells, 20/143 in patients with no-cells, and 20/68 in patients with rare cells (p= 0.70). The most frequent diagnoses with cells included Bardet Biedl syndrome, Leber congenital amaurosis (LCA), and retinitis pigmentosa. The most frequent diagnoses without cells included congenital stationary night blindness, LCA, Stargardt disease, and blue cone monochromacy.
Discussion
A non-random subset of pediatric retinal degenerations exhibit vitritis. Cells were present in 5/5 BBS patients (a progressive degeneration) whereas cells were not detected in any of the 12 patients with CSNB (a stable dysfunction).
Conclusion
Studying vitritis in pediatric retinal degenerations may reveal whether inflammation accompanies progressive vision loss in certain sub-types. Potentially, inflammation could be treated. SLAV may also aid in clinical diagnosis.
INTRODUCTION
Albrecht von Graefe described the ophthalmoscopic appearance and clinical features of retinitis pigmentosa in detail in 1856, and postulated that heredity played a role. However it was Donders who, one year later, coined the term Retinitis Pigmentosa (RP), suggesting an infectious or inflammatory etiology, and thus started a discussion about the etiology of this condition which continues today1. Burstenbinder detected lymphocytes in the retina of pathologic specimens from RP patients early in the 20th century, and his contemporaries described “particles” in the vitreous of living patients, leading some ophthalmologists to espouse an inflammatory etiology even as others pronounced “retinitis pigmentosa” a misnomer1. The Ocular Immunology and Uveitis Foundations defines vitritis as “the accumulation of inflammatory cells or exudates in the vitreous humor” (http://www.uveitis.org/patients/education/glossary/t-z#VITRITIS). While one cannot say with certainty whether the particulate matter seen on slit lamp examination of the vitreous represents cells or debris from cell death, the appearance is similar to that in true inflammatory conditions such as uveitis. This reaction has been reported to decrease in response to local and systemic immunosuppression in the retinal degeneration seen in Juvenile Neuronal Ceroid Lipofuscinosis2. The finding that a secondary inflammatory response can exacerbate tissue injury that has a non-inflammatory initial cause is well known in other diseases of the nervous system, including stroke, Alzheimer disease, Parkinson disease, juvenile neuronal ceroid lipofuscinosis and ischemia reperfusion injury 3–8. It is thus similarly possible that the vitritis seen in retinal degenerations may play a role in the progression of the disease.
While the presence of anti-retinal autoantibodies has been described in some cases of retinitis pigmentosa 9–11 and lymphocytes have been detected in vitreous samples from eyes of adults with this disease12 there are few investigations into the quantity and timing of vitreous cells, especially as it relates to disease and genotype. Yoshida et al. 13 recently reported that 37% of adult patients with retinitis pigmentosa have an observable anterior vitreous cellular reaction and that stronger inflammatory reactions were more frequently associated with younger RP patients. In the current report, we studied the incidence of anterior vitritis in pediatric retinal degeneration patients with RP as well as other forms of inherited retinal disorders and correlated the presence or absence and intensity of the vitritis with molecular genetic diagnosis. These results suggest that vitreous cells occur in specific genotypes of inherited retinal diseases. Vitritis may have diagnostic, prognostic and therapeutic significance in pediatric retinal degenerations and dystrophies.
MATERIALS AND METHODS
Chart Review
Institutional Review Board approval was obtained prior to chart identification. Patients from the pediatric genetic eye disease clinic at the University of Iowa Hospitals and Clinics presenting with suspected retinal degeneration to one of the authors (AVD) from 2008–2013 were identified. Suspected retinal degeneration was based on patients’ referral diagnoses and features such as nystagmus, night blindness, peripheral vision restriction, abnormal electroretinogram testing and/or characteristic ophthalmoscopic or physical findings. Data collected included: age, Snellen best corrected visual acuity (VA), and slit lamp examination of anterior vitreous (SLAV) at most recent visit, and documentation of clinical and molecular genetic diagnosis. Slit lamp photographs of anterior vitreous (SLAV photos) were analyzed when available. Grading of vitritis at the slit lamp by a single examiner was documented in a similar fashion as prior studies12,13 where 0 represented no visible cell, rare = 1–4 cells per field, 1+ = 5–9 cells per field, 2+ = 10–30 cells per field, 3+ = more than 30 cells per field, as is our routine in the pediatric genetic eye disease clinic. Patients with rare cells were examined as a separate category from patients with or without vitritis. Data were analyzed using Analysis of Variance and Fisher exact test.
SLAV Photographs
SLAV photographs were obtained in patients with visible cells who could cooperate with anterior vitreous slit lamp photography. Because it is often possible to obtain a photograph of vitreous cells when a child is not able to cooperate fully with a more prolonged slit lamp evaluation, we routinely obtain vitreous photographs when slit lamp examination suggests a cellular infiltrate. Taking standard vitreous photographs at consecutive visits also allows us to compare the vitreous reaction over time. Photographs were obtained by a single ophthalmic photographer using a standardized technique. SLAV photographs were taken on a Haag-Streit BX 900 photo slit lamp with a Canon 50D DSLR camera back for digital capture. Posterior cell flare images were taken with a slit beam length of 5 mm and a width of 2mm. All images were captured using the 10x setting at the slit lamp camera. The slit lamp beam was projected into the space just behind the crystalline lens at a 30–45 degree angle. The final images were at 16x magnification. In patients with anterior vitreous photographs, cells in the anterior vitreous were counted by an observer masked to diagnosis and genotype using Image J (http://imagej.nih.gov/ij/) to mark and count the cells in the magnified slit beam photographs.
