Abstract
BACKGROUND
Posttraumatic stress disorder (PTSD) is associated with endocrine and immune abnormalities that could increase risk for autoimmune disorders. However, little is known about the risk for autoimmune disorders among individuals with PTSD.
METHODS
We conducted a retrospective cohort study of 666,269 Iraq and Afghanistan veterans under age 55 who were enrolled in the Department of Veterans Affairs (VA) healthcare system between October 7, 2001 and March 31, 2011. Generalized linear models were used to examine if PTSD, other psychiatric disorders, and military sexual trauma exposure (MST) increase risk for autoimmune disorders, including thyroiditis, inflammatory bowel disease, rheumatoid arthritis, multiple sclerosis, and lupus erythematosus, adjusting for age, gender, race, and primary care visits.
RESULTS
PTSD was diagnosed in 203,766 (30.6%) veterans, and psychiatric disorders other than PTSD were diagnosed in an additional 129,704 (19.5%) veterans. Veterans diagnosed with PTSD had significantly higher adjusted relative risk (ARR) for diagnosis with any of the autoimmune disorders alone or in combination compared to veterans with no psychiatric diagnoses (ARR = 2.00, 95% CI, 1.91, 2.09), and compared to veterans diagnosed with psychiatric disorders other than PTSD (ARR = 1.51, 95% CI, 1.43, 1.59, p < .001). The magnitude of the PTSD-related increase in risk for autoimmune disorders was similar in women and men, and MST was independently associated with increased risk in both women and men.
CONCLUSIONS
Trauma exposure and PTSD may increase risk for autoimmune disorders. Altered immune function, lifestyle factors, or shared etiology may underlie this association.
Keywords: autoimmune disorders, glucocorticoids, immune system, inflammation, military sexual trauma, posttraumatic stress disorder, traumatic stress, veterans
INTRODUCTION
Posttraumatic stress disorder (PTSD) is associated with a number of biological abnormalities that could increase risk for autoimmune disorders. First, PTSD appears characterized by lower levels of the immunomodulatory glucocorticoid hormone cortisol and reduced signaling through anti-inflammatory glucocorticoid receptor transcriptional control pathways (1–5). Second, accumulating evidence links PTSD with increased inflammatory activity, as indexed by elevated levels of pro-inflammatory cytokines and higher signaling through pro-inflammatory nuclear factor-κB (NF-κB) transcriptional control pathways (4–7). Third, investigators have observed altered patterns of gene expression in immune cells (8, 9), and reduced methylation of immune-related genes (10) in patients with PTSD. Finally, emerging evidence suggests that PTSD is associated with accelerated immune cell aging as indexed by shorter age-adjusted telomere length (11, 12), which has been linked with elevated inflammation in vivo and in vitro (13, 14). This pattern of abnormalities in the HPA axis, immune system, and telomere maintenance system may increase risk for autoimmune disorders by increasing inflammation and impairing the function of immune cells (15–18). Nonetheless, relatively little is known about the risk for autoimmune disorders associated with PTSD.
In one previous study, PTSD was associated with higher prevalence of self-reported autoimmune disorders in a sample of 2,490 male Vietnam veterans (19). In another, PTSD was associated with increased risk for physician-diagnosed rheumatoid arthritis in a sample of 3,143 pairs of male twins (20). However, no prior study has examined if PTSD increases risk for a range of physician-diagnosed autoimmune disorders with definitive diagnostic criteria, and it is not known if the risk for autoimmune disorders is greater in individuals with PTSD compared to those with other psychiatric disorders. Moreover, although the risk for, or severity of, many autoimmune disorders is substantially higher in women compared with men (21–26), no studies have examined the risk for autoimmune disorders in women with PTSD.
To assess the risk for autoimmune disorders associated with PTSD and other psychiatric disorders, we conducted the present study in a national sample of Iraq and Afghanistan veterans enrolled in the Veterans Affairs (VA) healthcare system. Emerging data indicate high rates of PTSD and other psychiatric disorders (27, 28) as well as high rates of military sexual trauma exposure (MST) (29) in this population of veterans. In the present study, we assessed risk for autoimmune disorders associated with PTSD, other psychiatric disorders, and MST, focusing our analyses on the most prevalent autoimmune disorders in the United States that have definitive diagnostic criteria or diagnostic tests (i.e., thyroiditis, rheumatoid arthritis, inflammatory bowel disorders, multiple sclerosis, and lupus erythematosus) (30).
METHODS
STUDY POPULATION
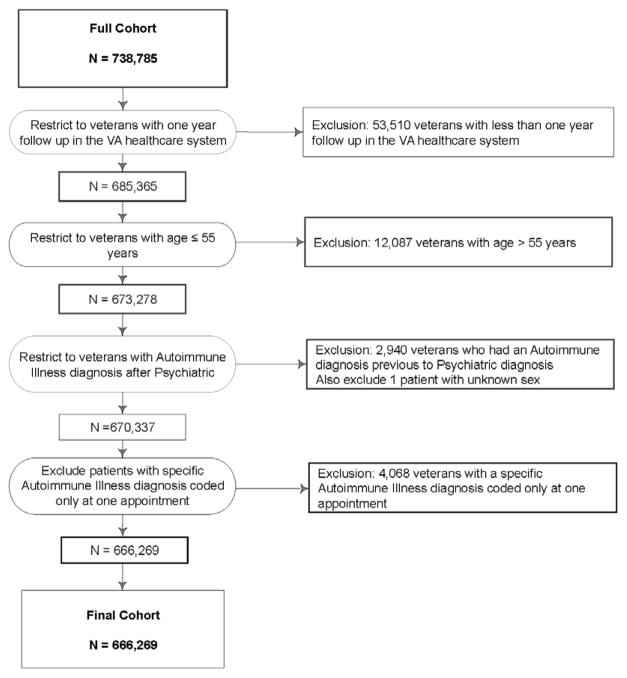
The Department of Veterans Affairs (VA) national Operation Enduring Freedom (OEF), Operation Iraqi Freedom (OIF) and Operation New Dawn (OND) Roster includes veterans deployed in OEF/OIF/OND who have separated from service and enrolled in the VA healthcare system. We identified 738,785 male and female Iraq and Afghanistan veterans in the OEF/OIF/OND Roster who first received VA healthcare from October 7, 2001 to March 31, 2011. We excluded veterans without at least one year of follow up within the VA and the study end date was therefore March 31, 2012. Veterans aged over 55 years (1.6%) were excluded from our analyses because our goal was to assess the risk for autoimmune disorders in a more homogenous group of veterans without confounds associated with older age. Veterans who remain in the military later in life – making them older than 55 during their first VA appointment following service in OEF/OIF/OND - may also differ from the general population of veterans because military service personnel are usually eligible for retirement after twenty years of service. Veterans who already had a diagnosis of one of the target autoimmune disorders before receiving a psychiatric diagnosis were excluded to avoid any confounding of psychiatric diagnoses with autoimmune disorder-related symptoms or distress. Finally, to exclude potential inaccurate or “rule-out” diagnoses, we excluded veterans who had received an autoimmune disorder diagnosis at only one appointment. See Figure 1 for a more complete description of exclusions. After exclusions, our study population included 666,269 veterans. The study was approved by the Committees on Human Research at the University of California, San Francisco, and the San Francisco VA Medical Center.
Figure 1.
