Abstract
Multistep processes likely underlie cholangiocarcinogenesis induced by chronic infection with the fish-borne liver fluke, Opisthorchis viverrini. One process appears to be cellular proliferation of the host bile duct epithelia driven by excretory-secretory (ES) products of this pathogen. Specifically, the secreted growth factor Ov-GRN-1, a liver fluke granulin, is a prominent component of ES and a known driver of hyper-proliferation of cultured human and mouse cells in vitro. We show potent hyper-proliferation of human cholangiocytes induced by low nanomolar levels of recombinant Ov-GRN-1 and similar growth produced by low microgram concentrations of ES products and soluble lysates of the adult worm. To further explore the influence of Ov-GRN-1 on the flukes and the host cells, expression of Ov-grn-1 was repressed using RNA interference. Expression of Ov-grn-1 was suppressed by 95% by day 3 and by ~100% by day 7. Co-culture of Ov-grn-1 suppressed flukes with human cholangiocyte (H-69) or human cholangiocarcinoma (KKU-M214) cell lines retarded cell hyper-proliferation by 25% and 92%, respectively. Intriguingly, flukes in which expression of Ov-grn-1 was repressed were less viable in culture, suggesting that Ov-GRN-1 is an essential growth factor for survival of the adult stage of O. viverrini survival, at least in vitro. To summarize, specific knock down of Ov-grn-1 reduced in vitro survival and capacity of ES products to drive host cell proliferation. These findings may help to contribute to a deeper understanding of liver fluke induced cholangiocarcinogenesis.
Keywords: O. viverrini, cholangiocarcinoma, RNA interference, granulin, liver fluke, cellular proliferation
1. Introduction
The International Agency for Research on Cancer recognizes infection with three helminth parasites as definitive causes of cancer as members of its Group 1 Carcinogens - the liver flukes Opisthorchis viverrini and Clonorchis sinensis and the blood fluke Schistosoma haematobium (Bouvard et al., 2009, de Martel et al., 2012, IARC, 2012). Liver fluke infection is of major public health concern in East Asia and Eastern Europe, where more than 40 million people are infected. O. viverrini infection is endemic in Thailand, Laos, Vietnam and Cambodia (Sithithaworn et al., 2012, Sripa et al., 2011). People acquire opisthorchiasis following ingestion of raw cyprinid fishes containing the metacercarial stage of O. viverrini (Sripa, 2012). Infected people may not exhibit clinical signs and symptoms; however, 5-10% of patients with chronic infection will progress to hepatobiliary disease including obstructive jaundice, hepatomegaly, cholecystitis, and/or cholangiocarcinoma (CCA) (bile duct cancer) (Smout et al., 2011, Sripa et al., 2012, Sripa et al., 2007). CCA is the eminent type of liver cancer in Thailand and Laos where consumption of uncooked fishes harboring the infectious larvae of this liver fluke is a dietary staple (Mairiang et al., 2012, Sripa and Pairojkul, 2008). Problematically, whereas the strong link between opisthorchiasis and CCA has been known for decades, the incidence of CCA remains high (Mairiang et al., 2012, Sripa et al., 2007). In resource limited settings such as rural Thailand and Laos, medical and surgical options are limited for CCA, and these options and care are complicated (Malhi and Gores, 2006, Razumilava and Gores, 2014).
Cancer of the bile ducts induced by infection with O. viverrini is thought to be a culmination of multi-factorial chronic inflammation and related disease processes (Plieskatt et al., 2013, Porta et al., 2011, Sripa et al., 2012). Of particular relevance to this study is the role of O. viverrini excretory-secretory products (ES) in establishing a carcinogenic microenvironment, including stimulating cellular proliferation of cholangiocytes lining the infected bile ducts (Smout et al., 2011, Sripa et al., 2007, Thuwajit et al., 2004). ES from O. viverrini stimulates the proliferation of both NIH-3T3 fibroblasts (Thuwajit et al., 2004) and the human KKU-100 cell line from a liver-fluke induced CCA (Smout et al., 2009). Using proteomic and transcriptomic approaches, O. viverrini ES and surface membrane proteins were characterized and shown to include a complex mixture of proteins, some of which have been associated with cancers, including proteases, protease inhibitors, orthologues of mammalian growth factors and anti-apoptotic proteins (Laha et al., 2007, Mulvenna et al., 2010). Of particular note was the identification of Ov-GRN-1, a protein with sequence similarity to the mammalian growth factor, granulin (Mulvenna et al., 2010, Smout et al., 2009). Ov-GRN-1 was expressed in diverse organs and tissues of the liver fluke, was detected on adjacent biliary epithelial cells of experimentally infected hamsters, and antibodies raised to recombinant Ov-GRN-1 inhibited ES of O. viverrini from driving cellular proliferation (Smout et al., 2009). Hence it has become clear that Ov-GRN-1 is the major factor of ES, at large, that induces cell proliferation and, ultimately, likely promotes cholangiocarcinogenesis.
RNA interference (RNAi) is a natural mechanism involved with silencing the expression of genes to control homeostasis and other mechanisms (Shuey et al., 2002). This phenomenon has been co-opted in the laboratory in functional genomic approaches to analyze gene function in numerous eukaryotic species, including parasitic worms (Knox, 2012). Indeed, we successfully deployed RNAi to investigate genes in liver flukes (Rinaldi et al., 2008, Thanasuwan et al., 2014). It is notable, also, that suppression of human granulin-epithelin precursor by RNAi inhibits growth of hepatocellular carcinoma cells and shows promise as a treatment for this disease (Park et al., 2011). To further explore the role of Ov-GRN-1 in pathogenesis of opisthorchiasis, here we used RNAi to suppress the expression of Ov-grn-1 in adult flukes, which lead to decreased viability of cultured flukes and compromised ability of ES to stimulate proliferation of cholangiocytes and related tumor cells.