RESULTS
Chart Review
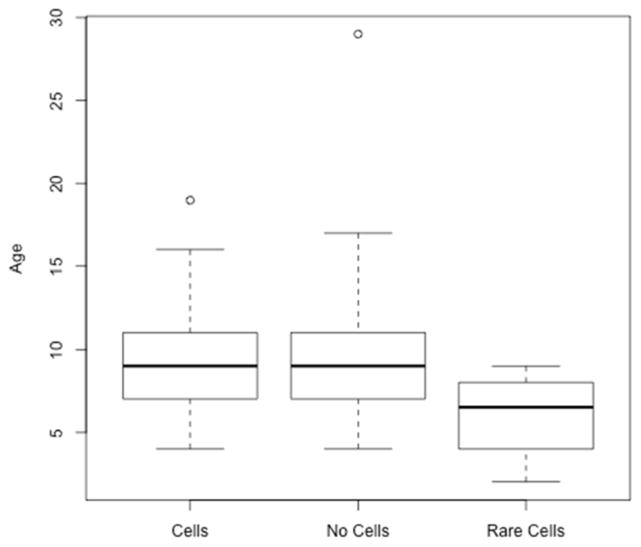
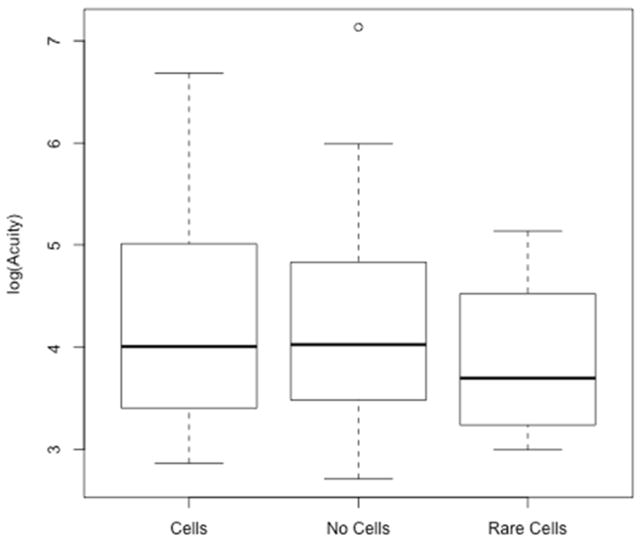
One hundred and seven charts were identified from children referred to the pediatric genetic eye disease service for a suspected genetic retinal disorder. Two were excluded as asymptomatic, unaffected siblings of patients with retinal degeneration. Clinical data were recorded from the most recent clinical visit, and the age at that visit was documented. SLAV data were available from 68/105 (64.4%). SLE could not be accomplished in 37/105 (35.6%) due to young age (the average age of patients with SLE of AV was 9.3 years while the average age of patients without SLE of AV was 3.3 years, p<10−6). 42/68 (62.7%) had no vitritis. 4/68 (6.0%) had rare cells. 22/68 (31.3%) had 1+ or greater cells. The average age of patients with cells, no-cells, and rare cell (9.5 years, 9.5 years, 6 years, respectively) did not differ significantly (ANOVA p=0.255; see Figure 1). Snellen VA averaged 20/124 in the vitritis group, 20/143 in the non-vitritis group, and 20/68 in the rare cells group. Logarithm-transformed best corrected VA means were 4.338, 4.222, and 3.881 respectively which were not significantly different when analyzed by ANOVA (p=0.70; see Figure 2). Diagnoses of patients with cells included: Bardet Biedl syndrome (BBS) (5/5), Leber congenital amaurosis (LCA) (7/15), retinitis pigmentosa (RP) (5/7), Usher syndrome (2/2), Batten disease (CLN3) (1/1), juvenile X-linked retinoschisis (JXLR) (1/3), Stargardt disease (1/6). Diagnoses of patients without cells included: Congenital stationary night blindness (CSNB) (12/12), LCA(6/15), Stargardt(5/6), blue cone monochromacy(4/4), RP(2/7), achromatopsia(2/2), JXLR (2/3), congenital nystagmus(4/4), Batten disease (CLN1) (1/1), Joubert syndrome (1/1), Cobalamin G deficiency(1/1), autism/obesity without RP(1/1), optic nerve hypoplasia (1/1). Diagnoses in the rare cell group included: LCA (2/15), choroideremia (1/1), and ocular histoplasmosis (1/1). A summary of study patients including their molecularly confirmed genetic diagnoses is shown in Table 1.
Figure 1.
Boxplots of the distribution of age in years in the presence or absence of vitreous cells. ANOVA p=0.25, 2 degrees of freedom.
Figure 2.
