Abstract
The UCS family of proteins regulate cellular functions through their interactions with myosin. Here we show that one member of this family, UNC45A, is also a novel centrosomal protein. UNC45A is required for cellular proliferation of cancer cell in vitro and for tumor growth in vivo through its ability to bind and regulate ChK1 nuclear-cytoplasmic localization in an Hsp90-independent manner. Immunocytochemical and biochemical fractionation studies revealed that UNC45A and ChK1 co-localize to the centrosome. Inhibition of UNC45A expression reduced ChK1 activation and its tethering to the centrosome, events required for proper centrosome function. Lack of UNC45A caused the accumulation of multi-nucleated cells, consistent with a defect in Chk1 regulation of centrosomes. These findings identify a novel centrosomal function for UNC45A and its role in cell proliferation and tumorigenesis.
Keywords: Tumorigenesis, UNC45A, Hsp90, chaperone, ChK1, Centrosome
1. Introduction
UNC45 is a member of the UCS (UNC-45/Cro1/She4p) family of myosin-interacting proteins (1). Two isoforms of UNC45 have been described. UNC45B is muscle specific and its function has been well established in the proper folding and maturation of myosin and myofibril organization (2–10). In contrast, UNC45A, which shares only 50% sequence identity (9, 10), is more generally expressed in all organs except muscles, and its function is largely unknown. Both isoforms contain an N-terminal tetratricopeptide repeat (TPR) that interacts with the chaperone heat shock protein (Hsp) 90 (4, 11, 12), repressing its ATPase and slowing the Hsp90 chaperoning cycle to promote optimum chaperoning (12, 13). In support of this idea, silencing UNC45A reduces the Hsp90-dependent transcriptional activity of the progesterone receptor in vivo (12).
As suggested by their differential tissue specific-expression and their limited sequence homology, UNC45A and UNC45B might have significant functional differences(8, 9, 14). Accumulating evidence indicates that UNC45A contributes to tumorigenesis, with overexpression in ovarian and breast carcinoma correlating with stage and grade of diseases (15, 16). Previous studies using ovarian cancer cell lines have shown that UNC45A may play a role in cell cycle progression through regulation of cytokinesis. Immunocytochemical staining revealed that UNC45A co-localizes with myosin II in the cleavage furrow (15). This was in line with genetic and biochemical studies showing that orthologs of UNC45A in yeast and nematodes chaperone myosin II, which culminates in furrow cleavage and drives cytokinesis (17). Intriguingly, however, silencing UNC45A did not cause myosin II protein degradation (15) as it would be expected from a chaperone client protein, thus suggesting that UNC45A regulates proliferation by another mechanism rather than insuring the stability of Myosin II.
A key regulator of proliferation is the checkpoint kinase ChK1. ChK1 is best known for it roles in coordinating responses to exogenous genotoxic stresses. However, recent reports have shown that ChK1 has additional functions. It is required for the progression and mitotic entry of unperturbed cells (18, 19) and it regulates the centrosome to maintain cell ploidy (20). These studies showed that inhibition of ChK1 induces premature centrosome separation through inhibition of cyclin-B1-Cdk1 activation (20) and that tethering ChK1 to centrosomes causes endoreplication and the appearance of enlarged polyploid nuclei (20). In this this report we show that UNC45A is a centrosome-associated protein that recruits ChK1 to the centrosome, thereby preventing the accumulation of multi-nucleated cells and cell death.
2. Materials and Methods
2.1 Knockdown of UNC45A by transient transfection
HeLa cells were transfected with 100 nanomolar concentration of siRNA against UNC45A as previously described (12). Non-targeting siRNA #1 from DharmaFECT was used as a negative control. Cells were harvested 96 hours after transient transfection.
2.2 Stable transfection of HeLa cells with UNC45A shRNA
HeLa cells were transfected with a lentivirus encoding the doxycycline-inducible UNC45A KD shRNA or scrambled shRNA. UNC45A depletion was achieved by exposing cells that stably harbored UNC45A shRNA cells to 1 μg/mL of doxycycline for 96–144 hours.