Flow chart detailing exclusion criteria applied to identify study population
DATA SOURCES
We used the VA OEF/OIF/OND Roster to obtain basic demographic and military service information for Iraq and Afghanistan veterans (31), and the VA’s electronic medical record database, the National Patient Care Database (NPCD), to obtain information on VA clinical visits and clinical diagnoses based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes.
SOCIODEMOGRAPHIC AND CLINICAL INFORMATION
The OEF/OIF/OND Roster was used to identify socio-demographic information including age, gender and race as well as military service information including military rank, component type, service branch, and multiple deployments. The VA NPCD was used to obtain clinical information including clinical diagnoses based on ICD-9-CM and number of primary care visits. We also used the VA NPCD to assess for the presence of MST-related clinical encounters and we used the presence of these encounters as an index of MST. Basic sociodemographic, military service and clinical information for our full sample is provided in Table 1, and stratified by gender in Supplementary Table S1.
Table 1.
Sample characteristics
| Characteristic | Total N 666,269 |
No Psychiatric Disorder N (%) 332,799 (49.9) |
Other Psychiatric Disorders N (%) 129,704 (19.5) |
PTSD N (%) 203,766 (30.6) |
P value |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 79,356 | 40,141 (12.1) | 19,310 (14.9) | 19,905 (9.8) | <.001 |
| Male | 589,913 | 292,658 (87.9) | 110,394 (85.1) | 183,861 (90.2) | <.001 |
| Age: M (SD)a | 31.2 (8.7) | 32.2 (9.0) | 30.8 (8.5) | 30.0 (8.0) | <.001 |
| Age Groupa | |||||
| 18–24 | 186,736 | 84,076 (25.3) | 37,847 (29.2) | 64,813 (31.8) | <.001 |
| 25–34 | 263,743 | 124,286 (37.3) | 53,353 (41.1) | 86,104 (42.3) | <.001 |
| 35–44 | 149,104 | 84,056 (25.3) | 26,502 (20.4) | 38,546 (18.9) | <.001 |
| 45–54 | 66,686 | 40,381 (12.1) | 12,002 (9.3) | 14,303 (7.0) | <.001 |
| Race/Ethnicity | |||||
| White | 326,101 | 158,349 (47.6) | 64,941 (50.1) | 102,811 (50.5) | <.001 |
| Non-White | 340,168 | 174,450 (52.4) | 64,763 (49.9) | 100,955 (49.5) | <.001 |
| Marital Status | |||||
| Married | 293,775 | 153,482 (46.1) | 52,881 (40.8) | 87,412 (42.9) | <.001 |
| Never Married | 341,868 | 164,598 (49.5) | 70,483 (54.4) | 106,787 (52.4) | <.001 |
| Divorced/Widowed/Other | 30,211 | 14,494 (4.4) | 6,249 (4.8) | 9,468 (4.6) | <.001 |
| Military Rank | |||||
| Enlisted | 610,646 | 292,609 (87.9) | 122,056 (94.1) | 195,981 (96.2) | <.001 |
| Other | 55,623 | 40,190 (12.1) | 7,648 (5.9) | 7,785 (3.8) | <.001 |
| Active Duty or Reserve | |||||
| Active Duty | 371,342 | 184,043 (55.3) | 70,365 (54.3) | 116,934 (57.4) | <.001 |
| Reserve/National Guard | 294,927 | 148,756 (44.7) | 59,339 (45.7) | 86,832 (42.6) | <.001 |
| Military Branch | |||||
| Army | 407,870 | 182,187 (54.7) | 78,166 (60.3) | 147,517 (72.4) | <.001 |
| Air Force | 79,184 | 55,187 (16.6) | 14,489 (11.2) | 9,508 (4.7) | <.001 |
| Marines | 91,928 | 41,746 (12.5) | 15,981 (12.3) | 34,201 (16.8) | <.001 |
| Navy | 87,287 | 53,679 (16.1) | 21,068 (16.2) | 12,540 (6.2) | <.001 |
| Multiple Deployments | 265,833 | 132,555 (39.9) | 48,242 (37.2) | 85,036 (41.8) | <.001 |
| Urban | 370,146 | 180,437 (62.0) | 75,026 (62.8) | 114,683 (60.0) | <.001 |
| Primary Care Visits: M (SD)b | 1.6 (2.0) | 1.1 (1.4) | 2.0 (2.2) | 2.3 (2.4) | <.001 |
| Mental Health Visits: M (SD)b | 2.5 (9.4) | 0.1 (0.6) | 2.4 (7.7) | 6.7 (15.0) | <.001 |
| Military Sexual Traumac | 13,650 | 1,548 (0.5) | 3,187 (2.5) | 8,915 (4.4) | <.001 |
| Psychiatric Diagnoses | |||||
| PTSD | 203,766 | 203,766 (100.0) | <.001 | ||
| Depression | 184,109 | 57,313 (44.2) | 126,796 (62.2) | <.001 | |
| Anxiety Disorder | 123,819 | 44,632 (34.4) | 79,187 (38.9) | <.001 | |
| Adjustment Disorder | 110,626 | 47,516 (36.6) | 63,110 (31.0) | <.001 | |
| Psychosis | 9,570 | 2,377 (1.8) | 7,193 (3.5) | <.001 | |
| Alcohol Use Disorder | 86,082 | 25,913 (20.0) | 60,169 (29.5) | <.001 | |
| Substance Use Disorder | 41,370 | 10,382 (8.0) | 30,988 (15.2) | <.001 | |
| Comorbid psychiatric dxs: M (SD)d | 1.7 (1.2) | 1.5 (1.0) | 1.8 (1.3) | <.001 | |
Notes: dxs = disorders; M = mean; N = number of participants; SD = standard deviation;
Age refers to age at initial VA appointment
Visits refer to mean annual number of primary care or mental health visits
Military sexual trauma exposure is based on VA clinical encounters coded for military sexual trauma
Refers to number of psychiatric diagnoses excluding PTSD. Total column reflects 333,470 patients with one or more psychiatric diagnosis.
PSYCHIATRIC DISORDERS
Based on psychiatric diagnoses received within the VA system, we classified patients into three groups: 1) veterans with PTSD alone or combined with other psychiatric disorders; 2) veterans with psychiatric disorders other than PTSD; and 3) veterans with no psychiatric disorders. Psychiatric diagnoses were identified by ICD-9-CM codes from the VA NPCD database and the codes used were as described previously (31).
AUTOIMMUNE DISORDERS
We identified the most prevalent autoimmune disorders that have definitive diagnostic criteria and/or diagnostic tests based on epidemiologic data and clinical diagnostic criteria (30). These disorders included thyroiditis, rheumatoid arthritis, inflammatory bowel disorders, multiple sclerosis, and lupus erythematosus. The VA NPCD was then used to obtain information on diagnoses of these autoimmune disorders in our population, based on ICD-9-CM codes (see Supplementary Table S2).
COVARIATES
The VA OEF/OIF/OND Roster was used to ascertain age, gender and race, and the NPCD was used to ascertain number of primary care visits. Due to the frequent misclassification of race/ethnicity in administrative data (33, 34), we adjusted only for White versus Non-White in our models. Because greater healthcare utilization in patients with psychiatric disorders produces a potential ascertainment bias, we adjusted for the number of primary care visits in the year prior to the autoimmune disorder diagnosis for each patient diagnosed with an autoimmune disorder. For veterans without an autoimmune disorder diagnosis, we adjusted for the number of primary care visits in the year prior to their most recent VA encounter.