2. Materials and methods
2.1. Opisthorchis viverrini
Metacercariae of O. viverrini were recovered from flesh of naturally infected fishes, as described (Laha et al., 2007, Sithithaworn et al., 1997). Syrian hamsters (Mesocricetus auratas) were infected with 50 metacercariae each via an intragastric tube (Sripa and Kaewkes, 2002). Hamsters were reared at the animal facility, Faculty of Medicine, Khon Kaen University. Study design protocols and standard operating procedures were approved by the Animal Ethics Committee of Khon Kaen University according to the Ethics of Animal Experimentation of the National Research Council of Thailand, approval number AEKKU43/2555. Hamsters were euthanized at six weeks after infection, at which point adult worms were recovered from the in gall bladders and bile ducts.
2.2. O. viverrini excretory-secretory products (ES) and lysate of adult worms
A soluble lysate of adult worms, which we termed somatic adult worm extract (SAE) was prepared from frozen and homogenized adult worms (Smout et al., 2009). ES was prepared from viable adult worms washed in antibiotics and incubated in modified RPMI-1640 (Life Technologies) at 37°C/5% CO2 and the supernatant harvested daily for three days and stored at −80°C (Sripa and Kaewkes, 2000).
2.3. Recombinant protein production
Purification of Ov-GRN-1 recombinant protein was as our previously optimized protocol using BL21 Escherichia coli bacterial insoluble expression with refolding followed by pure monomer isolation (Smout et al., 2011).
2.4. Human cell culture
The non-malignant cholangiocyte cell line H69 is a SV40-transformed human bile duct epithelial cell line originally derived from a normal liver harvested for transplantation (Grubman et al., 1994, Reid and Jefferson, 1984) and was obtained in 2010 from Dr. Gregory J. Gores, Mayo Clinic, Rochester, Minnesota. H69s were grown under similar conditions with growth factor supplemented specialized complete media (Matsumura et al., 2004); DMEM/F12 with high glucose, 10% fetal bovine serum (FBS), 1x antibiotic/antimycotic, 25 μg/ml adenine, 5 μg/ml insulin, 1 μg/ml epinephrine, 8.3 μg/ml holo-transferrin, 0.62 μg/ml, hydrocortisone, 13.6 ng/ml T3 and 10 ng/ml EGF (Life Technologies). H69 cells were grown in complete medium as described (Grubman et al., 1994). The KKU-M214 cell line, derived from a moderately differentiated type human CCA was cultured in T75cm2 vented monolayer flasks (Corning) in complete media [RPMI 1640 (Sigma) with 10% FBS and 1x antibiotic/antimycotic as previously described (Sripa et al., 2005).
Cell lines were incubated in 5% CO2 at 37°C, and Trypan blue staining was used to determine cell viability. Cells were gown to ~80% confluence for further experiments. All growth assays for cells were performed in low nutrient media-5% complete media. For H69s this is 0.5% with FCS with 1/20th of the previously listed growth factors for complete media. For the KKU-214 cell lines, this was RPMI 1640 medium with 0.5% with FCS.
2.5. Growth assays with xCELLigence
Cells were seeded at 3,000 cells/well in 200 μl of complete media (above) in E-plates (ACEA Biosciences, San Diego, California) and grown overnight while monitored with an xCELLigence SP system (ACEA Biosciences) which monitors cellular events in real time by measuring electrical impedance across inter-digitated gold micro-electrodes integrated on the bottom of tissue culture plates (Ke et al., 2011). Cells were washed three times with PBS and replaced with 180 μl of relevant low nutrient media and incubated for a minimum of six hours before further treatments. Treatments were prepared at 10× concentration in low nutrient media and added to a final in-well concentration of 1× (i.e., 20 μl into each well). The xCELLigence system recorded cell index (CI) readings hourly for six days post treatment. GraphPad Prism 6.02 was used for cubic or quadratic non-linear best fit regression (intercept constrained to 1) with least squares fit with comparisons between treatments and TRX controls performed with extra sum of squares F test with P values of ≤ 0.05 and P ≤ 0.001 deemed significant and highly significant, respectively.
2.6. Ov-grn-1 gene and double-strand RNAs (dsRNAs) synthesis
The fragment of Ov-grn-1of 285 bp (spanning nt no. 49-333, GenBank accession FJ436341) was amplified by PCR from a cDNA library assembled from adult flukes (Laha et al., 2007). The target locus was amplified using specific primers flanked with T7 RNA polymerase promoter sequence (indicated in bold and italicized font) at the 5’ ends; Ov-dsRNA-T7-Gra-F, 5’-TAATACGACTCACTATAGGGGTGTTGACGGTGATTTCAC-3’ as forward primer and Ov dsRNA-T7-Gra-R, 5’-TAATACGACTCACTATAGGGCTTTCGAGCGTTGAGCATAA-3’) as reverse primer. PCR reactions were carried out as follows: 100 ng of O. viverrini cDNA library as template, 0.2 mM dNTP, 1.5 mM MgCl2 performed with 1 unit Taq polymerase (Invitrogen, Germany) with 35 cycles of denaturation at 95°C for 30 sec, annealing at 55°C for 30 sec, extension at 72°C for 45 sec and a final extension at 72°C for 10 min. PCR products were identified by agarose gel electrophoresis and purified by gel extraction kit (Fermentas, EU). PCR products were ligated into the pGEM-T Easy vector (Promega, USA), after which Escherichia coli JM109 competent cells (Promega) were transformed with the ligation products. Recombinant clones were screened on ampicillin with blue/white selection. White colonies were grown in LB, and positive inserts identified by PCR using primers corresponding to the multiple cloning site promoter sequences, T7 and SP6. The extracted plasmids were subjected to sequencing using the BigDye terminator method (1st BASE, Singapore).