2.3 Western blot
Lysates were made using 1 x RIPA buffer (150 mM NaCl, 0.1% Triton-X-100, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris-HCl) and supplemented with 10 mM NaF, 2 mM β-glycerophosphate, 2 mM sodium pyrophosphate, 1 mM sodium orthovanadate, and a protease inhibitor mixture (Roche Applied Science). Lysates were analyzed by Western blot using the following antibodies: the monoclonal antibody for UNC45A (12), a polyclonal antibody against the peptide DKAVEYGLIQPNQDGE at the C-terminus of UNC45A, the antibody H90.10 for Hsp90β, β-actin from Santa Cruz Biotechnology (catalog no. sc-477786), myosin II, PARP (a generous gift from Dr. Scott Kaufmann of Mayo Clinic, MN), ChK1 (Cell Signaling, catalog no. 2345P) against a peptide surrounding serine 296, ChK1 (Santa Cruz, catalog no. sc-8408) against amino acids 1–476, ChK2 (Cell Signaling, catalog no. 2662P), pChK1(S345) (Cell Signaling, catalog no. 2348P), γ-tubulin (Sigma-Aldrich, catalog no. T6557), polyclonal antibody against nonmuscle myosin II heavy chain Isoform A (MHC-A, NMHC IIA, Myosin-9) (BioLegend, Catalog no: PRB-440P) and pericentrin (Abcam, catalog no. ab28144).
2.4 Immunocytochemistry and Fluorescence Microscopy
HeLa cells were grown in 24-well plates on 22-mm diameter to 60% confluency. UNC45A was silenced by transient transfection with UNC45A siRNA or by exposure to doxycycline. Cells were partially fixed in a 3% para-formaldehyde solution for 2–3 minutes, washed twice with PBS, permeabilized with 0.1% Triton-X-100 for 3 min, fixed for 10 minutes at −20°C in methanol, and dried at room temperature. The coverslips were then blocked in PBS containing 10% fetal bovine serum and 5% glycerol overnight at 4°C. Primary and secondary antibodies were prepared in the blocking buffer. Coverslips were mounted using Prolong Gold™ (Life Technologies, catalog no. P-36931). Cells were analyzed using the Zeiss Axio Imager.M1 microscope. Deconvolution of Z-stack images was done using an inverse filter algorithm with autolinear normalization. Counts of multi-nucleated and rosette-shaped cells were done in triplicate on different slides by hand.
2.5 Nuclear Fractionation
Cells stably harboring scrambled or shRNA to UNC45A were grown and treated with 1 μg/mL of doxycycline for 144 hours. Cells were harvested and washed in PBS, spun down at 2000 rcf for 10 minutes at 4°C, and then resuspended in a hypotonic lysis buffer (10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 1 mM, DTT, 10 mM NaF, 2 mM sodium pyrophosphate, 2 mM β-glycerophosphate, 1 mM sodium orthovanadate, and protease inhibitors (Roche)). The cells were incubated on ice for 10 minutes and then snap-frozen in liquid nitrogen and thawed three times. The cell suspension was transferred to a Dounce homogenizer and lysed with 15–25 strokes. The supernatant was recovered as the cytosolic fraction and the pellet as the raw nuclei. The pellet was then washed twice with the hypotonic buffer. The nuclear pellet was then resuspended in a hypertonic buffer (20 mM HEPES, pH 7.9, 1.5 mM MgCl2, 0.5 M NaCl, 25% glycerol, 0.5 mM EDTA, 1 mM DTT, 10 mM NaF, 2 mM sodium pyrophosphate, 2 mM β-glycerophosphate, 1 mM sodium orthovanadate, and protease inhibitors (Roche)) and placed in a rotor/shaker at 4°C for 30 minutes. The suspension was then spun down and the supernatant recovered.
2.6 ChK1 Immunoprecipitation
Cytosolic or nuclear extracts (300 μg protein) from HeLa cells with either scrambled or UNC45A shRNA were incubated with ChK1 antibody (Cell Signaling) overnight 4°C with gentle rotation. The next day, a 30-μL slurry of protein A/protein G agarose beads (Pierce® Recombinant Protein A Agarose, catalog no. 20366 and Pierce® Protein G Agarose, catalog no. 20399) was added to each sample, and the samples were incubated at 4°C with gentle rotation for 2 hours. The samples were then washed three times with 20 mM Tris, pH 7.4, 150 mM KCl, 10 mM 1-thioglycerol, 0.03% NP40, 10% glycerol, protease inhibitors (Roche), 10 mM NaF, 2 mM sodium pyrophosphate, 2 mM β-glycerophosphate, and 1 mM sodium orthovanadate and prepared for Western blot.