Boxplot of logarithm-transformed best corrected visual acuity in the presence or absence of vitreous cells. ANOVA p=0.70, 2 degrees of freedom.
Table 1.
Genotypes and clinical characteristics
| Case | Gender | Age at last visit (years) | Diagnosis | Gene affected | Documented vitreous cells | Visual acuity (best corrected)
|
|
|---|---|---|---|---|---|---|---|
| OD | OS | ||||||
| 1 | M | 11 | Stargardt | ABCA4 | 1+ cells OU | 20/80 | 20/30 |
| 2 | F | 4 | LCA | CRX | 1+ cells | 20/800 | 20/800 |
| 3 | F | 8 | BBS | ARL6/BBS3 | 2+ cells | 20/30 | 20/30 |
| 4 | F | 8 | Batten | CLN3 | 1+ cells OD; 2+ cells OS | 20/500 | 20/300 |
| 5 | M | 19 | LCA | NMNAT1 | <1+ cells OU | LP | 20/560 |
| 6 | M | 9 | Usher | USH1c | 2+ cells OU | 20/30 | 20/25 |
| 7 | F | 5 | LCA | RPE65 | no cells OS; rare cells OD | 20/667 | 20/200 |
| 8 | F | 14 | BBS | BBS10 | <1+ cells OU | 20/300 | 20/100 |
| 9 | F | 4 | LCA | RDH12 | 3+ cells OU | 20/50 | 20/250 |
| 10 | M | 9 | cone-rod dystrophy | * | 1+ cells OD; 1/2+cells OS | 20/30 | 20/30 |
| 11 | F | 5 | BBS | BBS1 | 1+ cells OD; 2+ cells OS | 20/40 | 20/40 |
| 12 | F | 11 | BBS | BBS1 | 1+ cells OD; 2+ cells OS | 20/70 | 20/40 |
| 13 | F | 14 | retinal dystrophy | * | 1+ cells OD; 2+ cells OS | 20/15 | 20/20 |
| 14 | M | 7 | RP | Rho | 2–3+ cells OD; 1–2+ cells OS | 20/30 | 20/30 |
| 15 | M | 9 | LCA | RDH12 | rare cells OD; 1+few cells OS | 20/100 | 20/40 |
| 16 | M | 6 | LCA with+ Coat’s-like reaction | * | 1–2+ cells in vitreous | 20/80 | 20/80 |
| 17 | M | 10 | Usher | * | 1+ cells OU | 20/20 | 20/20 |
| 18 | M | 11 | RP | * | 1+ cells OD; 2+ cells OS | 20/40 | 20/40 |
| 19 | M | 10 | RP | * | 2+ cells OD; 1+ cell OS | 20/50 | 20/60 |
| 20 | M | 16 | BBS | BBS1 | 2+ cells OD; 1+ cell OS | 20/40 | 20/40 |
| 21 | M | 7 | JXLR | RS1 | 1+ cells OD; rare cells OS | 20/150 | 20/100 |
| 22 | F | 11 | LCA | RPE65 | 1+ cells | 20/200 | 20/160 |
|
| |||||||
| 23 | F | 11 | Stargardt | ABCA4 | No cells | 20/400 | 20/200 |
| 24 | F | 11 | Stargardt | ABCA4 | No cells | 20/30 | 20/30 |
| 25 | F | 15 | Stargardt | ABCA4 | No cells | 20/200 | 20/100 |
| 26 | M | 7 | Blue cone monochromacy | OPN1LW | No cells | 20/50 | 20/40 |
| 27 | F | 12 | LCA | GUCY2D | No cells | 20/250 | unable to do distance |
| 28 | F | 7 | CSNB | ‡ | No cells | 20/60 | 20/100 |
| 29 | M | 12 | cone dystrophy | ‡ | No cells | 20/40 | 20/40 |
| 30 | M | 6 | CSNB | NYX | No cells | 20/30 | 20/25 |
| 31 | M | 9 | CSNB | CACNA1F | No cells | 20/40 | 20/40 |
| 32 | M | 9 | CSNB | TRPM1 | No cells | 20/60 | 20/70 |
| 33 | M | 11 | CSNB | TRPM1 | No cells | 20/50 | 20/40 |
| 34 | M | 6 | LCA | AIPL1 | No cells | 20/1250 | 20/3000 |
| 35 | F | 12 | CSNB | TRPM1 | No cells | 20/25 | 20/20 |
| 36 | F | 7 | LCA | CEP290 | No cells | NLP | NLP |
| 37 | M | 14 | CSNB | GUCY2D# | No cells | 20/20 | 20/20 |
| 38 | F | 10 | Joubert syndrome | * | No cells | 20/125 | 20/100 |
| 39 | M | 10 | CSNB | * | No cells | 20/20 | 20/25 |
| 40 | M | 7 | CSNB | ‡ | No cells | 20/125 | 20/125 |
| 41 | M | 29 | Blue cone monochromacy | ‡ | No cells | 20/20 | 20/25 |
| 42 | M | 10 | retinal dystrophy | * | No cells | 20/40 | 20/40 |
| 43 | M | 9 | congenital nystagmus | * | No cells | 20/70 | 20/100 |
| 44 | F | 15 | LCA | CEP290 | No cells | LP | LP |
| 45 | M | 9 | congenital nystagmus | * | No cells | 20/100 | 20/100 |
| 46 | M | 4 | Blue cone monochromacy | OPN1LW | No cells | 20/400 | 20/400 |
| 47 | M | 9 | autism/obesity | * | No cells | 20/15 | 20/15 |