To synthesize Ov-grn-1double stranded RNA (dsRNA), amplicons were generated by PCR from the pGEM-T plasmid clone of Ov-grn-1 (above). The PCR conditions were 35 cycles of denaturation at 95°C for 30 sec, annealing at 55°C for 30 sec, extension at 72°C for 45 sec and a final extension at 72°C for 10 min. A 285 bp fragment of Ov-grn-1 (as above) was generated by in vitro transcription using specific primers tailed with the T7 RNA polymerase promoter sequence Ov-dsRNA-T7-Gra-F and Ov-dsRNA-T7-Gra-R (described above). An irrelevant negative control firefly luciferase (Luc) dsRNA derived from pGL3 was also produced (Promega, USA). Luc dsRNA was amplified using primers ds-LUC_T7-F5’-TAATACGACTCACTATAGGGTGCGCCCGCGAACGACATTTA and ds-LUC_T7-R5’-TAATACGACTCACTATAGGGGCAACCGCTTCCCCGACTTCCTTA (Rinaldi et al., 2008). The PCR products were in vitro single transcribed to produce dsRNAs and purified using the Megascript RNAi kit (Ambion, USA) accorded to the manufacturer's instructions. Integrities of the dsRNAs were assessed on a 1.5% agarose gel and concentrations determined by spectrophotometer (NanoVue, GE Healthcare, USA).
2.7. Delivery of Ov-grn-1 dsRNA into O. viverrini adult flukes
Adult stage O. viverrini flukes from hamsters were washed several times with sterile normal saline and then washed five times in DMEM (Gibco, USA) containing 2× antibiotics. Flukes were incubated for 1 hour at 37°C in 5% CO2 atmosphere with DMEM containing 2× antibiotics. The flukes were resuspended in 100 μl of DMEM plus 1x antibiotic and 50 μg of Ov-grn-1 dsRNA in 4 mm gap cuvettes (Bio-Rad, Hercules, CA, USA) and subjected to a single pulse of square wave electroporation at 125 V for 20 milliseconds (Electroporator Gene Pulser Xcell, Bio-Rad). The parasites were soaked with 50 μg of Ov-grn-1 dsRNA in 10 ml of 1× DMEM for two days and maintained at 37°C and 5% CO2 in air before downstream processing.
2.8. RNA extraction and quantitative real time-PCR
To determine RNA expression levels, Ov-grn-1 dsRNA treated worms (n = 30) were cultured in RPMI-1640 supplemented with 1x antibiotic and antimycotic, and single worms in triplicate were harvested at days 1, 2, 3, 5 and 7 to evaluate the endogenous expression of Ov grn-1 mRNA. Flukes that were not exposed to dsRNA but were electroporated in media served as mock controls, and worms exposed to Luc dsRNA were used as negative, irrelevant controls. Total RNA of flukes was extracted with TRIZOL (Invitrogen, USA) following the manufacturer's instructions, and contaminating genomic DNA was removed by treatment with DNase I (Thermo-scientific, EU). Concentrations of RNA were determined with a spectrophotometer. cDNAs were synthesized from 1 μg of adult RNA using a cDNA synthesis kit (Fermentas). Ov-grn-1 cDNA was amplified using specific primers as follows: forward primer, Ov-GRN-EXF: 5'-GGGATCGGTTAGTCTAATCTCC-3', and reverse primer, Ov-GRN EXR: 5'-GATCATGGGGGTTCACTGTC) (spanning Ov-grn-1 coding DNA position 6-365) amplifying a 359 bp product which is larger than the dsRNA used for electroporation and therefore cannot be amplified from exogenous dsRNA. The endogenous actin gene (GenBank AY005475) was used as a housekeeping control (Piratae et al., 2012). Real time PCR was carried out using an ABI7500 thermal cycler and the SYBR Green assay. PCR reactions consisted of 12.5 μl of SYBR Green Master Mix (TAKARA Perfect Real-time Kit),0.5 μl (10 mM) each of forward and reverse primers, 0.5 μl of reference Dye (ROX), 1 μl (equivalent to 50 ng of total RNA) of first-stand cDNA and water to a final volume of 25 μl. PCR cycling conditions consisted of initiation with pre-heat for one cycle at 95°C for 10 min followed by 40 cycles of denaturation at 95°C for30 sec, annealing at 55°C for 30 sec, extension at72°C for 45 sec, and a final extension at 72°C for 10 min. The endogenous Ov-grn-1 mRNA levels were normalized with actin mRNA. Data is presented as the unit value of 2-ΔΔCt where ΔΔCt = ΔCt (treated worms)-ΔCt (non-treated worms); ΔCt of each group = Ctgrn 1,2,3 – Ctactin(average). In addition, RT PCR products amplified from total RNA of adult flukes were run on 1.5% agarose gels in order to visually assess the integrity of the bands.
2.9. Viability of parasites
To determine the viability of parasites during in vitro culture, twenty worms treated with Ov-grn-1 dsRNA were cultured in DMEM containing 1x antibiotic; media were changed every two days. Viability of flukes was determined on day 5 by observing movement of oral or ventral suckers using light microscopy in the presence of the vital dye, Trypan blue at 0.1% in PBS for 10 min at 37°C, 5% CO2. Flukes were scored as dead if the worm stained blue, or as alive if not stained or stained weakly with Trypan blue and the oral or ventral suckers was still moving (alive), or dead, if no movement was apparent during observation for 10 min duration (Gold, 1997, Rinaldi et al., 2012). Worm survival among treatment groups was compared using independent t-tests.
2.10. Cell proliferation assay using a non-contact co-culture technique
To evaluate the effect on cell proliferation of suppressing Ov-grn-1, viable O. viverrini adult flukes that had been electroporated once and soaked with Ov-grn-1 dsRNAs for 24 hours and cultured in culture media for another day. Flukes were then co-cultured with either H-69 or KKU-M214 cells using 24-well Transwell plates with a 4 μm pore size membrane separating the upper and lower chambers (Corning, USA). Briefly, 10,000 of either H-69 or KKU-M214 cells were seeded into the lower chamber of the Transwell plate and cultured for 24 h with complete medium. Complete medium was removed and replaced with incomplete medium, which consisted of complete medium diluted to 3:20 in DMEM/F12 for H-69, and Ham's F-12 supplemented with 1% FBS for KKU-M214 for overnight and culture medium was renewed with incomplete medium prior to the next experiment. Five active and viable O. viverrini adult flukes that had been exposed to Ov-grn-1 dsRNAs were transferred to the upper chamber of each well. In addition, five adult flukes that had been electroporated/soaked with no dsRNAs were used as a mock control. Wells without flukes served as a negative control. Four replicates of treatments were conducted. The fluke ES but not the fluke itself could reach the lower chamber, which was separated from the upper chamber by a membrane a pose size of 4 μm diameter. The number of cells in each well was determined at days 1, 2 and 3 using the WST-1 cell proliferation reagent (Roche, Germany), where 10 μl of WST-1 was added to cells at 37°C for up to 30 min for KKU M214 and 2 h for H-69 cells. Absorbance at 450 nm was determined and cell number calculated from a standard curve (following the manufacturer's instructions) before converting into relative growth compared to the negative control. Independent t-tests were used to compare treatment with Ov-grn-1 dsRNA versus the mock control.