3. Results
3.1 UNC45A is essential for cancer cell proliferation in vitro and tumor growth in vivo
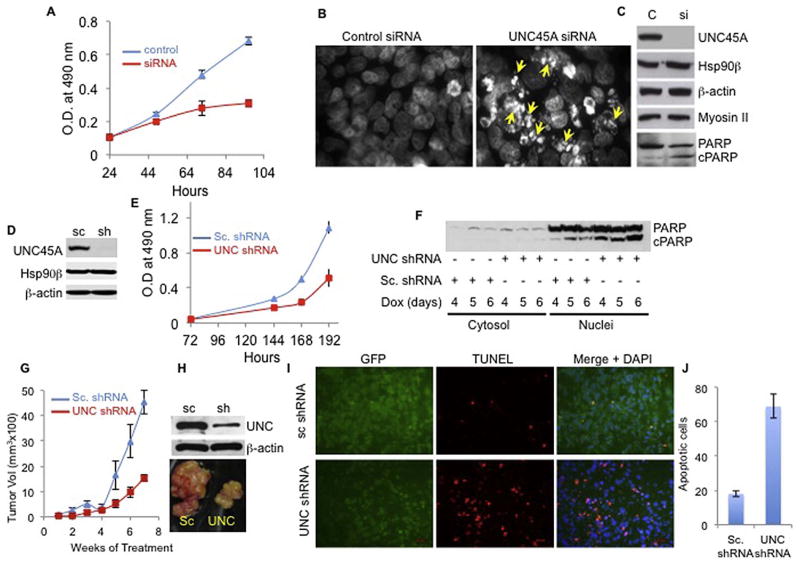
Previous studies showed that UNC45A is overexpressed in human tumors (15, 16) and is essential for cancer cell proliferation and motility in vitro using ovarian and breast cancer cell lines (15, 16). Consistent with this, we found that transiently silencing UNC45A expression with an siRNA blocked HeLa cell proliferation (Fig 1A) and induced molecular characteristics of apoptosis. These include cell membrane blebbing (Fig. S1A), DNA fragmentation (Fig. 1B), and poly(ADP-ribose) polymerase (PARP) cleavage (Fig. 1C). Similarly, we also observed that doxycycline-induced depletion of UNC45A from HeLa cells (Fig. 1D) that stably harbor doxycycline-inducible UNC45A shRNA slowed growth (Fig. 1E) and triggered apoptosis in vitro, as demonstrated by PARP cleavage (Fig. 1F) and TUNEL analysis (Fig. S1B,C).
Figure 1. UNC45A is essential for cancer cell proliferation in vitro and for tumor growth in vivo.
(A) MTT assay assessing cell growth of HeLa cells transfected with 100 nM of siRNA against UNC45A (red) or non-targeting siRNA control (blue). (B) Hoechst staining of DNA from HeLa cells treated with non-targeting siRNA control (control siRNA) or UNC45A siRNA. Yellow arrows indicate fragmented DNA. (C) Total cell lysates were made from cells in (B) and Western blotted for UNC45A, Hsp90β, β-actin, Myosin II, full length PARP (PARP), and cleaved PARP (cPARP). (D) Western blot analysis of cytosol from HeLa cells stably harboring shRNA against UNC45A (sh) or non-targeting scramble shRNA (sc) cultured in MEM media containing 1 μg/ml doxycycline. (E) MTT assay assessing growth of HeLa cells stably harboring shRNA against UNC45A (red) or non-targeting scramble shRNA (blue) cultured in MEM media containing 1 μg/ml doxycycline. Error bars in A and E represents the standard deviation of mean of six wells. All experiments are repeated at least three times. (F) Total cell lysates of HeLa cells cultured in doxycycline for indicated time were used to assess full-length PARP (PARP) and cleaved PARP (cPARP) levels in cytoplasmic and nuclear fractions. (G) HeLa cells (1 × 106) harboring doxycycline-inducible shRNA against UNC45A (red) or non-targeting shRNA control (blue) were injected subcutaneously into nude mice. Three weeks after injection, tumors grew to about 100 mm3. Mice, six for each group, were then fed doxycycline in drinking water and injected intraperitoneally; tumor volume was monitored using calipers. When tumors in the control group reached about 4.5 cm3, animals were sacrificed and tumors were extracted for final photograph (H, bottom panel). Parts of the tumors were used for Western blot (H) and for analysis by TUNEL (I and J). Average number of apoptotic cells found in three microscopic fields comparing the TUNEL positivity in scrambled shRNA and UNC45A shRNA tumors. All animals received doxycycline for 7 weeks. Data are presented as mean ± SD. Statistical significance was assessed using Student’s t-test considering P<0.05 as an indicator of significance.
Because previous studies suggested that UNC45A played a role in cell proliferation through co-localization with myosin II at the cleavage furrow and regulation of cytokinesis (15), we also assessed myosin II levels in the UNC45A-depleted cells. As shown in Figure 1C, robust UNC45A depletion did not affect myosin II levels, thus indicating that UNC45A affects proliferation independently of myosin II depletion in these cells.