| 48 | M | 11 | achromatopsia | * | No cells | 20/150 | 20/125 |
| 49 | M | 4 | congenital nystagmus | * | No cells | 20/63 | 20/63 |
| 50 | M | 5.5 | Optic nerve hypoplasia | * | No cells | 20/40 | 20/40 |
| 51 | F | 9 | achromatopsia | * | No cells | 20/300 | 20/200 |
| 52 | F | 6.5 | congenital nystagmus | ‡ | No cells | 20/40 | 20/40 |
| 53 | F | 11 | Stargardt | ABCA4 | No cells | 20/100 | 20/100 |
| 54 | M | 8 | CSNB | ‡ | No cells | 20/70 | 20/60 |
| 55 | F | 17 | Stargardt | ABCA4 | No cells | 20/200 | 20/400 |
| 56 | M | 8.5 | JXLR | RS1 | No cells | 20/25 | 20/25 |
| 57 | M | 6.5 | JXLR | RS1 | No cells | 20/40 | 20/40 |
| 58 | M | 6 | Batten | CLN1 | No cells | unable to fix or follow | |
| 59 | M | 12 | Cobalamin G deficiency | MTR | No cells | 20/40 | 20/60 |
| 60 | M | 9 | LCA | CEP290 | No cells | LP | LP |
| 61 | M | 8 | LCA | GUCY2D | No cells | 20/125 | 20/125 |
| 62 | M | 6 | Blue cone monochromacy | ‡ | No cells | 20/150 | 20/100 |
| 63 | F | 8 | CSNB | TRPM1 | No cells | 20/40 | 20/25 |
| 64 | F | 6 | CSNB | TRPM1 | No cells | 20/20 | 20/25 |
|
| |||||||
| 65 | F | 2 | LCA | IQCB1 | no cells OD; 1 pigmented cell OS | 20/170 | 20/170 |
| 66 | M | 7 | Choroideremia | CHM | rare pigmented cells OU | 20/20 | 20/20 |
| 67 | F | 9 | Ocular histoplasmosis | N/A | no cell OD; rare cell OS | 20/80 | 20/20 |
| 68 | M | 6 | LCA | * | rare cell OD; no cells OS | 20/40 | 20/25 |
Genetic testing was negative for all genes tested that corresponded with phenotype
GeneGc tesGng not done due to parent/patient wishes or lost to follow up; Patients 54 and 62 had positive family histories for their disorders, therefore molecular genetic testing was not performed.
Patient number 37 is designated here as CSNB because he has normal acuity and full peripheral vision but an almost non-recordable ERG and severe nightblindness since infancy. Clinically he is best described as CSNB, however the ERG, infantile onset and GUCY2D mutations also make atypical LCA a valid clinical diagnosis.
All patients in this table with recessive disorders had 2 alleles found with the exception of #20 who had one mutated allele found in BBS1.
5/22 patients with cells had BBS vs. 0/42 patients without (p=0.003 by Fisher exact test). 12/42 patients without cells had CSNB compared to 0/22 patients with cells (p=0.003). Each p-value is still significant at 0.05 even after correction for multiple testing using the Bonferroni method. Although LCA patients as a whole showed variability in the presence of cells, when stratified by genotype the genetic subtypes of LCA did not overlap. Patients with LCA due to mutations in RDH12(2/2), CRX(1/1), and RPE65(2/2) exhibited cells, whereas patients with mutations in CEP290(3/3), GUCY2D(2/2), AIPL1(1/1) did not. One patient with IQCB1-associated LCA presented in the rare cell group. A few diagnoses were represented by patients in both the cells and no cells groups: JXLR and Stargardt disease each had one patient with rare to 1+ vitreous cells while the other patients had none. Patients with RP and LCA without an identified mutation were present in both the no cells and with cells groups. The distribution of patients in the cells vs. no cells categories can be seen in figure 3.
Figure 3.
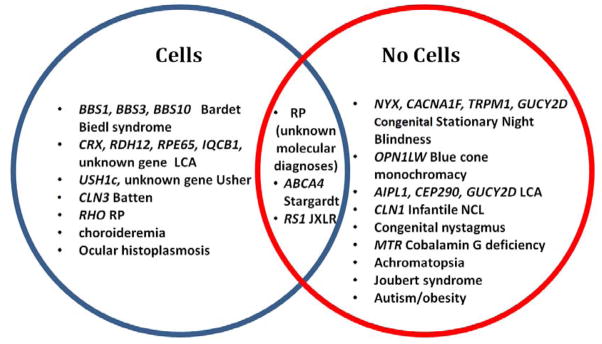
Venn diagram of patient diagnoses with genotypes, between cell and no cell populations. Patients with diagnoses of cone-rod dystrophy, retinal dystrophy, cone dystrophy, and RP were included under RP in this diagram.