STATISTICAL ANALYSES
Generalized linear models with Poisson distribution and robust error variance were used to estimate relative risks (RR), adjusted relative risks (ARR) and 95% confidence intervals (CIs). In our primary models, we estimated RR and ARR for any of the autoimmune disorders alone or in combination as well as risk for each autoimmune disorder separately in veterans with a diagnosis of PTSD compared to: 1) veterans without any psychiatric diagnoses; and 2) veterans with psychiatric diagnoses other than PTSD. In follow-up analyses, we stratified by gender and assessed the interaction between gender and psychiatric disorder group. We also used generalized linear models to examine the contribution of common comorbid psychiatric disorders and MST. In follow-up analyses, we assessed risk for autoimmune disorders associated with depressive, anxiety, adjustment, psychotic, and alcohol and substance use disorders with and without PTSD. We did not conduct separate analyses for veterans with PTSD alone without any comorbid psychiatric disorders because the symptoms of PTSD overlap with other common psychiatric disorders (e.g., depression and anxiety). In fact, individuals with severe PTSD who have most or all of the symptoms must by definition also meet criteria for major depression. Nonetheless, there is a lot of variability in the number of comorbid diagnoses received by individuals with PTSD and other psychiatric disorders, and this “psychiatric burden” may also predict outcomes (32). Thus, we computed a “psychiatric burden” variable based on the sum of depression, anxiety, adjustment, psychotic, alcohol use, and substance use disorders (Range 0 – 6 disorders) in order to examine the effects of number of comorbidities on risk for autoimmune disorders. Finally, in follow-up analyses testing the reverse of our primary hypothesis, we examined the RR and ARR for diagnosis with PTSD and other psychiatric disorders in veterans with versus without autoimmune disorders, excluding veterans who had a diagnosis of a psychiatric disorder before their autoimmune disorder. Models were adjusted for age, gender, race and number of primary care visits. Analyses were conducted using SAS software 9.3 (SAS Institute Inc, Cary, NC, USA) and STATA 13.1 (Statacorp, College Station, TX, USA). All tests of significance were two-sided with an α of p < .05.
RESULTS
SAMPLE CHARACTERISTICS
Table 1 describes the sample characteristics. PTSD with and without other psychiatric diagnoses was diagnosed in 203,766 (30.6%) veterans, and psychiatric disorders other than PTSD were diagnosed in an additional 129,704 (19.5%) veterans. Veterans with psychiatric disorders were younger (p < .001), more likely to be male (p < .001) and non-white (p < .001), and had a higher number of visits to primary care in the prior year (p < .001) than veterans with no psychiatric disorders. The median follow-up time from first appointment to the study end date was 1,502 days (interquartile range, 913 – 2186 days) with a minimum of 366 and a maximum of 3819 days.
AUTOIMMUNE DISORDERS
Within the sample of 666,269 veterans, 9,743 (1.5%) received a diagnosis of an autoimmune diagnosis at two or more separate VA clinical encounters: 6,963 (1.0%) with thyroiditis, 1,460 (0.2%) with inflammatory bowel diseases, 562 (0.1%) with rheumatoid arthritis, 535 (0.1%) with multiple sclerosis, and 339 (0.1%) with lupus erythematosus. Based on previous epidemiologic studies that use a wider age range of participants and balanced samples of males and females, our relatively young and mostly male veteran sample had slightly lower rates of thyroiditis (1% versus 1.95%) (35), inflammatory bowel diseases (0.2% versus 0.4%) (36), and rheumatoid arthritis (0.1% versus 0.5–1%), (37), but similar rates of systemic lupus erythematosus (0.1% versus 0.1%) (38) and higher rates of multiple sclerosis (0.1% versus 0.01%) (39). The median time between first psychiatric diagnosis and first autoimmune disorder diagnosis was 220 days (interquartile range, 6 – 807 days).
PTSD AND RISK FOR AUTOIMMUNE DISORDERS
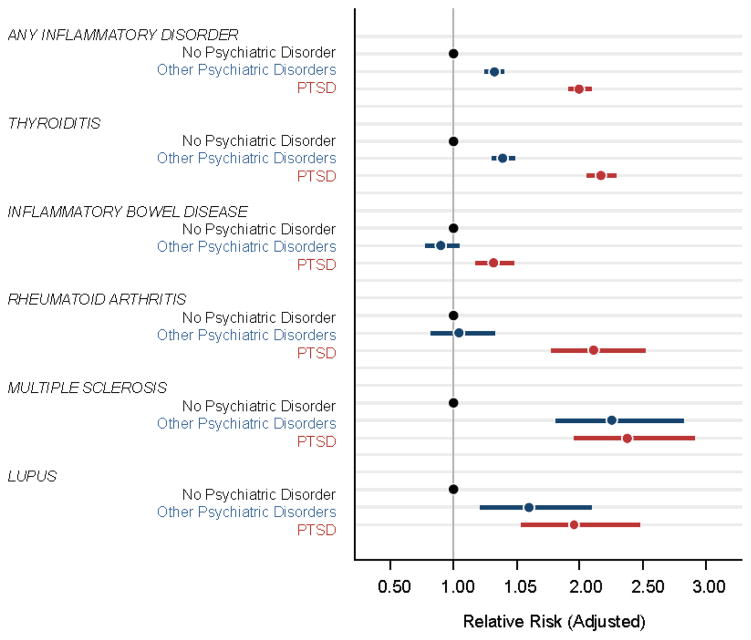
Veterans diagnosed with PTSD had significantly higher risk for diagnosis with any of the autoimmune disorders alone or in combination and for all of the autoimmune disorders considered individually, compared to veterans with no psychiatric disorders (Table 2 and Fig. 2). Veterans with psychiatric disorders other than PTSD also had significantly higher risk for any of the autoimmune disorders alone or in combination with other autoimmune disorders, as well as for thyroiditis, multiple sclerosis, and lupus erythematosus, compared to veterans with no psychiatric disorders (Table 2 and Fig. 2).
Table 2.