2.11. Statistical analysis
Mean and standard deviation were calculated using SPSS for windows version 21 or GraphPad Prism software; ANOVA and Student's t-test were used to compare groups. P ≤ 0.05 was considered to be statistically significant.
3. Results
3.1. Fluke proteins stimulate hyper-proliferation of human cholangiocytes
We compared the effect of Ov-GRN-1 in stimulating human cholangiocytes (H69s) using recombinant Ov-GRN-1, in comparison with a soluble lysate of worms and with ES of O. viverrini, using real time cell growth analysis using the xCELLigence system (Figure 1). All four treatments induced cellular proliferation, when compared with controls (PBS or TRX protein) (F(DFn, DFd) = 487.5-5253 (2,1478)). Cellular growth was monitored for up to 140 hours after treatment, but only up until 90 hours was used for comparisons since the low nutrient media causes control cells numbers to decline. The two controls, PBS vehicle control and the expression matched bacterial TRX, showed similar growth rate curves. It is noted that three of the treatments showed similar profiles, 12 nM Ov-GRN-1, 14 μg/ml ES of O. viverrini and lysate of worms (SAE), climbed above controls after about one day and reached 27-38% over controls at 90 h. These comparable rates of induction of proliferation allowed approximation that the proportion of Ov-GRN-1 to represent ~0.8% of the total protein in ES. This assumes that recombinant Ov-GRN-1 was fully active and that are no other factors from the flukes influenced cell growth-as shown previously by Smout et al. (Smout et al., 2009). The lower dose treatment of 3 nM Ov-GRN-1 also significantly induced growth, compared to control wells, but at a far lower rate of 13% at 90 h (F(DFn, DFd) = 487.5 (2,1478)).
Figure 1. Proteins from Opisthorchis viverrini stimulate the proliferation of human cholangiocytes in vitro.
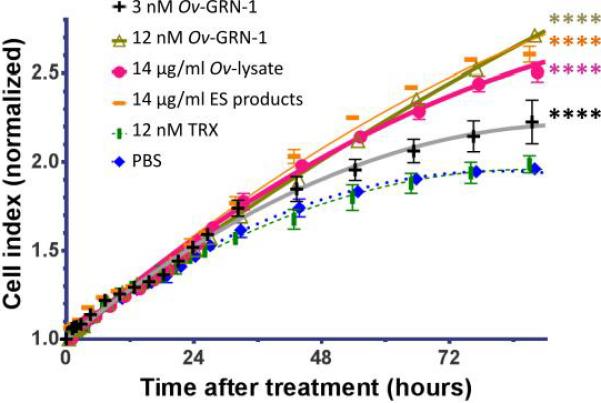
Normalized Cell Index is the output from the xCELLigence instrument. Ov-GRN-1 is produced in E. coli expressed and refolded into an active growth factor. The lysate of O. viverrini represents a soluble extract of adult Opisthorchis viverrini worms. OvES (excreted/secreted products) is the supernatant from cultured adult O. viverrini. TRX is thioredoxin from E. coli and is an expression-matched control for Ov-GRN-1. Error bars reveal the standard error of triplicate biological replicates. **** denotes statistical significance at the level of P ≤ 0.0001.
3.2. Suppression of Ov-grn-1 expression in Opisthorchis viverrini by RNAi
In order to investigate the role of Ov-grn-1 by suppressing its expression via RNAi, we subjected adult flukes to square wave electroporation of Ov-grn-1 or Luc dsRNA (or media alone) and then, in addition, soaked the worms for two days in dsRNA. Total RNA was extracted from the flukes and used as template for quantitative RT-PCR. Potent knockdown of Ov-grn-1 resulted in flukes treated with Ov-grn-1 dsRNA compared to controls (Figure 2). Significant knockdown of Ov-grn-1 gene expression compared to mock controls (P ≤ 0.001) was evident as early as 48 h after electroporation (91.4% reduced expression), which progressively increased to complete knockdown by day 7 (Figure 2). Thereafter, the majority of the flukes electroporated with Ov-grn-1 dsRNA were dead; and accordingly transcript levels were only considered for the first seven days of culture (below). A reduction in the expression of Ov-grn-1 expression in flukes that were electroporated in Luc dsRNA (Figure 2) was not seen. Moreover, differences in expression of the actin gene among the groups were not apparent (not shown). RT-PCR products amplified from total RNA of adult that were analyzed on 1.5% agarose gel and showed less intensity corresponding to knockdown level compared to actin which were not different (Figure 2). These findings confirmed the experimental RNAi silencing of Ov-grn-1 at the transcript level.
Figure 2. Gene-specific silencing of Ov-grn-1 in Opisthorchis viverrini following delivery of dsRNA by square wave electroporation.
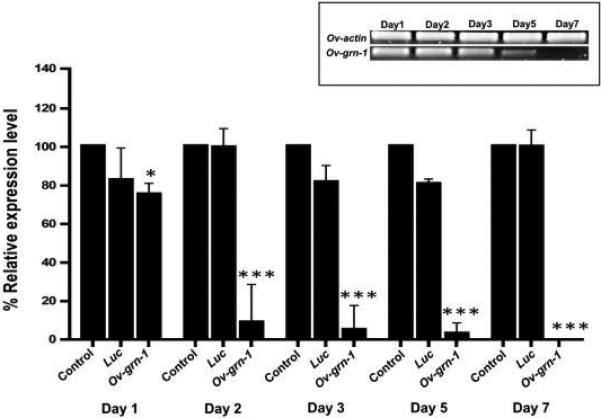
Real-time qPCR analysis revealed the transcript levels of Ov-grn-1 normalized to levels of Ov-actin in flukes after electroporation and soaking with Ov-grn-1 or luciferase (Luc) dsRNAs. Ov-grn-1 transcript levels had decreased significantly by day 1 and were not detectable by day 7. Ov-grn-1 RT-PCR products amplified from total RNA of adult flukes after electroporation with either Ov-grn-1or Luc dsRNAs (inset). Data are represented by mean ± 1 standard deviation; *** denotes statistical significance at the level of P ≤ 0.001.