Slit lamp photographs
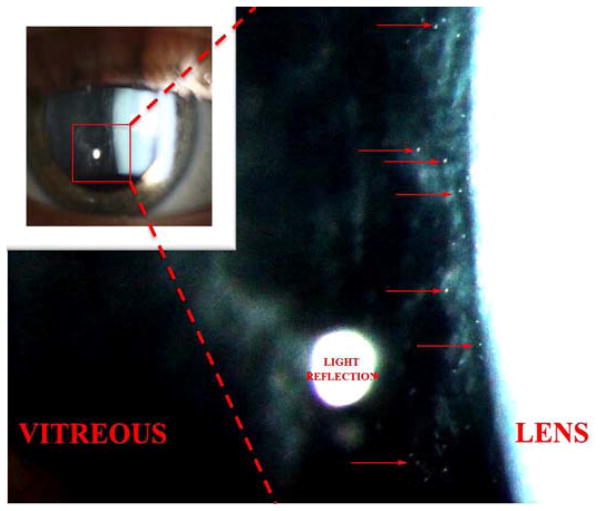
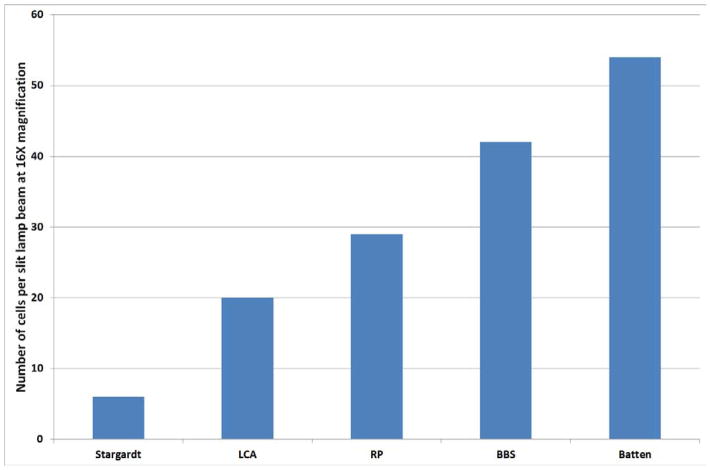
A typical slit lamp photograph taken at 16X magnification is shown in Figure 4 with an enlarged field showing visible cells pictured on the right. Thirteen patients had SLAV photographs. The number of cells per eye were counted and an average was calculated for the two eyes of each patient. When more than one patient with a diagnosis was available, the values for all eyes of patients with that diagnosis were averaged (Table 2). Diagnoses for patients with SLAV photographs are as follows: RP (5), LCA (4), BBS (2), Stargardt (1), Batten/CLN3 (1). Based on averaged cell counts from SLAV photographs, the number of cells per slit beam in the Batten patient was 53, vs. the BBS patients which was 42, vs. 29 for the RP patients, 20 for the LCA patients with cells, and 6 for the Stargardt patient with cells (Figure 5). The cell counts obtained at the slit lamp in clinic were compared to those obtained from photographs. Most eyes had similar counts by the physician counting them at the slit lamp vs. counted on a photograph, but some were discordant (see Table 2). This likely reflects suboptimal patient cooperation at the slit lamp exam, and/or eye movement during photography.
Figure 4.
Slit lamp photograph demonstrating cells in the anterior vitreous of a patient with Bardet Biedl Syndrome type 1 (BBS1). Photograph by Brice Critser, CRA.
Table 2.
Cell counts at slit lamp photos by individual patients
| Diagnosis (mutation) | # cells in photo | Clinical Grading | |
|---|---|---|---|
| OD | OS | ||
| LCA (IQCB1) | 3 | 1 | rare cell OD; no cells OS |
| Stargardt (ABCA4) | 5 | 6 | few cells OU |
| RP (Rho) | 13 | 0 | 2–3+ OD, 1–2+ OS |
| RP | 15 | 23 | 1+ OD, 2+ OS |
| RP | 26 | 27 | few cells OU; more cells OS |
| LCA (RPE65) | 28 | 12 | 1+ OU |
| BBS (BBS1) | 37 | 16 | 1+ OD, 2+ OS |
| LCA (RPE65) | 39 | 14 | rare cells OD; no cells OS |
| LCA (RDH12) | 50 | 13 | rare cells OD, few cells OS |
| RP | 16 | 56 | 1+ OD, 1/2+ OS |
| Batten (CLN3) | 76 | 31 | 1+ OD, 2+ OS |
| BBS (BBS1) | 101 | 13 | 2+ OD, 1+ OS; pigmented cells |
| RP | 8 | 103 | 2+ pigmented cells OD; 1+ cells OS |
Vitreous cell counts by individual patient starting with least number of cells and proceeding to highest.
Figure 5.
Average number of cells per slit lamp beam photograph by diagnosis. The counts for both eyes of each patient were averaged into one value per patient. In cases with more than one patient per diagnosis, cell counts for all eyes of all patients with that diagnosis were averaged. Stargardt = Stargardt disease. LCA = Leber congenital amaurosis. RP = retinitis pigmentosa. BBS = Bardet Biedl syndrome. Batten = Batten disease (juvenile neuronal ceroid lipofuscinosis).