Unadjusted and age adjusted relative risk for each autoimmune disorder according to psychiatric status
| Relative Risk (95% CI) | Adjusteda Relative Risk (95% CI) | |||||
|---|---|---|---|---|---|---|
| Psychiatric status | No Psychiatric Disorder | Other Psychiatric Disorders | PTSD | No Psychiatric Disorder | Other Psychiatric Disorders | PTSD |
| All Veterans | ||||||
| Any Autoimmune Disorder | 1.00 [reference] | 1.33 (1.26, 1.41)** | 1.81 (1.73, 1.89)** | 1.00 [reference] | 1.32 (1.25, 1.39)** | 2.00 (1.91, 2.09)** |
| Thyroiditis | 1.00 [reference] | 1.40 (1.31, 1.49)** | 1.92 (1.82, 2.03)** | 1.00 [reference] | 1.39 (1.30, 1.48)** | 2.17 (2.06, 2.29)** |
| Inflammatory Bowel Diseases | 1.00 [reference] | 0.92 (0.79, 1.06) | 1.34 (1.20, 1.50)** | 1.00 [reference] | 0.89 (0.77, 1.04) | 1.30 (1.16, 1.46)** |
| Multiple Sclerosis | 1.00 [reference] | 2.35 (1.88, 2.93)** | 2.23 (1.83, 2.73)** | 1.00 [reference] | 2.29 (1.83, 2.86)** | 2.36 (1.93, 2.89)** |
| Rheumatoid Arthritis | 1.00 [reference] | 1.00 (0.78, 1.28) | 1.70 (1.42, 2.04)** | 1.00 [reference] | 1.01 (0.79, 1.30) | 2.04 (1.70, 2.45)** |
| Lupus Erythematosus | 1.00 [reference] | 1.66 (1.26, 2.19)** | 1.63 (1.28, 2.09)** | 1.00 [reference] | 1.57 (1.19, 2.08)* | 1.85 (1.45, 2.37)** |
| Female | ||||||
| Any Autoimmune Disorder | 1.00 [reference] | 1.26 (1.14, 1.39)** | 2.14 (1.97, 2.33)** | 1.00 [reference] | 1.27 (1.14, 1.40)** | 2.09 (1.92, 2.29)** |
| Thyroiditis | 1.00 [reference] | 1.20 (1.07, 1.34)* | 2.10 (1.91, 2.31)** | 1.00 [reference] | 1.22 (1.09, 1.37)** | 2.09 (1.89, 2.31)** |
| Inflammatory Bowel Diseases | 1.00 [reference] | 1.31 (0.90, 1.93) | 1.99 (1.42, 2.78)** | 1.00 [reference] | 1.28 (0.86, 1.92) | 1.92 (1.34, 2.76)** |
| Multiple Sclerosis | 1.00 [reference] | 2.63 (1.72, 4.02)** | 2.92 (1.93, 4.41)** | 1.00 [reference] | 2.68 (1.73, 4.15)** | 2.90 (1.91, 4.40)** |
| Rheumatoid Arthritis | 1.00 [reference] | 1.22 (0.81, 1.81) | 2.58 (1.86, 3.56)** | 1.00 [reference] | 1.20 (0.80, 1.80) | 2.39 (1.72, 3.33)** |
| Lupus Erythematosus | 1.00 [reference] | 1.37 (0.92, 2.06) | 2.05 (1.43, 2.94)** | 1.00 [reference] | 1.34 (0.89, 2.02) | 1.89 (1.30, 2.75)** |
| Male | ||||||
| Any Autoimmune Disorder | 1.00 [reference] | 1.29 (1.21, 1.38)** | 1.79 (1.70, 1.88)** | 1.00 [reference] | 1.34 (1.26, 1.44)** | 1.95 (1.85, 2.06)** |
| Thyroiditis | 1.00 [reference] | 1.41 (1.30, 1.52)** | 1.97 (1.85, 2.10)** | 1.00 [reference] | 1.48 (1.37, 1.60)** | 2.22 (2.08, 2.36)** |
| Inflammatory Bowel Diseases | 1.00 [reference] | 0.87 (0.74, 1.02) | 1.28 (1.13, 1.44)** | 1.00 [reference] | 0.84 (0.72, 0.99)* | 1.23 (1.09, 1.39)** |
| Multiple Sclerosis | 1.00 [reference] | 2.14 (1.65, 2.78)** | 2.14 (1.70, 2.70)** | 1.00 [reference] | 2.16 (1.65, 2.81)** | 2.18 (1.73, 2.76)** |
| Rheumatoid Arthritis | 1.00 [reference] | 0.83 (0.61, 1.13) | 1.52 (1.22, 1.88)** | 1.00 [reference] | 0.91 (0.66, 1.25) | 1.88 (1.50, 2.43)** |
| Lupus Erythematosus | 1.00 [reference] | 1.69 (1.16, 2.47)* | 1.57 (1.12, 2.19)* | 1.00 [reference] | 1.78 (1.21, 2.62)* | 1.73 (1.22, 2.45)* |
0.001 < p < 0.05 and
p ≤ 0.001 when compared to veterans with no psychiatric disorder
Relative risk adjusted for age, gender, race and primary care visits
Figure 2.
Adjusted relative risk (ARR) for autoimmune disorder diagnoses in veterans without any psychiatric disorder (black), with psychiatric disorders other than PTSD (blue), and with PTSD with and without other psychiatric disorders (red) adjusted for age, gender, race and primary care visits. Dots represent ARR and lines represent 95% confidence intervals.
In all cases, the effect size for the ARR associated with psychiatric disorders other than PTSD was smaller than that associated with PTSD. Moreover, compared to veterans with other psychiatric disorders, veterans with PTSD had significantly higher risk for diagnosis with any autoimmune disorder alone or in combination (ARR = 1.51, 95% CI, 1.43, 1.59, p < .001), as well as for thyroiditis (ARR = 1.56, 95% CI, 1.47, 1.66, p < .001), inflammatory bowel disorders (ARR = 1.45, 95% CI, 1.23, 1.67, p < .001), and rheumatoid arthritis, (ARR = 2.01, 95% CI, 1.52, 2.51, p < .001), but not multiple sclerosis (ARR = 1.03, 95% CI, 0.82, 1.25, p = .63) or lupus erythematosus (ARR = 1.18, 95% CI, 0.85, 1.51, p = .12) individually.
In follow-up analyses, we examined if the addition of comorbid PTSD was associated with elevated risk for autoimmune disorders in veterans with other specific psychiatric diagnoses including depressive, anxiety, adjustment, psychotic, substance use and alcohol use disorders. These analyses indicated that in each case, the addition of PTSD conferred significantly higher risk for developing an autoimmune disorder (Table 3). Veterans with PTSD in our sample had a higher number of comorbid psychiatric diagnoses than veterans with other psychiatric disorders (Table 1 and Supplementary Table S1, p’s < .001). Thus, we reran our analyses adjusting for number of comorbid psychiatric disorders (excluding PTSD) and found that PTSD-related increased risk for autoimmune disorders remained significant in analyses adjusted for age, gender, race/ethnicity and number of primary care visits (ARR = 1.36, 95% CI, 1.29, 1.42, p < .001). In the same model, number of comorbidities itself was an independent risk factor for autoimmune disorders (ARR = 1.22, 95% CI, 1.20, 1.25, p < .001).
Table 3.
Unadjusted and age adjusted relative risk for diagnosis of any autoimmune disorder with specific psychiatric diagnoses without versus with comorbid PTSD
| Psychiatric diagnoses without and with PTSD | Total No. | AI Disorder Diagnosis No. (%) | Relative Risk (95% CI) | Adjusted Relative Riska (95% CI) |
|---|---|---|---|---|
|
Depression
| ||||
| Without PTSD | 57,313 | 1,245 (2.2) | 1.00 [reference] | 1.00 [reference] |
| With PTSD | 126,796 | 3,183 (2.5) | 1.16 (1.08, 1.23) ** | 1.34 (1.25, 1.43) ** |
|
| ||||
|
Anxiety
| ||||
| Without PTSD | 44,632 | 775 (1.7) | 1.00 [reference] | 1.00 [reference] |
| With PTSD | 79,187 | 2,024 (2.6) | 1.47 (1.36, 1.60) ** | 1.60 (1.47,1.73) ** |
|
| ||||
|
Adjustment
Disorder
| ||||
| Without PTSD | 47,516 | 692 (1.5) | 1.00 [reference] | 1.00 [reference] |
| With PTSD | 63,110 | 1,520 (2.4) | 1.65 (1.51, 1.81) ** | 1.78 (1.63, 1.95) ** |
|
| ||||
|
Psychosis
| ||||
| Without PTSD | 2,377 | 76 (3.2) | 1.00 [reference] | 1.00 [reference] |
| With PTSD | 7,193 | 255 (3.5) | 1.11 (0.86, 1.43) | 1.16 (0.89, 1.51) |
|
| ||||
|
Alcohol Use
Disorder
| ||||
| Without PTSD | 25,913 | 282 (1.1) | 1.00 [reference] | 1.00 [reference] |
| With PTSD | 60,169 | 1,157 (1.9) | 1.77 (1.55, 2.01) ** | 1.81 (1.61, 2.11) ** |
|
| ||||
|
Substance Use
Disorder
| ||||
| Without PTSD | 10,382 | 126 (1.2) | 1.00 [reference] | 1.00 [reference] |
| With PTSD | 30,988 | 660 (2.1) | 1.75 (1.45, 2.12) ** | 1.88 (1.54, 2.30) ** |
0.001 < p < 0.05 and
p ≤ 0.001 when compared to veterans with no psychiatric disorder
Relative risk adjusted for age, gender, race and primary care visits
We also reran our analyses for lupus erythematosus excluding patients with lupus erythematosus (ICD-9 code 695.4) and focusing only on those specifically diagnosed with systemic lupus erythematosus (ICD-9 code 710.0). Results indicated that the pattern of results for the combined lupus erythematosus category was the same as that for systemic lupus erythematosus alone. In fact, the effect size for PTSD-related increased risk was larger for systemic lupus erythematosus alone than for the combination of lupus erythematosus and systemic lupus erythematosus (ARR = 1.65, 95% CI, 1.14, 2.40, p = .008).