3.3. Suppression of Ov-grn-1 mRNA hastened death of worms
To determine the effect of silencing the expression of Ov-grn-1 on viability of the flukes, we determined viability rates by observing motility and death by Trypan blue exclusion. Viability decreased significantly in Ov-grn-1 dsRNA-treated worms compared to the mock control group. More specifically, Ov-grn-1 dsRNA-treated flukes exhibited significantly decreased (40%) viability when compared to intact, control worms by five days of culture in standard medium (not containing dsRNAs) (Figure 3).
Figure 3. Survival rate of Opisthorchis viverrini after RNAi-based silencing of Ov-grn-1.
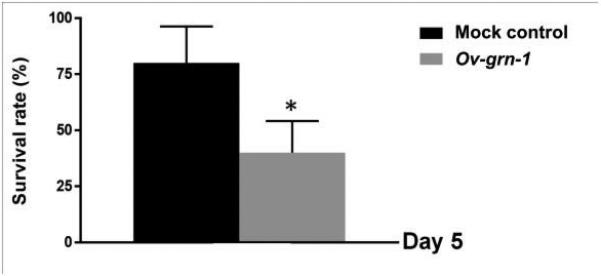
Twenty adult flukes were cultured for five days. The worms were examined the viability with Trypan blue by light microscopy. Data were represented by mean ± 1 standard deviation. Significance at the level of P ≤ 0.05 level denoted with * in a comparison with flukes not exposed to dsRNA but subjected to identical electroporation (mock).
3.4. Silencing of Ov-grn-1 results in reduced ability of O. viverrini to drive cell proliferation in Transwell co-culture
To further explore the role of Ov-GRN-1 in driving proliferation of human host cells, Ov- grn-1 knocked-down worms were co-cultured with cholangiocytes (H-69) and a line of CCA cells (KKU-M214). Compared to electroporation mock control parasites, Ov-grn-1-suppressed parasites induced far less cell proliferation of H-69 (P ≤ 0.001) and KKU-M214 (P ≤ 0.001) after as brief a period as 24 h in culture (Figure 4A and 4B). After three days of cell culture, highly significant inhibition of proliferation continued (P ≤ 0.001).
Figure 4. Proliferation of cholangiocytes repressed after exposure to Opisthorchis viverrini subjected to gene knockdown of Ov-grn-1.
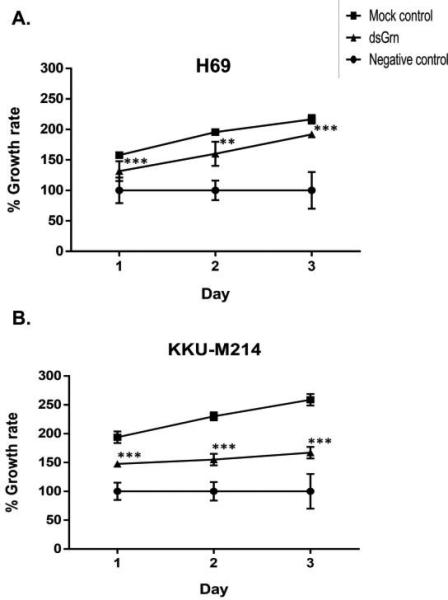
Growth expressed as the ratio of cell numbers per well between treatment and control groups (“no parasites” used as 100% negative control). The effect of specific repression of expression of Ov-grn-1 on growth of lines of immortalized cholangiocytes (H69 cell line) (panel A) and cholangiocarcinoma cells (KKU-M214 cells) (Panel B) resulted in significantly decreased proliferation compared to controls during cultivation for three days. Findings presented as the means of biological quadruplicates, with the standard deviation shown as bars. Asterisks indicate significant differences between mock control and Ov-grn-1 dsRNA treatments: ** and *** represent P ≤ 0.01 and 0.001, respectively.
4. Discussion
The mechanisms by which infection with O. viverrini results in liver disease and chronic infection can ultimately cause CCA are undoubtedly complex, involving multi-factorial mechanisms (Plieskatt et al., 2013, Sripa et al., 2012). Chronic irritation caused by O. viverrini induces inflammation of the bile ducts, resulting in hyperplasia of exposed biliary epithelial cells. The induction of cell proliferation is an important step in the genesis of cancer because hyperplastic cells are vulnerable to a carcinogen that can easily induce DNA damage during mitosis and proliferation (Watanapa and Williamson, 1993, Williamson and Rainey, 1984). The mechanisms involved in these phenomena are multifactorial, and may include 1) mechanical, where direct contact of parasites with the bile duct epithelium results in constant abrasion and irritation, and 2) direct and varied effects of ES from the flukes (Sripa et al., 2007) stimuli, among others (Porta et al., 2011). ES from O. viverrini can induce proliferation of fibroblasts (Thuwajit et al., 2004) as well as in KKU-100 human cholangiocarcinoma cells (Smout et al., 2009). ES includes a complex catalogue of proteins that have been associated with cancers, particularly the potent growth factor Ov-GRN-1 (Mulvenna et al., 2010).
Human granulin is expressed as pro-granulin, PGRN, and is a secreted glycoprotein comprised of seven tandem repeats of a 12-cysteine module called granulin A-G or epithelin domains. It is sensitive to hydrolysis by elastase, which liberates peptides of 6 kDa corresponding to individual granulin modules that maintain diverse biological activity (Bateman and Bennett, 1998, Tolkatchev et al., 2008). In addition to the granulin units initiating signal cascades, intact PGRN has key biological activities, including regulation of early embryogenesis, repair of adult tissues and modulation of inflammation. PGRN is involved in multiple steps in the tumor progression cascade, including cellular proliferation, tissue repair, and tumorigenesis (He and Bateman, 2003). For example, treatment of CCA cells with recombinant PGRN results in increased cell proliferation in vitro, and suppression of PGRN expression in CCA cells decreases the expression of proliferating cellular nuclear antigen, a marker of proliferative capacity, and retards tumor growth in vivo (Frampton et al., 2012). Moreover, PGRN promotes migration of 5637 bladder cancer cells and stimulates wound closure and invasion (Monami et al., 2006). Nonetheless, the signaling cascade is not fully understood since either or both of PGRN or the individual granulin A-G units may be responsible for initiating proliferation.