DISCUSSION
Genetic pediatric retinal disorders are a major cause of blindness and low vision in children. They represent a complex and often clinically overlapping group of non-syndromic as well as syndromic ocular diseases that can be either stable or progressive in nature. The presence and clinical grading of vitreous cells in adults with retinitis pigmentosa has been documented; 13 however, the presence and quantity of cells has not been correlated with molecular diagnosis or a clinical subset of retinal degeneration, and has not to our knowledge been documented in children with retinal disease. In this study we demonstrate (1) that a discrete subset of pediatric retinal degenerations exhibit vitritis and (2) a standardized clinical method for quantifying the increased number of cells in the vitreous of patients with more progressive ocular disease.
One hundred percent (5/5) of BBS patients (i.e., with a progressive retinal degeneration) demonstrated cells, whereas vitreous cells were detected in none of the 12 patients with CSNB (a stable condition) (p=0.003). Comparing the clinical course of these two disorders reveals that a salient difference is natural history: BBS retinal degeneration is rapidly progressive resulting in loss of most vision by 20–30 years of age15,16. Patients experience night blindness in the first decade with abnormal ERG, and by age 20–30 years the ERG is usually non-recordable and most vision is lost. In the mouse model of the most common form of BBS, Bbs1M390R/M390R, histology of the retina at various stages shows a steady loss of photoreceptors with the outer nuclear layer reduced to one layer of nuclei by 8–9 months of age17. BBS is a pleomorphic disorder which can be caused by mutations in at least 19 genes and which is characterized by cilia dysfunction18–20. A cellular response in the vitreous of these patients would suggest that this degeneration is augmented by inflammation, and/or causes inflammation. This suggests that vitritis is associated with progression of retinopathy, and perhaps with photoreceptor cell loss. CSNB, on the other hand, also presents with night blindness in the first decade of life, but it is generally non-progressive with maintenance of VA unless retinal detachment or other complications develop21. Patients with CSNB have gross abnormalities on ERG, but in most cases do not have progressive retinal degeneration. CSNB patients exhibit a transduction deficit and would not be expected to have attendant inflammation, which agrees with what was found in our study. When eyes are grouped by diagnosis and the average number of cells seen in each patient, the numbers of cells roughly correlate with extent of retinal disease and rapidity of vision loss with Stargardt disease eyes having the fewest cells, followed by LCA, RP, BBS, and CLN3 Batten Disease (figure 5).
Interestingly all patients with RDH12 and RPE65 subtypes of LCA had vitritis, while all patients with CEP290 and GUCY2D genetic subtypes of LCA did not. The groups did not overlap when analyzing the vitritis phenotype. This suggests that either mechanism or temporal features of disease (or both) may be more similar between CEP290 and GUCY2D LCA than RPE65 and RDH12 LCA. It is noteworthy that both the RDH12 and RPE65 genes participate in the retinoid cycle 22.
The difference in VA between groups is not significant, but the averages are skewed due to several outliers. Visual acuity for 4/42 individuals in the no cells group was documented as “no light perception”, “light perception”, or “unable to fix or follow”, whereas only one eye of one patient in the cell group had vision as poor as LP. Patients with vision worse than optotype identification were of necessity not included in the visual acuity averages. The 4 patients with the worst acuity in the no-cells group represent three molecularly confirmed cases of CEP290 associated LCA as well as an individual with molecularly confirmed CLN1 Batten disease. These children with CEP290 LCA had very little vision from birth, and their SLAV exam was performed at an average age of 8.77 years. The child with CLN1 Batten disease had lost all visual responses by age 21 months, and was most recently examined at age 6 years. It is possible that in very early retinal degenerations no vitreous cellular reaction is mounted or that it resolves before the child is old enough to be examined.
Only one patient with ABCA4 associated Stargardt disease had vitreous cells (Case 1, Table 1). The most recent slit lamp examination for this patient was shortly after diagnosis when visual acuity was dropping rapidly. Vision was 20/80 right eye and 20/30 left eye on this examination, but dropped to 20/100 left eye over the ensuing 6 months. 4/5 other Stargardt patients in the study had already lost vision to the 20/100–20/400 range before the recorded examination (Cases 23, 25, 53, 55 Table 1). Case 24 had an affected family member and therefore had genetic testing performed at a very early stage of disease when her vision had fallen from 20/20 to 20/30, and Ishihara color vision testing became abnormal. Stargardt patients who had already lost central vision or who had barely started to lose vision had no cells in the vitreous; the patient who was in the phase of rapid vision loss did. JXLR case 21 (Table 1) had vitreous cells and much worse vision (average 20/125) than the 2 JXLR cases (56 and 57, Table 1) without cells (average 20/30), perhaps signifying more active disease.
Yoshida et al. recently reported on the incidence of vitreous cells in adults with RP13. They report that a stronger inflammatory reaction (e.g. 2+ cells) is more frequently associated with younger age in RP patients (<30 years old) than older RP patients. We did not find an age difference between cells or no cells in the pediatric age group. These authors found approximately 37% of adult RP patients have cells in the anterior vitreous. Our study of much younger patients found that 38% had vitreous cells when those with rare cells and 1+ or greater cells are considered together. In the adult RP patients younger age may be associated with disease progression, while older age represents a plateau.