Although these in vitro findings indicated a role for UNC45A in proliferation, the relevance of UNC45A for tumor growth in vivo has not been directly tested. To address this role, athymic Nu/Nu mice were injected subcutaneously with 1 × 106 HeLa cells harboring UNC45A shRNA or the control scramble shRNA. After tumors grew to about 100 mm3, animals were administered doxycycline via daily intraperitoneal injections, and tumor growth was monitored using calipers. As shown in Figure 1G and H, silencing UNC45A drastically reduced tumor growth in mice. TUNEL analysis showed that tumors lacking UNC45A underwent extensive apoptotic cell death (Fig. 1I,J). Taken together, these findings demonstrate that UNC45A plays a critical role in cell proliferation and that lack of UNC45A triggers apoptosis of HeLa cells in vitro and in vivo.
3.2 UNC45A is a centrosomal protein
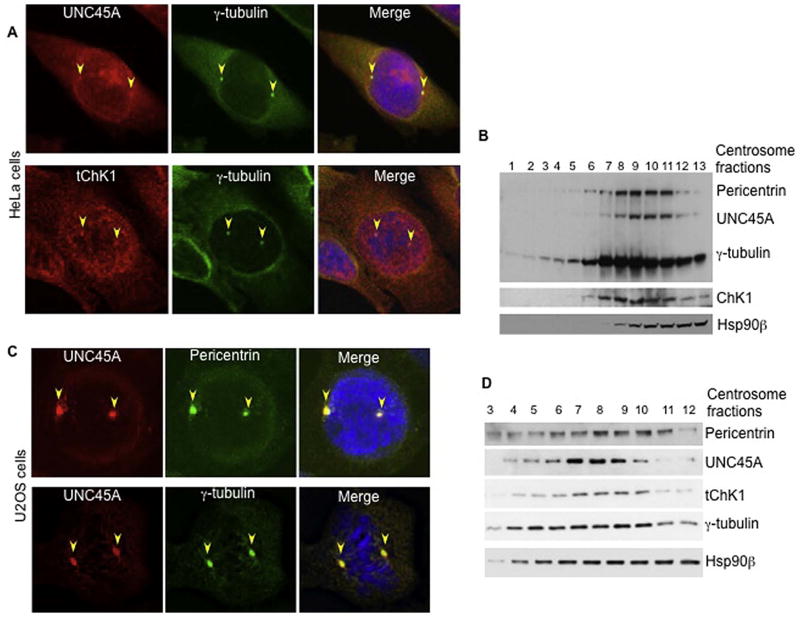
To gain insight into how UNC45A regulates cell proliferation, we performed immunocytochemical staining using a polyclonal antibody recognizing the C-terminus of UNC45A (Fig. 2A, upper panel). These studies revealed that UNC45A was widely distributed throughout HeLa cells, along with focal localization in structures that resembled centrosomes (Fig. 2A). Indeed, co-staining with the centrosomal marker γ-tubulin revealed that UNC45A and γ-tubulin co-localized (Fig. 2A and S2). The antibody we have used was raised against the extreme C-terminus of UNC45A (DKAVEYGLIQPNQDGE), which has has little identity with UNC45B (IKCMDYGFIKPVS). More importantly, this antibody recognizes a single, 103-kDa band corresponding to the molecular weight of UNC45A. Furthermore, as shown in Figures 1C,D, 3C, 4B and Fig. S7, the siRNA against UNC45A causes near-complete depletion of this unique band, thus demonstrating that this antibody specifically recognizes only UNC45A. UNC45A centrosomal localization was further confirmed in U2OS cells where UNC45A perfectly co-localized with pericentrin and γ-tubulin (Fig. 2C and S3 upper panels). Consistent with these immunofluorescence data, we also observed that UNC45A co-purified with centrosomes on sucrose gradients from HeLa (Fig. 2B) and U2OS (Fig. 2D) cells. Similarly, Hsp90 beta also co-purified with the centrosome (Fig 2C and D), thus confirming a previous report indicating a key role for this co-chaperone in centrosomal functions (21, 22). Interestingly, Myosin II does not localize to the centrosome (Fig. S7) suggesting that UNC45A function in the centrosome is independent of myosin II.
Figure 2.
UNC45A and ChK1 co-localize to centrosomes in HeLa and U2OS cell lines. Asynchronous HeLa (A) or U2OS (C) cells were stained with DAPI and the polyclonal antibody (red) to UNC45A. Centrosomes are visualized with monoclonal antibody against centrosomal proteins γ-tubulin (green) and pericentrin (green). Shown are representative images (more images are shown in supplementary data). Yellow arrowheads indicate centrosomes. (B) and (D) Western blot analysis of centrosomal fractions prepared from HeLa (B) and U2OS (D) cells as described in Material and Methods. UNC45A, ChK1, and Hsp90β were co-isolated with centrosomes from both cell lines. γ-tubulin and pericentrin were used as centrosome markers.
Figure 3.