Earlier findings revealed that Ov-GRN-1 stimulates proliferation of murine fibroblasts, and proliferation is inhibited by the MAPK kinase inhibitor, U0126 (Smout et al., 2009). Antibodies raised to recombinant Ov-GRN-1 inhibit the ability of ES to induce proliferation of murine fibroblasts and a human CCA cell line in vitro, indicating that Ov-GRN-1 is the major growth factor present in O. viverrini ES products (Smout et al., 2009). In the present study, we silenced the expression of the Ov-grn-1 gene in adult flukes using RNA interference, and noted a suppression of mitogenicity in the ES products of the fluke. An intriguing finding was the increased lethality that follows silencing of Ov-grn-1. This implies that Ov-GRN-1 acts as a growth factor for both host cells and the fluke itself. Indeed, to our knowledge, this is the first report describing the silencing of a growth factor gene in a parasitic helminth. The ubiquitous expression of Ov-GRN-1 in organs and tissues of adult O. viverrini (Smout et al., 2009) supports the notion that this fluke mitogen functions as an essential, endogenous growth factor for O. viverrini. It is not unexpected that Ov-GRN-1 functions as a growth factor for both the liver fluke and its host cells since granulins evolved in all branches of the animal kingdom and are known to promote proliferation of different cell types from phylogenetically distant taxa (Hanington et al., 2008). Future studies will explore the effects of introducing Ov-grn-1 dsRNA-treated metacercariae (the infectious stage to mammals) to hamsters to assess survival in vivo.
In terms of infiltration of dsRNAs throughout fluke tissues during the RNAi process, notably, electroporation with as little as 50 μg of dsRNA per 100 μl of media followed by soaking in 50 μg/10 ml for two days was sufficient to see significant knock down of Ov-grn-1 in adult flukes, and resulted in death of some flukes by five days of culture. Andrew et al (1998) reported that only a few molecules of injected double-stranded RNA were required per affected cell, arguing against stoichiometric interference with endogenous mRNA and suggesting that there could be a catalytic or amplification component in the interference process (Fire et al., 1998). Previously, electroporation of Cy3-siRNA into adult O. viverrini revealed the location of the reporter siRNA throughout the parenchyma of transduced worms, with accumulation in the gut (Sripa et al., 2011). Furthermore, Ov-GRN-1 was detected in fluke gut tissue and ES products, as well as on the surface of biliary epithelial cells of hamsters that were experimentally infected with O. viverrini (Smout et al., 2009), underscoring extracellular roles for the protein at the host-parasite interface. Taken together, we suggest that dsRNAs introduced into O. viverrini adults enter into apertures on the body of the fluke including the tegument, genital pore and mouth from where they pass through the gut. Presumably the affected cells amplify the component in the interference process, interfering with target gene expression.
In the human liver, differentiated cholangiocytes are mitotically dormant (Priester et al., 2010). Cholangiocyte proliferation may include some combination of proliferation of pre existing ductules, progenitor cell activation, and appearance of intermediate hepatocytes (Priester et al., 2010). Proliferation of cholangiocytes induced by the fluke infection may serve to compensate for cells damaged by the fluke and maintain secretory function (Leite and Nathanson, 2005). Therefore, inhibiting the activity of Ov-GRN-1 may decrease excess cell proliferation and potentially inhibit the development of biliary fibrosis or malignancy (Ho et al., 2008, Smout et al., 2009). Hence, Ov-GRN-1 may be plausible target of a potential anti-parasitic and/or anti-pathogenic vaccine to control the opisthorchiasis and opisthorchiasis-induced cholangiocarcinoma.
HIGHLIGHTS.
>Granulin of Opisthorchis viverrini is susceptible to experimental RNAinterference
>Suppression of granulin reduced ability of ES products to drive cell proliferation
>Knock down of granulin hastened death of cultured liver flukes
Acknowledgements
This work was supported by The Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, through the Health Cluster (SHeP-GMS), Khon Kaen University to AP and TL, a Tropical Medicine Research Center award number P50AI098639 (TL, BS) from the National Institute of Allergy and Infectious Diseases (NIAID), and award number R01CA CA164719 (AL, PJB, TL) from the National Cancer Institute (NCI), US National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID, the NCI or the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bateman A, Bennett HP. Granulins: the structure and function of an emerging family of growth factors. J Endocrinol. 1998;158:145–151. doi: 10.1677/joe.0.1580145. [DOI] [PubMed] [Google Scholar]
- Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009;10:321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, Plummer M. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607–615. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Frampton G, Invernizzi P, Bernuzzi F, Pae HY, Quinn M, Horvat D, Galindo C, Huang L, McMillin M, Cooper B. Interleukin-6-driven progranulin expression increases cholangiocarcinoma growth by an Akt-dependent mechanism. Gut. 2012;61:268–277. doi: 10.1136/gutjnl-2011-300643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold D. Assessment of the viability of Schistosoma mansoni schistosomula by comparative uptake of various vital dyes. Parasitol Res. 1997;83:163–169. doi: 10.1007/s004360050227. [DOI] [PubMed] [Google Scholar]