Standardized anterior vitreous cell photographs allow us to quanitify vitreous cells in children without requiring prolonged cooperation at the SL. However, the photographs must also be obtained quickly. Focus and orientation may be compromised in some photographs. In some cases the correlation between clinical slit lamp cell counts and photographs was not strong; this likely represents patient inability to cooperate. It is likely that for a given patient the slit lamp photograph cell count is the more accurate number. In this retrospective study we counted the cells in the illuminated slit beam even if the focus was not ideal due to a patient’s inablility to cooperate, which may have caused under- or over-counting in some cases. Even with these shortcomings, presence or absence of vitreous cells could be ascertained by some method in a majority of patients. A prospective study in which all patients had SLAV using strict criteria for which photographs were of adequate quality and using only SL photographic data rather than estimated counts at the slit lamp would greatly add to our understanding of this topic. Yoshida, et al. recently reported increased protein levels of proinflammatory cytokines in the vitreous of RP patients13, suggesting that chronic inflammation is a component of the pathophysiology of RP. Similarly, Mullins and co-workers recently performed postmortem microarray analysis of the retina of a patient with severe retinal degeneration due to ABCA4 mutations and showed upregulation of genes associated with immune processes23, consistent with recruitment of inflammatory cells.
Since vitritis is not well characterized in these populations, a better understanding of the physiology of cellular infiltrates may be obtained by studying a corresponding mouse model of disease. A mouse model of the most common type of BBS, Bbs1M390R/M390R recapitulates most of the human phenotypes, including obesity and photoreceptor degeneration. The retinal degeneration in particular is similar to the human condition with an abnormal ERG within the first months of life and photoreceptor dropout and loss of all detectable retinal electrical activity by 8 months of age17. The most rapid part of the degeneration occurs between 4–8 months of age. This mouse model provides an opportunity to determine whether treating the inflammatory component will affect the course of the retinal degeneration and to study this component more fully. It has recently been demonstrated that treatment of the rd10 mouse with N acetyl cysteine, an antioxidant, slows retinal degeneration24. We previously demonstrated that treatment with TUDCA, which has antiapoptotic as well as antioxidant effects, slows retinal degeneration in Bbs1 mice25.
The clinical presentation of several subtypes of genetic pediatric retinal disorders represent a dilemma for the pediatric ophthalmologist as several non-syndromic and syndromic ocular phenotypes may overlap. The underlying pathology, visual prognosis, and future treatments are different for each diagnosis. Accurate clinical diagnosis is therefore essential. Examining the anterior vitreous for the presence of cells may give an early indication of whether a progressive or static retinal disorder is present.
Acknowledgments
Financial support: U. of Iowa Carver College of Medicine NIH T35 Training Grant 2T35HL007485 (Stunkel), Marjorie Carr Adams Career Development Award, Foundation Fighting Blindness (Drack), Research to Prevent Blindness Physician-Scientist Award (Drack), Hope for Vision (Drack), Howard Hughes Medical Institute (Stone).
Footnotes
Conflict of interest: The authors have no proprietary or commercial interest in any of the materials discussed in this article.
Meeting presentation: The Association for Research in Vision and Ophthalmology, Annual Meeting, May 2013.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shoemaker WT, Swan JM. Retinitis pigmentosa with an analysis of seventeen cases occurring in deaf-mutes. 2–10. Philadelphia, PA: Lippincott; 1909. pp. 94–6. [Google Scholar]
- 2.Drack AV, Mullins RF, Pfeifer WL, et al. Immunosuppressive treatment for retinal degeneration in juvenile neuronal ceroid lipofuscinosis (juvenile Batten disease) Ophthalmic Genet. doi: 10.3109/13816810.2014.886271. In press. [DOI] [PubMed] [Google Scholar]
- 3.Kreutzberg GW. Microglia: a sensor for the pathological events in the CNS. Trends Neurosci. 1996;19:312–8. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 4.Kim YS, Joh TH. Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson’s disease. Exp Mol Med. 2006;38:333–47. doi: 10.1038/emm.2006.40. [DOI] [PubMed] [Google Scholar]
- 5.Pearce DA, Atkinson M, Tagle DA. Glutamic acid decarboxylase autoimmunity in Batten disease and other disorders. Neurology. 2004;63:2001–5. doi: 10.1212/01.wnl.0000145836.72059.3b. [DOI] [PubMed] [Google Scholar]