(A) UNC45A, Hsp90β, and ChK1 are in complex in HeLa cells. Cytoplasmic and nuclear fractions of HeLa cells were immunoprecipitated using polyclonal total ChK1 (tChK1) antibody. Rabbit immunoglobulins (RIgG) were used as control. Immunoprecipitated proteins were Western blotted for total ChK1 (tChK1), UNC45A, and Hsp90β. (B) HeLa cells were treated with DMSO control or 1μM 17-AAG for 24h. Whole cell lysate were analyzed for UNC45A, total ChK1 (tChk1) and Hsp70. GAPDH was used as loading control. Overexpression of Hsp70 indicates inactivation of Hsp90. (C) Silencing UNC45A causes overexpression of ChK1 and its localization in the nucleus. HeLa cells expressing shRNA to UNC45A or control were treated with 1 μM doxycycline for 120–144 h. Cytoplasmic and nuclear fractions (15 μg protein/samples) were analyzed by Western blot using indicated antibodies. The histone H2A-X was used as a nuclear marker. As expected, Hsp90β is mostly cytoplasmic with a small fraction localized to the nucleus. The experiments were repeated at least three times.
Figure 4.
UNC45 is important for ChK1 localization to centrosomes. HeLa cells were transfected with non-targeting siRNA control (control siRNA) or with siRNA to UNC45A (UNC siRNA) for 72h. Cells were fixed, and immunocytochemistry was performed using polyclonal antibodies to total ChK1 (tChK1) and with a monoclonal antibody to γ-tubulin (green). DAPI was used to stain DNA. (B) Cells surrounding the coverslips in A were Western blotted for UNC45A. β-actin was used as a loading control. (C) Multinucleated and rosette-shaped cells were counted in non-targeting control siRNA (control) and UNC45A siRNA (UNC KD) samples. Statistical significance versus control by 2-tailed t-test are p = 0.000234 for multinucleated and p = 0.02811465 for the rosette-shaped nuclei. (D) In Western blot analysis of centrosomal fractions from HeLa cells stably harboring doxycycline-inducible scrambled shRNA control (sc shRNA) or shRNA to UNC45A (UNC shRNA), membranes were immunoblotted using antibodies against UNC45A, ChK1, and Hsp90β. β-tubulin and pericentrin were used as centrosomal markers. All experiments were repeated at least three times.
3.3 UNC45A co-purifies and interacts with ChK1
Previous studies showed that Chk1 associates with centrosomes and prevents premature centrosome duplication during interphase in unperturbed cells. Consistent with these reports, we observed that ChK1 localized to centrosomes in HeLa and U2OS cells using immunocytochemistry with multiple antibodies (Fig. 2A, lower panels, Fig. S3, S4 and S5). We also found that ChK1 co-purified with UNC45A in centrosomes on sucrose gradients in HeLa (Fig. 2B) and U2OS (Fig 2D) cells, and we observed that Chk1 co-immunoprecipitated with UNC45A from HeLa cell cytosols (Fig. 3A, lane 5).
3.4 UNC45A does not participate in Hsp90-mediated chaperoning of Chk1
Given that 1) ChK1 co-precipitated with UNC45A (Fig. 3A), 2) ChK1 is an Hsp90 client (23), and 3) UNC45A is an Hsp90 co-chaperone for some clients (12), we next asked whether UNC45A participated in ChK1 chaperoning. Consistent with previous reports, the Hsp90 inhibitor 17-AAG caused ChK1 loss (Fig. 3B). Surprisingly, however, depletion of UNC45A did not reduce ChK1 levels (Fig. 3C). Instead, we observed increased total ChK1 levels in the cytoplasmic fraction and a more pronounced ChK1 up-regulation in the nuclear fraction of UNC45A knockdown cells (Fig. 3C). No significant and reproducible changes in the expression or cellular localization of ChK2 were observed (Fig. 3C), indicating that UNC45A regulation is less global. Taken together, these findings show that UNC45A does not participate in ChK1 chaperoning by the Hsp90 machine.
3.5 UNC45A regulates ChK1 levels and phosphorylation
Increased accumulation of ChK1 in the nucleus has been linked to reduced ChK1 phosphorylation on multiple residues including S345 (24–28). Thus, we hypothesized that UNC45A depletion might affect S345 ChK1 phosphorylation. Western blot analysis showed that ChK1 phosphorylation at S345 was dramatically reduced in the UNC45A knockdown cells, even though the total ChK1 protein level has increased (Fig. 3C). Taken together, our findings suggest that UNC45A is essential for ChK1 phosphorylation on S345 in unperturbed cells.