GENDER DIFFERENCES
Supplementary Table S1 describes sample characteristics stratified by gender. Women had significantly higher risk for autoimmune disorders overall with 4.6% of women versus 1.7% of men being diagnosed with an autoimmune disorder. In models adjusted for age, race, and primary care visits, women relative to men had more than three times higher risk for any of the autoimmune disorders alone or in combination (ARR = 3.03, 95% CI, 2.89, 3.16, p < .001), and significantly elevated risk for thyroiditis (ARR = 3.48, 95% CI, 3.31, 3.67, p < .001), rheumatoid arthritis (ARR = 3.94, 95% CI, 3.30, 4.69, p < .001), multiple sclerosis (ARR = 2.63, 95% CI, 2.16, 3.19, p < .001), and lupus erythematosus (ARR = 6.23, 95% CI, 5.01, 7.73, p < .001), but not inflammatory bowel disorders (ARR = 1.04, 95% CI, 0.89, 1.22, p = .59) considered individually.
Relative risks for autoimmune disorders in PTSD, other psychiatric disorders, and no psychiatric disorder are shown separately for women and men in Table 2. PTSD-related increased risk for autoimmune disorders was similar in both genders, and we did not find evidence of a PTSD by gender interaction. However, due to the increased risk for autoimmune disorders in women overall, the absolute prevalence of autoimmune disorders was highest in women with PTSD (Fig. 3).
Figure 3.
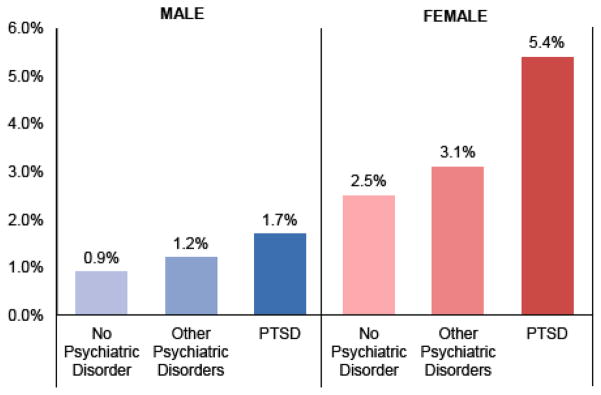
Percentage of female and male veterans with any autoimmune disorder. The absolute prevalence of autoimmune disorders was highest in women with PTSD at 5.4%, followed by women with psychiatric disorders other than PTSD at 3.1%, and women with no psychiatric disorders at 2.5%. Men with PTSD had the next highest prevalence at 1.7%, followed by men with psychiatric disorders other than PTSD at 1.2%, and finally men with no psychiatric disorders with the lowest prevalence of the autoimmune disorders at 0.9%
MILITARY SEXUAL TRAUMA AND AUTOIMMUNE DISORDERS
One factor that differed markedly between women and men was MST, which was much more common in women (13%) than men (0.5%). Table 1 shows rates of MST across the sample and Supplementary Table S1 shows MST rates stratified by gender. In order to examine if MST was contributing to our finding of elevated risk for autoimmune disorders in veterans we PTSD, we examined the risk for autoimmune disorders in veterans with MST without PTSD, PTSD without MST, and with both MST and PTSD compared to all other veterans. In the combined sample of males and females, both MST without PTSD (ARR = 1.64, 95% CI, 1.41, 1.92, p < .001) and PTSD without MST (ARR = 1.84, 95% CI, 1.75, 1.93, p < .001) were significant risk factors for autoimmune disorder diagnoses and the combination of PTSD and MST was associated with even higher risk (ARR = 2.40, 95% CI, 2.17, 2.65, p < .001). When we repeated these analyses in the stratified samples of women and men, the pattern of results was similar. The only difference was that in women, the risk for autoimmune disorders with PTSD without MST was higher than risk associated with MST without PTSD (ARR = 1.95 versus 1.65), whereas in men, the risk for autoimmune disorders associated with MST without PTSD was higher than risk associated with PTSD without MST (ARR = 2.24 versus 1.80). The combination of MST and PTSD was associated with the highest risk in both women (ARR = 2.57, 95% CI, 2.28, 2.89, p < .001) and men (ARR = 2.40, 95% CI, 1.81, 3.20, p < .001). Thus, MST was an independent risk factor for autoimmune disorders in our sample, but it did not fully account for our findings of PTSD-related increased risk.
AUTOIMMUNE DISORDERS AND RISK FOR PSYCHIATRIC DISORDERS
A total of 2,940 veterans received a diagnosis of an autoimmune disorder prior to being diagnosed with a psychiatric disorder. Surprisingly, veterans with autoimmune disorders had lower risk for subsequent diagnosis with PTSD and other psychiatric disorders compared to those without autoimmune disorders (ARR = 0.59, 95% CI, 0.57, 0.62, p < .001).
DISCUSSION
This study of 666,269 Iraq and Afghanistan veterans indicates that PTSD is associated with increased risk for diagnosis with autoimmune disorders. Specifically, our results showed that veterans with PTSD had twice the risk of being diagnosed with an autoimmune disorder compared to those without any psychiatric disorders, and 51% increased risk compared to veterans with psychiatric disorders other than PTSD. Veterans with a higher number of comorbid psychiatric diagnoses were also at increased risk for autoimmune disorders, but high levels of comorbidity did not entirely account for the effect of PTSD on risk. Although the magnitude of PTSD-related increased risk was similar in women and men, women overall had almost three times higher risk for diagnosis with an autoimmune disorder. Thus, the absolute prevalence of autoimmune disorders was highest in women with PTSD. MST was independently associated with risk for autoimmune disorders in both women and men.
Our findings expand upon prior studies in smaller samples that have indicated increased risk for autoimmune disorders in patients with PTSD (19, 20). Taken together with the two smaller previous studies, our data indicate that PTSD, and not cohort specific environmental exposures, may increase risk for autoimmune disorders. Our findings also contribute to a growing literature highlighting the increased risk for other chronic physical diseases in veterans with PTSD and other psychiatric disorders (40–42).