- 6.Seehafer SS, Ramirez-Montealegre D, Wong AM, et al. Immunosuppression alters disease severity in juvenile Batten disease mice. J Neuroimmunol. 2011;230:169–72. doi: 10.1016/j.jneuroim.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu PX, Wang SW, Yu XL, et al. Rutin improves spatial memory in Alzheimer’s disease transgenic mice by reducing Aβ oligomer level and attenuating oxidative stress and neuroinflammation. Behav Brain Res. 2014;264:173–80. doi: 10.1016/j.bbr.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Wu Y, Satkunendrarajah K, Fehlings MG. Riluzole improves outcome following ischemia-reperfusion injury to the spinal cord by preventing delayed paraplegia. Neuroscience. 2014;265:302–12. doi: 10.1016/j.neuroscience.2014.01.059. [DOI] [PubMed] [Google Scholar]
- 9.Heckenlively JR, Aptsiauri N, Nusinowitz S, et al. Investigations of antiretinal antibodies in pigmentary retinopathy and other retinal degenerations. Trans Am Ophthalmol Soc. 1996;94:179–200. discussion 200–6. [PMC free article] [PubMed] [Google Scholar]
- 10.Heckenlively JR, Jordan BL, Aptsiauri N. Association of antiretinal antibodies and cystoid macular edema in patients with retinitis pigmentosa. Am J Ophthalmol. 1999;127:565–73. doi: 10.1016/s0002-9394(98)00446-2. [DOI] [PubMed] [Google Scholar]
- 11.Ko AC, Brinton JP, Mahajan VB, et al. Seroreactivity against aqueous-soluble and detergent-soluble retinal proteins in posterior uveitis. Arch Ophthalmol. 2011;129:415–20. doi: 10.1001/archophthalmol.2011.65. [DOI] [PubMed] [Google Scholar]
- 12.Newsome DA, Michels RG. Detection of lymphocytes in the vitreous gel of patients with retinitis pigmentosa. Am J Ophthalmol. 1988;105:596–602. doi: 10.1016/0002-9394(88)90050-5. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida N, Ikeda Y, Notomi S, et al. Clinical evidence of sustained chronic inflammatory reaction in retinitis pigmentosa. Ophthalmology. 2013;120:100–5. doi: 10.1016/j.ophtha.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Boldrey EE. Vitreous cells as an indicator of retinal tears in asymptomatic or not recently symptomatic eyes. Am J Ophthalmol. 1997;123:263–4. doi: 10.1016/s0002-9394(14)71050-5. [DOI] [PubMed] [Google Scholar]
- 15.Drack AV, Mullins RF, Seo S. Retinitis pigmentosa syndromes: Bardet-Biedl. In: Hartnett ME, editor. Pediatric Retina. 2. Philadelphia, PA: Lippincott Williams & Wilkins; 2013. pp. 259–268. AQ: must supply specific inclusive pagination for chapter Done. [Google Scholar]
- 16.Heon E, Westall C, Carmi R, et al. Ocular phenotypes of three genetic variants of Bardet-Biedl syndrome. Am J Med Genet A. 2005;132A:283–7. doi: 10.1002/ajmg.a.30466. [DOI] [PubMed] [Google Scholar]
- 17.Davis RE, Swiderski RE, Rahmouni K, et al. A knockin mouse model of the Bardet-Biedl syndrome 1 M390R mutation has cilia defects, ventriculomegaly, retinopathy, and obesity. Proc Natl Acad Sci U S A. 2007;104:19422–7. doi: 10.1073/pnas.0708571104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox KF, Kerr NC, Kedrov M, et al. Phenotypic expression of Bardet-Biedl syndrome in patients homozygous for the common M390R mutation in the BBS1 gene. Vison Res. 2012;75:77–87. doi: 10.1016/j.visres.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Deveault C, Billingsley G, Duncan JL, et al. BBS genotype-phenotype assessment of a multiethnic patient cohort calls for a revision of the disease definition. Hum Mutat. 2011;32:610–9. doi: 10.1002/humu.21480. [DOI] [PubMed] [Google Scholar]
- 20.Abu SL, Aldahmesh MA, Shamseldin H, et al. Clinical and molecular characterization of Bardet-Biedl syndrome in consanguineous populations: the power of homozygosity mapping. J Med Genet. 2010;47:236–41. doi: 10.1136/jmg.2009.070755. [DOI] [PubMed] [Google Scholar]
- 21.Bijveld MM, Florijn RJ, Bergen AA, et al. Genotype and phenotype of 101 Dutch patients with congenital stationary night blindness. Ophthalmology. 2013;120:2072–81. doi: 10.1016/j.ophtha.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 22.den Hollander AI, Roepman R, Koenekoop RK, Cremers FP. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog Retin Eye Res. 2008;27:391–419. doi: 10.1016/j.preteyeres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Mullins RF, Kuehn MH, Radu RA, et al. Autosomal recessive retinitis pigmentosa due to ABCA4 mutations: clinical, pathologic, and molecular characterization. Invest Ophthalmol Vis Sci. 2012;53:1883–94. doi: 10.1167/iovs.12-9477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshida N, Ikeda Y, Notomi S, et al. Laboratory evidence of sustained chronic inflammatory reaction in retinitis pigmentosa [report online] Ophthalmology. 2013;120:e5–12. doi: 10.1016/j.ophtha.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Drack AV, Dumitrescu AV, Bhattarai S, et al. TUDCA slows retinal degeneration in two different mouse models of retinitis pigmentosa and prevents obesity in Bardet-Biedl syndrome type 1 mice. Invest Ophthalmol Vis Sci. 2012;53:100–6. doi: 10.1167/iovs.11-8544. [DOI] [PMC free article] [PubMed] [Google Scholar]