3.6 UNC45A is essential for ChK1 centrosomal localization and function
Given that 1) ChK1 localized to centrosomes (Figs. 1 and 2), and 2) centrosomal ChK1 was phosphorylated on S345 (Fig. S3 and S4), and 3) UNC45A depletion reduced Chk1 S345 (Fig. 3C), an event that regulates centrosomes, we tested the hypothesis that UNC45A might be important for ChK1 localization to centrosomes. Depletion of UNC45A (Fig. 4B) reduced ChK1 centrosomal localization (Fig. 4A); indeed, it was difficult to find cells with any significant co-localization in the knockdown samples. These findings were confirmed by biochemical analysis comparing the centrosomal fractions purified from control and UN45A-depleted HeLa cells (Fig. 4D). Although comparable amounts of proteins were loaded, silencing UNC45A decreased ChK1 and γ-tubulin levels in the centrosomal fractions (Fig. 4D). However, levels of Hsp90 and pericentrin in centrosomal fractions were overall very similar in knockdown cells compared to controls.
Because UNC45A depletion disrupted Chk1 centrosomal localization, and because centrosomal Chk1 regulates the activity of this organelle, we reasoned that depleting UNC45A would disrupt centrosome function. Consistent with this hypothesis, depleting UNC45A caused a large fraction of cells to divide abnormally and become multinucleated with rosette-shaped nuclei, indicating dysfunctional centrosomes (Fig. 4A, C). Taken together, these results suggest that UNC45A regulates centrosome function by recruiting Chk1 to centrosomes.
4. Discussion
In this report we confirm previous findings showing that UNC45A is essential for cancer cell proliferation in vitro. We also established for the first time that UNC45A is essential for tumor growth in vivo and that lack of UNC45A triggers apoptosis in vitro and in vivo. Furthermore, our molecular studies demonstrate UNC45A is a centrosomal protein that is required for ChK1 localization to centrosomes through regulation of ChK1 activation. Because initial ChK1 centrosomal localization (20) was questioned in a recent report (29), we verified that ChK1 localizes to centrosomes using several mono- and polyclonal antibodies with various epitopes (Fig 2B and Fig. S3, S4 and S5), extending the list of antibodies used by others for this purpose (19, 30–32). Furthermore, methods independent of immunofluorescence, including stringent biochemical purifications, have also demonstrated that ChK1 co-purifies with centrosomes in various cell lines (Fig. 2C,D) (33, 34). Taken together, these multiple studies firmly establish that Chk1 localizes to centrosomes.
Pharmacological inhibition of Hsp90β reduces the level of total ChK1 through proteasomal degradation (Fig 3B) (23, 35, 36). However, the stability of Chk1 is independent of the co-chaperone UNC45A, thus demonstrating that UNC45A plays a different role in regulating Chk1 at the centrosome. Importantly, activation of Chk1 requires UNC45A, because knockdown of UNC45A abolishes key phosphorylations of ChK1(37), without reducing Chk1 level. This indicates that UNC45A regulation of ChK1 phosphorylation is independent of the Hsp90 chaperoning activity required for ChK1 cellular stability. As our immunofluorescence studies show, only a small portion of UNC45A is localized to centrosomes. This is consistent with UNC45A having other centrosome-independent functions in the cell including regulation of steroid receptor signaling (12) and myosin functions (8–10, 14).
Taken together our results demonstrate that UNC45A is a new novel centrosomal protein essential for ChK1 phosphorylation and centrosomal localization. This explains, at least in part, the essential role of UNC45A in cancer cell proliferation, and further establishes the key role of UNC45A in tumorigenesis.
Supplementary Material
Acknowledgments
This work was supported by NIH R01 grant GM102443-01 for Ahmed Chadli.
We thank Dr. Sara Felts for the critical reading of the manuscript and Dr. Lisa Middleton for the editing of the manuscript.