- Grubman SA, Perrone RD, Lee DW, Murray SL, Rogers LC, Wolkoff LI, Mulberg AE, Cherington V, Jefferson DM. Regulation of intracellular pH by immortalized human intrahepatic biliary epithelial cell lines. Am J Physiol. 1994;266:G1060–1070. doi: 10.1152/ajpgi.1994.266.6.G1060. [DOI] [PubMed] [Google Scholar]
- Hanington PC, Brennan LJ, Belosevic M, Andrew Keddie B. Molecular and functional characterization of granulin-like molecules of insects. Insect Biochem Mol Biol. 2008;38:596–603. doi: 10.1016/j.ibmb.2008.02.002. [DOI] [PubMed] [Google Scholar]
- He Z, Bateman A. Progranulin (granulin-epithelin precursor, PC-cell-derived growth factor, acrogranin) mediates tissue repair and tumorigenesis. J Mol Med (Berl) 2003;81:600–612. doi: 10.1007/s00109-003-0474-3. [DOI] [PubMed] [Google Scholar]
- Ho JC, Ip YC, Cheung ST, Lee YT, Chan KF, Wong SY, Fan ST. Granulin-epithelin precursor as a therapeutic target for hepatocellular carcinoma. Hepatology. 2008;47:1524–1532. doi: 10.1002/hep.22191. [DOI] [PubMed] [Google Scholar]
- IARC Biological agents. Volume 100 B. A review of human carcinogens. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 2012;100:1–441. [PMC free article] [PubMed] [Google Scholar]
- Ke N, Wang X, Xu X, Abassi YA. The xCELLigence system for real-time and label-free monitoring of cell viability. Methods Mol Biol. 2011;740:33–43. doi: 10.1007/978-1-61779-108-6_6. [DOI] [PubMed] [Google Scholar]
- Knox DP. Developments in RNA interference and genetic transformation to define gene function in parasitic helminths. Parasitology. 2012;139:557–559. doi: 10.1017/S0031182012000108. [DOI] [PubMed] [Google Scholar]
- Laha T, Pinlaor P, Mulvenna J, Sripa B, Sripa M, Smout MJ, Gasser RB, Brindley PJ, Loukas A. Gene discovery for the carcinogenic human liver fluke, Opisthorchis viverrini. BMC Genomics. 2007;8:189. doi: 10.1186/1471-2164-8-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite MF, Nathanson MH. Signaling Pathways in Biliary Epithelial Cells. Signaling Pathways in Liver Diseases; Springer: 2005. pp. 17–26. [Google Scholar]
- Mairiang E, Laha T, Bethony JM, Thinkhamrop B, Kaewkes S, Sithithaworn P, Tesana S, Loukas A, Brindley PJ, Sripa B. Ultrasonography assessment of hepatobiliary abnormalities in 3359 subjects with Opisthorchis viverrini infection in endemic areas of Thailand. Parasitol Int. 2012;61:208–211. doi: 10.1016/j.parint.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi H, Gores GJ. Cholangiocarcinoma: modern advances in understanding a deadly old disease. J Hepatol. 2006;45:856–867. doi: 10.1016/j.jhep.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura T, Takesue M, Westerman KA, Okitsu T, Sakaguchi M, Fukazawa T, Totsugawa T, Noguchi H, Yamamoto S, Stolz DB, Tanaka N, Leboulch P, Kobayashi N. Establishment of an immortalized human-liver endothelial cell line with SV40T and hTERT. Transplantation. 2004;77:1357–1365. doi: 10.1097/01.tp.0000124286.82961.7e. [DOI] [PubMed] [Google Scholar]
- Monami G, Gonzalez EM, Hellman M, Gomella LG, Baffa R, Iozzo RV, Morrione A. Proepithelin promotes migration and invasion of 5637 bladder cancer cells through the activation of ERK1/2 and the formation of a paxillin/FAK/ERK complex. Cancer Res. 2006;66:7103–7110. doi: 10.1158/0008-5472.CAN-06-0633. [DOI] [PubMed] [Google Scholar]
- Mulvenna J, Sripa B, Brindley PJ, Gorman J, Jones MK, Colgrave ML, Jones A, Nawaratna S, Laha T, Suttiprapa S, Smout MJ, Loukas A. The secreted and surface proteomes of the adult stage of the carcinogenic human liver fluke Opisthorchis viverrini. Proteomics. 2010;10:1063–1078. doi: 10.1002/pmic.200900393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MY, Park YS, Nam JH. RNA interference against granulin-epithelin precursor prevents hepatocellular carcinoma growth: its application as a therapeutic agent. Int J Oncol. 2011;39:853–861. doi: 10.3892/ijo.2011.1095. [DOI] [PubMed] [Google Scholar]
- Piratae S, Tesana S, Jones MK, Brindley PJ, Loukas A, Lovas E, Eursitthichai V, Sripa B, Thanasuwan S, Laha T. Molecular characterization of a tetraspanin from the human liver fluke, Opisthorchis viverrini. PLoS Negl Trop Dis. 2012;6:e1939. doi: 10.1371/journal.pntd.0001939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plieskatt JL, Deenonpoe R, Mulvenna JP, Krause L, Sripa B, Bethony JM, Brindley PJ. Infection with the carcinogenic liver fluke Opisthorchis viverrini modifies intestinal and biliary microbiome. FASEB J. 2013;27:4572–4584. doi: 10.1096/fj.13-232751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porta C, Riboldi E, Sica A. Mechanisms linking pathogens-associated inflammation and cancer. Cancer Lett. 2011;305:250–262. doi: 10.1016/j.canlet.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Priester S, Wise C, Glaser SS. Involvement of cholangiocyte proliferation in biliary fibrosis. World journal of gastrointestinal pathophysiology. 2010;1:30. doi: 10.4291/wjgp.v1.i2.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168–2179. doi: 10.1016/S0140-6736(13)61903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid LM, Jefferson DM. Culturing hepatocytes and other differentiated cells. Hepatology. 1984;4:548–559. doi: 10.1002/hep.1840040332. [DOI] [PubMed] [Google Scholar]