In the present cohort, women had almost three-fold higher risk for autoimmune disorders compared with men, which is likely due to sex differences in immunomodulation. Compared to men, women tend to show elevated antibody response to infection, vaccination, and physical trauma, which is thought to confer protection from infectious diseases but greater risk for autoimmune disorders (43, 44). Moreover, previous studies have shown sex differences in stress-response systems that may lead to differences in biological and health outcomes in women versus men with PTSD (45–47). However, women and men with PTSD in our population had roughly equivalent levels of increased risk for autoimmune disorders than same-sex veterans without PTSD. Thus, our results suggest no interaction between PTSD and gender in risk for autoimmune disorders. Women had much higher levels of MST exposure compared to men and MST was itself a risk factor for autoimmune disorder diagnoses. However, PTSD without MST remained significantly associated with increased risk for autoimmune disorders in both women and men.
The mechanisms of the observed association between PTSD and autoimmune disorders remain unclear. However, a dysregulated HPA axis, elevated inflammation, accelerated immune cell aging, and altered immune cell gene expression patterns may play a mechanistic role (1–6, 8, 10, 48). In addition, increased tobacco use, impaired sleep, poor diet, and substance and alcohol use may contribute to both biological abnormalities and risk for autoimmune disorders observed in individuals with PTSD (49–52). It is also possible that shared genetic and environmental vulnerability factors, including dysregulated glucocorticoid signaling, accelerated immune cell aging, and childhood trauma, may contribute to the development of PTSD as well as autoimmune disorders (53–55). In fact, prior studies indicate that autoimmune disorders increase risk for subsequent diagnosis with mood disorders and schizophrenia (56, 57). Moreover, the autoimmune disorders that we have included in the present study have distinct as well as overlapping etiological mechanisms and the pathway from psychiatric disorders to autoimmune disorders may differ among them.
Although studies such as the present one indicate that psychiatric disorders and autoimmune disorders co-occur, causal direction is less clear. In our study, we focused on the risk for autoimmune disorders in individuals with PTSD. The hypothesis that trauma exposure and PTSD may increase risk for the development of autoimmune disorders is supported by some non-human experimental stress studies (58, 59). However, other investigators have proposed that stress may actually protect against the development of specific autoimmune disorders under some circumstances (60).
Our analyses indicated lower risk for subsequent diagnosis with psychiatric disorders in individuals previously diagnosed with autoimmune disorders, which may be due to diagnostic biases. Autoimmune disorders such as lupus and multiple sclerosis can be accompanied by psychiatric symptoms including depressive and even psychotic symptoms (61). In the present study, we relied on physician diagnoses made during routine clinical care as indices of psychiatric and autoimmune disorders, which may bias results. For example, once an autoimmune disorder has been diagnosed, psychiatric symptoms (e.g., fatigue, confusion, sadness, and anxiety) may be attributed to the autoimmune disorder rather than to a de novo psychiatric disorder. Further large-scale prospective research studies in different healthcare settings will be necessary to clarify this issue.
LIMITATIONS
The present study benefits from a large sample size comprising most of the population being studied, longitudinal data, physician rather than self-report diagnoses of psychiatric and autoimmune disorders, adjustment for many relevant confounds, and the ability to assess gender differences. However, the study also has several limitations. First, we cannot claim to have shown causal relationships in this retrospective observational study. Moreover, we cannot be confident about temporality because diagnoses were made at the point when VA providers first coded the condition using ICD-9CM criteria, which may not correspond with emergence of symptoms and psychiatric and autoimmune disorders may be present for years or even decades before an official diagnosis. Second, the use of codes rather than clinical diagnoses may lead to misclassification errors and our data may be at risk of bias due to under- or over-reporting of symptoms by veterans seeking VA care (62, 63). Third, veterans with PTSD are known to be at increased risk for other chronic physical diseases and we did not adjust for the presence of these disorders in our analytic models (64, 65). Fourth, our focus on Iraq and Afghanistan veterans who have served in conflict relatively recently means that we have a relatively short follow-up time. Later follow-up studies will be necessary to explore if the findings remain consistent over time as veterans age. Fifth, our data on MST may underestimate actual MST in the population because it is based on clinical visits related to MST. However, the rates observed in our sample are similar to those seen in studies that used the VA MST screen (29). Our study also lacks information on other traumatic events that may vary by gender, including other warzone trauma exposures. Finally, our study is based on treatment-seeking veterans using the VA healthcare system. Although the VA is the largest provider of healthcare to returning veterans, our results may not generalize to Iraq and Afghanistan veterans not enrolled in VA healthcare, other veterans, or civilians.
CONCLUSIONS
The present results underline high rates of PTSD and other psychiatric diagnoses in Iraq and Afghanistan veterans and highlight the knock-on effects of trauma exposure and PTSD on physical health. Our results indicate that young veterans diagnosed with PTSD have significantly increased risk for diagnosis with autoimmune disorders with definitive diagnostic criteria. Future prospective longitudinal cohort studies are needed to establish causality, measure endocrine and immune system activity in veterans with PTSD, and evaluate whether timely and successful treatment of PTSD reduces risk of autoimmune disorders. However, lower thresholds for evaluating the presence of autoimmune disorders may be warranted in veterans with PTSD and other psychiatric disorders immediately. Our data underscore the need to identify and treat PTSD and other psychiatric disorders in veterans in order to enhance not only mental but also physical health.
Supplementary Material
Acknowledgments
The authors acknowledge support for the present manuscript from “The Mental Illness Research, Education and Clinical Centers (MIRECC)”, “Society in Science – The Branco Weiss Fellowship”, and the National Institutes of Health as well as the Department of Veterans Affairs and the NCIRE – The Veterans Health Research Institute. We also thank Ashkan Ahmadian for his excellent assistance with this manuscript.