Footnotes
The authors declare no conflict of Interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hutagalung AH, Landsverk ML, Price MG, Epstein HF. The UCS family of myosin chaperones. J Cell Sci. 2002;115:3983–90. doi: 10.1242/jcs.00107. [DOI] [PubMed] [Google Scholar]
- 2.Hoppe T, Cassata G, Barral JM, Springer W, Hutagalung AH, Epstein HF, et al. Regulation of the myosin-directed chaperone UNC-45 by a novel E3/E4-multiubiquitylation complex in C. elegans. Cell. 2004;118:337–49. doi: 10.1016/j.cell.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 3.Epstein HF, Benian GM. Paradigm shifts in cardiovascular research from Caenorhabditis elegans muscle. Trends in cardiovascular medicine. 2012;22:201–9. doi: 10.1016/j.tcm.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 4.Barral JM, Bauer CC, Ortiz I, Epstein HF. Unc-45 mutations in Caenorhabditis elegans implicate a CRO1/She4p-like domain in myosin assembly. Journal of Cell Biology. 1998;143:1215–25. doi: 10.1083/jcb.143.5.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landsverk ML, Li S, Hutagalung AH, Najafov A, Hoppe T, Barral JM, et al. The UNC-45 chaperone mediates sarcomere assembly through myosin degradation in Caenorhabditis elegans. J Cell Biol. 2007;177:205–10. doi: 10.1083/jcb.200607084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernick EP, Zhang PJ, Du S. Knockdown and overexpression of Unc-45b result in defective myofibril organization in skeletal muscles of zebrafish embryos. BMC Cell Biol. 2010;11:70. doi: 10.1186/1471-2121-11-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wohlgemuth SL, Crawford BD, Pilgrim DB. The myosin co-chaperone UNC-45 is required for skeletal and cardiac muscle function in zebrafish. Dev Biol. 2007;303:483–92. doi: 10.1016/j.ydbio.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 8.Comyn SA, Pilgrim D. Lack of developmental redundancy between Unc45 proteins in zebrafish muscle development. PLoS One. 2012;7:e48861. doi: 10.1371/journal.pone.0048861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Price MG, Landsverk ML, Barral JM, Epstein HF. Two mammalian UNC-45 isoforms are related to distinct cytoskeletal and muscle-specific functions. Journal of Cell Science. 2002;115:4013–23. doi: 10.1242/jcs.00108. [DOI] [PubMed] [Google Scholar]
- 10.Anderson MJ, Pham VN, Vogel AM, Weinstein BM, Roman BL. Loss of unc45a precipitates arteriovenous shunting in the aortic arches. Dev Biol. 2008;318:258–67. doi: 10.1016/j.ydbio.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barral JM, Hutagalung AH, Brinker A, Hartl FU, Epstein HF. Role of the myosin assembly protein UNC-45 as a molecular chaperone for myosin. Science. 2002;295:669–71. doi: 10.1126/science.1066648. [DOI] [PubMed] [Google Scholar]
- 12.Chadli A, Graham JD, Abel MG, Jackson TA, Gordon DF, Wood WM, et al. GCUNC-45 is a novel regulator for the progesterone receptor/hsp90 chaperoning pathway. Molecular & Cellular Biology. 2006;26:1722–30. doi: 10.1128/MCB.26.5.1722-1730.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ni W, Hutagalung AH, Li S, Epstein HF. The myosin-binding UCS domain but not the Hsp90-binding TPR domain of the UNC-45 chaperone is essential for function in Caenorhabditis elegans. J Cell Sci. 2011;124:3164–73. doi: 10.1242/jcs.087320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu L, Srikakulam R, Winkelmann DA. Unc45 activates Hsp90-dependent folding of the myosin motor domain. J Biol Chem. 2008;283:13185–93. doi: 10.1074/jbc.M800757200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bazzaro M, Santillan A, Lin Z, Tang T, Lee MK, Bristow RE, et al. Myosin II co-chaperone general cell UNC-45 overexpression is associated with ovarian cancer, rapid proliferation, and motility. Am J Pathol. 2007;171:1640–9. doi: 10.2353/ajpath.2007.070325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo W, Chen D, Fan Z, Epstein HF. Differential turnover of myosin chaperone UNC-45A isoforms increases in metastatic human breast cancer. J Mol Biol. 2011;412:365–78. doi: 10.1016/j.jmb.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Hutagalung AH, Landsverk ML, Price MG, Epstein HF. The UCS family of myosin chaperones. Journal of Cell Science. 2002;115:3983–90. doi: 10.1242/jcs.00107. [DOI] [PubMed] [Google Scholar]
- 18.Zachos G, Gillespie DA. Exercising restraints: role of Chk1 in regulating the onset and progression of unperturbed mitosis in vertebrate cells. Cell Cycle. 2007;6:810–3. doi: 10.4161/cc.6.7.4048. [DOI] [PubMed] [Google Scholar]