- Rinaldi G, Morales ME, Cancela M, Castillo E, Brindley PJ, Tort JF. Development of functional genomic tools in trematodes: RNA interference and luciferase reporter gene activity in Fasciola hepatica. PLoS Negl Trop Dis. 2008;2:e260. doi: 10.1371/journal.pntd.0000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi G, Suttiprapa S, Tort JF, Folley AE, Skinner DE, Brindley PJ. An antibiotic selection marker for schistosome transgenesis. Int J Parasitol. 2012;42:123–130. doi: 10.1016/j.ijpara.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuey DJ, McCallus DE, Giordano T. RNAi: gene-silencing in therapeutic intervention. Drug Discov Today. 2002;7:1040–1046. doi: 10.1016/s1359-6446(02)02474-1. [DOI] [PubMed] [Google Scholar]
- Sithithaworn P, Andrews RH, Nguyen VD, Wongsaroj T, Sinuon M, Odermatt P, Nawa Y, Liang S, Brindley PJ, Sripa B. The current status of opisthorchiasis and clonorchiasis in the Mekong Basin. Parasitol Int. 2012;61:10–16. doi: 10.1016/j.parint.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sithithaworn P, Pipitgool V, Srisawangwong T, Elkins DB, Haswell-Elkins MR. Seasonal variation of Opisthorchis viverrini infection in cyprinoid fish in north-east Thailand: implications for parasite control and food safety. Bull World Health Organ. 1997;75:125–131. [PMC free article] [PubMed] [Google Scholar]
- Smout MJ, Laha T, Mulvenna J, Sripa B, Suttiprapa S, Jones A, Brindley PJ, Loukas A. A granulin-like growth factor secreted by the carcinogenic liver fluke, Opisthorchis viverrini, promotes proliferation of host cells. PLoS Pathog. 2009;5:e1000611. doi: 10.1371/journal.ppat.1000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smout MJ, Mulvenna JP, Jones MK, Loukas A. Expression, refolding and purification of Ov-GRN-1, a granulin-like growth factor from the carcinogenic liver fluke, that causes proliferation of mammalian host cells. Protein Expression and Purification. 2011;79:263–270. doi: 10.1016/j.pep.2011.06.018. [DOI] [PubMed] [Google Scholar]
- Smout MJ, Sripa B, Laha T, Mulvenna J, Gasser RB, Young ND, Bethony JM, Brindley PJ, Loukas A. Infection with the carcinogenic human liver fluke, Opisthorchis viverrini. Mol Biosyst. 2011;7:1367–1375. doi: 10.1039/c0mb00295j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripa B. Global burden of food-borne trematodiasis. Lancet Infect Dis. 2012;12:171–172. doi: 10.1016/S1473-3099(11)70321-8. [DOI] [PubMed] [Google Scholar]
- Sripa B, Bethony JM, Sithithaworn P, Kaewkes S, Mairiang E, Loukas A, Mulvenna J, Laha T, Hotez PJ, Brindley PJ. Opisthorchiasis and Opisthorchis-associated cholangiocarcinoma in Thailand and Laos. Acta Trop. 2011;120(Suppl 1):S158–168. doi: 10.1016/j.actatropica.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripa B, Brindley PJ, Mulvenna J, Laha T, Smout MJ, Mairiang E, Bethony JM, Loukas A. The tumorigenic liver fluke Opisthorchis viverrini--multiple pathways to cancer. Trends Parasitol. 2012;28:395–407. doi: 10.1016/j.pt.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripa B, Kaewkes S. Relationship between parasite-specific antibody responses and intensity of Opisthorchis viverrini infection in hamsters. Parasite Immunology. 2000;22:139–145. doi: 10.1046/j.1365-3024.2000.00286.x. [DOI] [PubMed] [Google Scholar]
- Sripa B, Kaewkes S. Gall bladder and extrahepatic bile duct changes in Opisthorchis viverrini-infected hamsters. Acta Trop. 2002;83:29–36. doi: 10.1016/s0001-706x(02)00052-9. [DOI] [PubMed] [Google Scholar]
- Sripa B, Kaewkes S, Sithithaworn P, Mairiang E, Laha T, Smout M, Pairojkul C, Bhudhisawasdi V, Tesana S, Thinkamrop B, Bethony JM, Loukas A, Brindley PJ. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007;4:e201. doi: 10.1371/journal.pmed.0040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripa B, Leungwattanawanit S, Nitta T, Wongkham C, Bhudhisawasdi V, Puapairoj A, Sripa C, Miwa M. Establishment and characterization of an opisthorchiasis-associated cholangiocarcinoma cell line (KKU-100). World J Gastroenterol. 2005;11:3392–3397. doi: 10.3748/wjg.v11.i22.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripa B, Pairojkul C. Cholangiocarcinoma: lessons from Thailand. Curr Opin Gastroenterol. 2008;24:349–356. doi: 10.1097/MOG.0b013e3282fbf9b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripa J, Pinlaor P, Brindley PJ, Sripa B, Kaewkes S, Robinson MW, Young ND, Gasser RB, Loukas A, Laha T. RNA interference targeting cathepsin B of the carcinogenic liver fluke, Opisthorchis viverrini. Parasitol Int. 2011;60:283–288. doi: 10.1016/j.parint.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanasuwan S, Piratae S, Brindley PJ, Loukas A, Kaewkes S, Laha T. Suppression of aquaporin, a mediator of water channel control in the carcinogenic liver fluke, Opisthorchis viverrini. Parasit Vectors. 2014;7:224. doi: 10.1186/1756-3305-7-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuwajit C, Thuwajit P, Kaewkes S, Sripa B, Uchida K, Miwa M, Wongkham S. Increased cell proliferation of mouse fibroblast NIH-3T3 in vitro induced by excretory/secretory product (s) from Opisthorchis viverrini. Parasitology. 2004;129:455–464. doi: 10.1017/s0031182004005815. [DOI] [PubMed] [Google Scholar]
- Tolkatchev D, Malik S, Vinogradova A, Wang P, Chen Z, Xu P, Bennett HP, Bateman A, Ni F. Structure dissection of human progranulin identifies well-folded granulin/epithelin modules with unique functional activities. Protein Sci. 2008;17:711–724. doi: 10.1110/ps.073295308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanapa P, Williamson RC. Experimental pancreatic hyperplasia and neoplasia: effects of dietary and surgical manipulation. Br J Cancer. 1993;67:877–884. doi: 10.1038/bjc.1993.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson RC, Rainey JB. The relationship between intestinal hyperplasia and carcinogenesis. Scand J Gastroenterol Suppl. 1984;104:57–76. [PubMed] [Google Scholar]