Footnotes
FINANCIAL DISCLOSURES
Dr. Aoife O’Donovan received salary support from Society in Science - The Branco Weiss Fellowship. Dr. Beth Cohen received salary support through NIH-K23 HL 094765-0. Mr. Dan Bertenthal received grant salary support through grants to Dr. Karen Seal, and MIRECC Program Director Dr. Thomas Neylan. Dr. Mary Margaretten received grant support through NIH-KL2TR000143. All other authors reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yehuda R, Teicher MH, Levengood RA, Trestman RL, Siever LJ. Circadian regulation of basal cortisol levels in posttraumatic stress disorder. Ann N Y Acad Sci. 1994;746:378–380. doi: 10.1111/j.1749-6632.1994.tb39260.x. [DOI] [PubMed] [Google Scholar]
- 2.Neylan TC, Brunet A, Pole N, Best SR, Metzler TJ, Yehuda R, et al. PTSD symptoms predict waking salivary cortisol levels in police officers. Psychoneuroendocrinology. 2005;30:373–381. doi: 10.1016/j.psyneuen.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Yehuda R, Golier JA, Kaufman S. Circadian rhythm of salivary cortisol in Holocaust survivors with and without PTSD. Am J Psychiatry. 2005;162:998–1000. doi: 10.1176/appi.ajp.162.5.998. [DOI] [PubMed] [Google Scholar]
- 4.O’Donovan A, Sun B, Cole S, Rempel H, Lenoci M, Pulliam L, et al. Transcriptional control of monocyte gene expression in post-traumatic stress disorder. Dis Markers. 2011;30:123–132. doi: 10.3233/DMA-2011-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pace TW, Wingenfeld K, Schmidt I, Meinlschmidt G, Hellhammer DH, Heim CM. Increased peripheral NF-kappaB pathway activity in women with childhood abuse-related posttraumatic stress disorder. Brain Behav Immun. 2012;26:13–17. doi: 10.1016/j.bbi.2011.07.232. [DOI] [PubMed] [Google Scholar]
- 6.Hoge EA, Brandstetter K, Moshier S, Pollack MH, Wong KK, Simon NM. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress Anxiety. 2009;26:447–455. doi: 10.1002/da.20564. [DOI] [PubMed] [Google Scholar]
- 7.Gola H, Engler H, Sommershof A, Adenauer H, Kolassa S, Schedlowski M, et al. Posttraumatic stress disorder is associated with an enhanced spontaneous production of pro-inflammatory cytokines by peripheral blood mononuclear cells. BMC Psychiatry. 2013;13:40. doi: 10.1186/1471-244X-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neylan TC, Sun B, Rempel H, Ross J, Lenoci M, O’Donovan A, et al. Suppressed monocyte gene expression profile in men versus women with PTSD. Brain Behav Immun. 2011;25:524–531. doi: 10.1016/j.bbi.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yehuda R, Cai G, Golier JA, Sarapas C, Galea S, Ising M, et al. Gene expression patterns associated with posttraumatic stress disorder following exposure to the World Trade Center attacks. Biol Psychiatry. 2009;66:708–711. doi: 10.1016/j.biopsych.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 10.Uddin M, Aiello AE, Wildman DE, Koenen KC, Pawelec G, de Los Santos R, et al. Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proc Natl Acad Sci U S A. 2010;107:9470–9475. doi: 10.1073/pnas.0910794107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malan S, Hemmings S, Kidd M, Martin L, Seedat S. Investigation of telomere length and psychological stress in rape victims. Depress Anxiety. 2011;28:1081–1085. doi: 10.1002/da.20903. [DOI] [PubMed] [Google Scholar]
- 12.O’Donovan A, Epel E, Lin J, Wolkowitz O, Cohen B, Maguen S, et al. Childhood trauma associated with short leukocyte telomere length in posttraumatic stress disorder. Biol Psychiatry. 2011;70:465–471. doi: 10.1016/j.biopsych.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Donovan A, Pantell MS, Puterman E, Dhabhar FS, Blackburn EH, Yaffe K, et al. Cumulative inflammatory load is associated with short leukocyte telomere length in the Health, Aging and Body Composition Study. PloS ONE. 2011;6:e19687. doi: 10.1371/journal.pone.0019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16:238–246. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eskandari F, Webster JI, Sternberg EM. Neural immune pathways and their connection to inflammatory diseases. Arthritis Res Ther. 2003;5:251–265. doi: 10.1186/ar1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goronzy JJ, Fujii H, Weyand CM. Telomeres, immune aging and autoimmunity. Exp Gerontol. 2006;41:246–251. doi: 10.1016/j.exger.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Georgin-Lavialle S, Aouba A, Mouthon L, Londono-Vallejo JA, Lepelletier Y, Gabet AS, et al. The telomere/telomerase system in autoimmune and systemic immune-mediated diseases. Autoimmun Rev. 2010;9:646–651. doi: 10.1016/j.autrev.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Costenbader KH, Prescott J, Zee RY, De Vivo I. Immunosenescence and rheumatoid arthritis: does telomere shortening predict impending disease? Autoimmun Rev. 2011;10:569–573. doi: 10.1016/j.autrev.2011.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boscarino JA. Posttraumatic stress disorder and physical illness: results from clinical and epidemiologic studies. Ann N Y Acad Sci. 2004;1032:141–153. doi: 10.1196/annals.1314.011. [DOI] [PubMed] [Google Scholar]
- 20.Boscarino JA, Forsberg CW, Goldberg J. A twin study of the association between PTSD symptoms and rheumatoid arthritis. Psychosom Med. 2010;72:481–486. doi: 10.1097/PSY.0b013e3181d9a80c. [DOI] [PubMed] [Google Scholar]
- 21.Kovacs WJ, Olsen NJ. Sexual dimorphism of RA manifestations: genes, hormones and behavior. Nat Rev Rheumatol. 2011;7:307–310. doi: 10.1038/nrrheum.2010.231. [DOI] [PubMed] [Google Scholar]
- 22.van Vollenhoven RF. Sex differences in rheumatoid arthritis: more than meets the eye. BMC Med. 2009;7:12. doi: 10.1186/1741-7015-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiovato L, Lapi P, Fiore E, Tonacchera M, Pinchera A. Thyroid autoimmunity and female gender. J Endocrinol Invest. 1993;16:384–391. doi: 10.1007/BF03348863. [DOI] [PubMed] [Google Scholar]
- 24.Casetta I, Riise T, Wamme Nortvedt M, Economou NT, De Gennaro R, Fazio P, et al. Gender differences in health-related quality of life in multiple sclerosis. Mult Scler. 2009;15:1339–1346. doi: 10.1177/1352458509107016. [DOI] [PubMed] [Google Scholar]
- 25.Schwartzman-Morris J, Putterman C. Gender differences in the pathogenesis and outcome of lupus and of lupus nephritis. Clin Dev Immunol. 2012;2012:604892. doi: 10.1155/2012/604892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saibeni S, Cortinovis I, Beretta L, Tatarella M, Ferraris L, Rondonotti E, et al. Gender and disease activity influence health-related quality of life in inflammatory bowel diseases. Hepatogastroenterology. 2005;52:509–515. [PubMed] [Google Scholar]
- 27.Seal KH, Metzler TJ, Gima KS, Bertenthal D, Maguen S, Marmar CR. Trends and risk factors for mental health diagnoses among Iraq and Afghanistan veterans using Department of Veterans Affairs health care, 2002–2008. Am J Public Health. 2009;99:1651–1658. doi: 10.2105/AJPH.2008.150284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seal KH, Maguen S, Cohen B, Gima KS, Metzler TJ, Ren L, et al. VA mental health services utilization in Iraq and Afghanistan veterans in the first year of receiving new mental health diagnoses. J Trauma Stress. 2010;23:5–16. doi: 10.1002/jts.20493. [DOI] [PubMed] [Google Scholar]
- 29.Maguen S, Cohen B, Ren L, Bosch J, Kimerling R, Seal K. Gender differences in military sexual trauma and mental health diagnoses among Iraq and Afghanistan veterans with posttraumatic stress disorder. Womens Health Issues. 2012;22:e61–66. doi: 10.1016/j.whi.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 30.Cooper GS, Stroehla BC. The epidemiology of autoimmune diseases. Autoimmun Rev. 2003;2:119–125. doi: 10.1016/s1568-9972(03)00006-5. [DOI] [PubMed] [Google Scholar]