- 19.Wilsker D, Petermann E, Helleday T, Bunz F. Essential function of Chk1 can be uncoupled from DNA damage checkpoint and replication control. Proc Natl Acad Sci U S A. 2008;105:20752–7. doi: 10.1073/pnas.0806917106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kramer A, Mailand N, Lukas C, Syljuasen RG, Wilkinson CJ, Nigg EA, et al. Centrosome-associated Chk1 prevents premature activation of cyclin-B-Cdk1 kinase. Nat Cell Biol. 2004;6:884–91. doi: 10.1038/ncb1165. [DOI] [PubMed] [Google Scholar]
- 21.Lange BM, Bachi A, Wilm M, Gonzalez C. Hsp90 is a core centrosomal component and is required at different stages of the centrosome cycle in Drosophila and vertebrates. EMBO Journal. 2000;19:1252–62. doi: 10.1093/emboj/19.6.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basto R, Gergely F, Draviam VM, Ohkura H, Liley K, Raff JW. Hsp90 is required to localise cyclin B and Msps/ch-TOG to the mitotic spindle in Drosophila and humans. J Cell Sci. 2007;120:1278–87. doi: 10.1242/jcs.000604. [DOI] [PubMed] [Google Scholar]
- 23.Arlander SJ, Eapen AK, Vroman BT, McDonald RJ, Toft DO, Karnitz LM. Hsp90 inhibition depletes Chk1 and sensitizes tumor cells to replication stress. Journal of Biological Chemistry. 2003;278:52572–7. doi: 10.1074/jbc.M309054200. [DOI] [PubMed] [Google Scholar]
- 24.Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K, et al. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 2000;14:1448–59. [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao H, Piwnica-Worms H. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol Cell Biol. 2001;21:4129–39. doi: 10.1128/MCB.21.13.4129-4139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo Z, Kumagai A, Wang SX, Dunphy WG. Requirement for Atr in phosphorylation of Chk1 and cell cycle regulation in response to DNA replication blocks and UV-damaged DNA in Xenopus egg extracts. Genes Dev. 2000;14:2745–56. doi: 10.1101/gad.842500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smits VA. Spreading the signal: dissociation of Chk1 from chromatin. Cell Cycle. 2006;5:1039–43. doi: 10.4161/cc.5.10.2761. [DOI] [PubMed] [Google Scholar]
- 28.Loffler H, Lukas J, Bartek J, Kramer A. Structure meets function--centrosomes, genome maintenance and the DNA damage response. Exp Cell Res. 2006;312:2633–40. doi: 10.1016/j.yexcr.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Matsuyama M, Goto H, Kasahara K, Kawakami Y, Nakanishi M, Kiyono T, et al. Nuclear Chk1 prevents premature mitotic entry. J Cell Sci. 2011;124:2113–9. doi: 10.1242/jcs.086488. [DOI] [PubMed] [Google Scholar]
- 30.Kim SH, Park ER, Joo HY, Shen YN, Hong SH, Kim CH, et al. RRM1 maintains centrosomal integrity via CHK1 and CDK1 signaling during replication stress. Cancer Lett. 2014;346:249–56. doi: 10.1016/j.canlet.2013.12.031. [DOI] [PubMed] [Google Scholar]
- 31.Niida H, Katsuno Y, Banerjee B, Hande MP, Nakanishi M. Specific role of Chk1 phosphorylations in cell survival and checkpoint activation. Mol Cell Biol. 2007;27:2572–81. doi: 10.1128/MCB.01611-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gruber R, Zhou Z, Sukchev M, Joerss T, Frappart PO, Wang ZQ. MCPH1 regulates the neuroprogenitor division mode by coupling the centrosomal cycle with mitotic entry through the Chk1-Cdc25 pathway. Nat Cell Biol. 2011;13:1325–34. doi: 10.1038/ncb2342. [DOI] [PubMed] [Google Scholar]
- 33.Tibelius A, Marhold J, Zentgraf H, Heilig CE, Neitzel H, Ducommun B, et al. Microcephalin and pericentrin regulate mitotic entry via centrosome-associated Chk1. J Cell Biol. 2009;185:1149–57. doi: 10.1083/jcb.200810159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sorino C, Bruno T, Desantis A, Di Certo MG, Iezzi S, De Nicola F, et al. Centrosomal Che-1 protein is involved in the regulation of mitosis and DNA damage response by mediating pericentrin (PCNT)-dependent Chk1 protein localization. J Biol Chem. 2013;288:23348–57. doi: 10.1074/jbc.M113.465302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ha K, Fiskus W, Rao R, Balusu R, Venkannagari S, Nalabothula NR, et al. Hsp90 inhibitor-mediated disruption of chaperone association of ATR with hsp90 sensitizes cancer cells to DNA damage. Mol Cancer Ther. 2011;10:1194–206. doi: 10.1158/1535-7163.MCT-11-0094. [DOI] [PubMed] [Google Scholar]
- 36.Arlander SJH, Felts SJ, Wagner JM, Stensgard B, Toft DO, Karnitz LM. Chaperoning checkpoint kinase 1 (Chk1), an Hsp90 client, with purified chaperones. Journal of Biological Chemistry. 2006;281:2989–98. doi: 10.1074/jbc.M508687200. [DOI] [PubMed] [Google Scholar]
- 37.Liu S, Shiotani B, Lahiri M, Marechal A, Tse A, Leung CC, et al. ATR autophosphorylation as a molecular switch for checkpoint activation. Mol Cell. 2011;43:192–202. doi: 10.1016/j.molcel.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.