- 31.Seal KH, Bertenthal D, Miner CR, Sen S, Marmar C. Bringing the war back home: mental health disorders among 103,788 US veterans returning from Iraq and Afghanistan seen at Department of Veterans Affairs facilities. Arch Intern Med. 2007;167:476–482. doi: 10.1001/archinte.167.5.476. [DOI] [PubMed] [Google Scholar]
- 32.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 33.Kressin NR, Chang BH, Hendricks A, Kazis LE. Agreement between administrative data and patients’ self-reports of race/ethnicity. Am J Public Health. 2003;93:1734–1739. doi: 10.2105/ajph.93.10.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jia H, Zheng YE, Cowper DC, Stansbury JP, Wu SS, Vogel WB, et al. Race/ethnicity: who is counting what? J Rehabil Res Dev. 2006;43:475–484. doi: 10.1682/jrrd.2005.05.0086. [DOI] [PubMed] [Google Scholar]
- 35.Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84:223–243. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- 36.Kappelman MD, Rifas-Shiman SL, Kleinman K, Ollendorf D, Bousvaros A, Grand RJ, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424–1429. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 37.Gabriel SE, Michaud K. Epidemiological studies in incidence, prevalence, mortality, and comorbidity of the rheumatic diseases. Arthritis Res Ther. 2009;11:229. doi: 10.1186/ar2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Danchenko N, Satia JA, Anthony MS. Epidemiology of systemic lupus erythematosus: a comparison of worldwide disease burden. Lupus. 2006;15:308–318. doi: 10.1191/0961203306lu2305xx. [DOI] [PubMed] [Google Scholar]
- 39.Olek MJ. Epidemiology and clinical features of multiple sclerosis in adults. In: Basow DS, editor. Up To Date. Waltham: 2012. http://www.uptodate.com/home/index.html. [Google Scholar]
- 40.Cohen BE, Marmar C, Ren L, Bertenthal D, Seal KH. Association of cardiovascular risk factors with mental health diagnoses in Iraq and Afghanistan war veterans using VA health care. JAMA. 2009;302:489–492. doi: 10.1001/jama.2009.1084. [DOI] [PubMed] [Google Scholar]
- 41.Yaffe K, Vittinghoff E, Lindquist K, Barnes D, Covinsky KE, Neylan T, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psych. 2010;67:608–613. doi: 10.1001/archgenpsychiatry.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kubzansky LD, Koenen KC, Spiro AI, Vokonas PS, Sparrow D. Prospective study of posttraumatic stress disorder symptoms and coronary heart disease in the Normative Aging Study. Arch Gen Psych. 2007;64:109–116. doi: 10.1001/archpsyc.64.1.109. [DOI] [PubMed] [Google Scholar]
- 43.Fairweather D, Frisancho-Kiss S, Rose NR. Sex differences in autoimmune disease from a pathological perspective. Am J Pathol. 2008;173:600–609. doi: 10.2353/ajpath.2008.071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fairweather D, Petri MA, Coronado MJ, Cooper LT. Autoimmune heart disease: role of sex hormones and autoantibodies in disease pathogenesis. Expert Rev Clin Immunol. 2012;8:269–284. doi: 10.1586/eci.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Almli LM, Mercer KB, Kerley K, Feng H, Bradley B, Conneely KN, et al. ADCYAP1R1 genotype associates with post-traumatic stress symptoms in highly traumatized African-American females. Am J Med Genet B Neuropsychiatr Genet. 2013;162B:262–272. doi: 10.1002/ajmg.b.32145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inslicht SS, Metzler TJ, Garcia NM, Pineles SL, Milad MR, Orr SP, et al. Sex differences in fear conditioning in posttraumatic stress disorder. J Psychiatr Res. 2013;47:64–71. doi: 10.1016/j.jpsychires.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.von Kanel R, Hepp U, Kraemer B, Traber R, Keel M, Mica L, et al. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J Psychiatr Res. 2007;41:744–752. doi: 10.1016/j.jpsychires.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 49.Padyukov L, Silva C, Stolt P, Alfredsson L, Klareskog L. A gene-environment interaction between smoking and shared epitope genes in HLA-DR provides a high risk of seropositive rheumatoid arthritis. Arthritis Rheum. 2004;50:3085–3092. doi: 10.1002/art.20553. [DOI] [PubMed] [Google Scholar]
- 50.Palma BD, Gabriel A, Jr, Colugnati FA, Tufik S. Effects of sleep deprivation on the development of autoimmune disease in an experimental model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1527–1532. doi: 10.1152/ajpregu.00186.2006. [DOI] [PubMed] [Google Scholar]
- 51.Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part II: Noninfectious factors. Ann Neurol. 2007;61:504–513. doi: 10.1002/ana.21141. [DOI] [PubMed] [Google Scholar]
- 52.Cook RT. Alcohol abuse, alcoholism, and damage to the immune system--a review. Alcohol Clin Exp Res. 1998;22:1927–1942. [PubMed] [Google Scholar]
- 53.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pace TW, Heim CM. A short review on the psychoneuroimmunology of posttraumatic stress disorder: from risk factors to medical comorbidities. Brain Behav Immun. 2011;25:6–13. doi: 10.1016/j.bbi.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 55.Dube SR, Fairweather D, Pearson WS, Felitti VJ, Anda RF, Croft JB. Cumulative childhood stress and autoimmune diseases in adults. Psychosom Med. 2009;71:243–250. doi: 10.1097/PSY.0b013e3181907888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Benros ME, Waltoft BL, Nordentoft M, Ostergaard SD, Eaton WW, Krogh J, et al. Autoimmune Diseases and Severe Infections as Risk Factors for Mood Disorders: A Nationwide Study. JAMA Psychiatry. 2013;60:812–820. doi: 10.1001/jamapsychiatry.2013.1111. [DOI] [PubMed] [Google Scholar]
- 57.Benros ME, Eaton WW, Mortensen PB. The epidemiologic evidence linking autoimmune diseases and psychosis. Biol Psychiatry. 2014;75:300–306. doi: 10.1016/j.biopsych.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson RR, Prentice TW, Bridegam P, Young CR, Steelman AJ, Welsh TH, et al. Social stress alters the severity and onset of the chronic phase of Theiler’s virus infection. J Neuroimmunol. 2006;175:39–51. doi: 10.1016/j.jneuroim.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 59.Welsh CJ, Steelman AJ, Mi W, Young CR, Storts R, Welsh TH, Jr, et al. Neuroimmune interactions in a model of multiple sclerosis. Ann N Y Acad Sci. 2009;1153:209–219. doi: 10.1111/j.1749-6632.2008.03984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dhabhar FS. Effects of stress on immune function: the good, the bad, and the beautiful. Immunol Res. 2014;58:193–210. doi: 10.1007/s12026-014-8517-0. [DOI] [PubMed] [Google Scholar]
- 61.Weiss DB, Dyrud J, House RM, Beresford TP. Psychiatric manifestations of autoimmune disorders. Current Treat Options Neurol. 2005;7:413–417. doi: 10.1007/s11940-005-0033-z. [DOI] [PubMed] [Google Scholar]
- 62.Gold PB, Frueh BC. Compensation-seeking and extreme exaggeration of psychopathology among combat veterans evaluated for posttraumatic stress disorder. J Nerv Ment Dis. 1999;187:680–684. doi: 10.1097/00005053-199911000-00005. [DOI] [PubMed] [Google Scholar]
- 63.McNally RJ, Frueh BC. Why are Iraq and Afghanistan War veterans seeking PTSD disability compensation at unprecedented rates? J Anxiety Disord. 2013;27:520–526. doi: 10.1016/j.janxdis.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 64.Spitzer C, Barnow S, Volzke H, John U, Freyberger HJ, Grabe HJ. Trauma, posttraumatic stress disorder, and physical illness: findings from the general population. Psychosom Med. 2009;71:1012–1017. doi: 10.1097/PSY.0b013e3181bc76b5. [DOI] [PubMed] [Google Scholar]
- 65.Boscarino JA. A prospective study of PTSD and early-age heart disease mortality among Vietnam veterans: implications for surveillance and prevention. Psychosom Med. 2008;70:668–676. doi: 10.1097/PSY.0b013e31817bccaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.