Abstract
Objectives
Our aim was to determine if chronic kidney disease (CKD) occurring in childhood impairs the normally vasoprotective functions of high-density lipoproteins (HDL).
Materials and Methods
HDL were isolated from children with end-stage renal disease on dialysis (ESRD), children with moderate CKD and controls with normal kidney function. Macrophage response to HDL was studied as expression of inflammatory markers (MCP-1, TNF-α, IL-1β) and chemotaxis. Human umbilical vein endothelial cells were used for expression of adhesion molecules (ICAM-1, VCAM-1, E-selectin) and adhesion. Cellular proliferation, apoptosis, and necrosis of endothelial cells was measured by MTS/PMS reagent-based assay, flow cytometry, and ELISA. Cholesterol efflux was assessed by gas chromatographic measurements of cholesterol in macrophages exposed to HDL.
Results
Compared with HDLControl, HDLCKD and HDLESRD heightened the cytokine response and disrupted macrophage chemotaxis. HDLControl reduced endothelial expression of ICAM-1, VCAM-1, E-selectin, whereas HDLCKD and HDLESRD were less effective and showed reduced capacity to protect endothelial cells against monocyte adhesion. Compared with a dramatically enhanced endothelial proliferation following injurious stimulus by HDLControl, neither HDLCKD nor HDLESRD caused proliferative effects. HDL of all three groups were equally protective against apoptosis assessed by flow cytometry and cleaved caspase-3 activity. Compared to HDLControl, HDLCKD and HDLESRD trended toward reduced capacity as cholesterol acceptors.
Conclusion
CKD in children impairs HDL function. Even in the absence of long-standing and concomitant risk factors, CKD alters specific HDL functions linked to control of inflammation and endothelial responses.
Keywords: HDL, CKD, child, macrophage, endothelial cell
1. INTRODUCTION
Chronic kidney disease (CKD) causes vascular dysfunction and increases risk of cardiovascular disease (CVD).1–6 Typically, individuals with CKD present with CVD risk factors and comorbidities (age, obesity, smoking, diabetes, and hypertension) which by themselves disrupt vascular integrity, including endothelial function, and cause CVD. The detrimental impact of CKD prevails among all ages, however, it is most pronounced in the younger age groups, 3,7,8 and thus the direct effects of CKD on CVD are best studied in pediatric populations. While in the general pediatric population CVD mortality is extremely low, more than 25% of deaths in children with CKD are due to CVD.9 Moreover, children with advanced CKD requiring dialysis who survive into adulthood live 40–50 years less than age-matched population.9 Although these observations underscore the direct impact of CKD on development of vascular complications, the pathogenesis remains unclear.
HDL have been shown to have a variety of beneficial vasoprotective actions.10–13 In addition to reverse cholesterol transport whereby HDL transfers cholesterol from the periphery to the liver for excretion, HDL has been shown to reduce inflammatory processes, limit oxidative stress, and inhibit blood clotting mechanisms.10,12,14 We and others have shown that adults with end stage renal disease (ESRD) requiring hemodialysis (HD) have a profoundly impaired HDL, which is less able to accept cholesterol from lipid-loaded macrophages and to control inflammation than HDL from normal controls.15–22 Predictably, the individuals in our study and adult subjects reported by others had high rates of CVD co-morbidities, including diabetes (40–50%); underlying CVD (30–60%); hypertension (>90%), and smoking (25%). 15–17,19 In the current study, we sought to evaluate the direct effects of CKD per se on HDL functionality. To this end we examined whether moderate or end-stage CKD in children affects HDL functions relevant to vasculopathic processes.
2. MATERIALS AND METHODS
2.1 Participants and HDL isolation
The study was approved by the institutional review board of Vanderbilt University Medical Center, and written informed consent was obtained from all participants and their parents. Forty-six children, 1–18 years of age, with chronic kidney disease (CKD stage 3–4), end-stage kidney disease requiring dialysis (CKD stage 5), or normal kidney function were recruited. The underlying causes of CKD included urological disorders and dysplasia (n=15), chronic glomerulosclerosis (n=12), hemolytic uremic syndrome (n=2), unknown (n=2). None of the children were receiving lipid lowering therapy and none had diabetes mellitus. Controls were recruited from children who had negative work-up for stones or other kidney disease. Five subjects were excluded, two samples were improperly handled and one yielded insufficient amount of HDL, one patient was maintained on hemodialysis, and a control subject turned out to have Hashimoto’s disease.
Blood samples were obtained by venipuncture collected into ethylenediaminetetraacetic acid-containing tubes, centrifuged at 1700 g for 15 min at 4°C. Aliquots were stored at −80°C for lipid/chemical determinations while the HDL fraction (d = 1.063 to 1.22 g/ml) was isolated from fresh plasma by density gradient ultracentrifugation (DGUC).23 Because of their size and small volume, we were initially concerned that the DGUR method would yield insufficient HDL fraction and began the studies using samples (three from each group) obtained by precipitating the apoB fraction with polyethylene glycol (PEG) solution as described.24 HDL isolation method did not influence results of migration and cytokine expression studies and results were combined. All other assays used HDL prepared by DGUC.
Plasma total cholesterol, triglycerides and HDL were measured enzymatically (Cliniqa, CA). High-sensitivity C-reactive protein (hsCRP) was measured by high sensitivity immunoturbidimetric assay (Roche Modular System, Indianapolis, IN).25 CKD stage was determined by calculating the estimated glomerular filtration rate (eGFR) by bedside Schwartz formula.26
2.2 Macrophage inflammatory cytokine response and chemotaxis assay
HDL modulation of inflammatory effect was measured using the established cytokine response in LPS-activated macrophages.19 Briefly, THP-1 cells (American Type Culture Collection, Manassas, VA) were plated and differentiated by RPMI 1670 containing 10% fetal bovine serum and 50 ng/ml phorbol 12-myristate 13-acetate. THP-1 macrophages were exposed to HDL (50 μg/ml) and LPS (50 ng/ml) for 4 h. Total RNA was extracted from cells with Trizol reagent (Life Technologies, Carlsbad, CA). Quantification of human interleukin (IL)-1β, monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor-α (TNF-α), and endogenous control human Euk 18S rRNA levels was performed by real-time reverse transcriptase polymerase chain reaction (PCR) using CFX96 Real-Time System (BIO-RAD, Hercules, CA). Probes for IL-1β (Hs99999029_m1), MCP-1 (Hs00234140_m1), TNF-α (Hs99999043_m1), and 18S rRNA were obtained from Applied Biosystems (Foster City, CA).
Macrophage migration studies were performed in a microchemotaxis chamber as described.19 THP-1 cells (5×105 /well) were exposed to HDL (50 μg/ml) for 1 h and then added to the upper compartment. MCP-1 0.1 μg/ml (Peprotech, Rocky Hill, NJ) was added to the lower compartment. Filters were fixed in methanol and stained with 1% crystal violet. Duplicate wells were used for each experimental condition, and > 5 fields (×40) were counted for each well in a blinded fashion.
2.3 Macrophage cholesterol efflux to HDL
THP-1 cells were plated and differentiated into macrophages then enriched with acetylated-low density lipoprotein (LDL) (100 μg/ml, Intracel, Frederick, MA) and exposed to HDL (50 μg/ml) and LPS (50 ng/ml) for 24 h.19 Cholesterol content was measured by the most demanding and rigorous gas chromatography method 23,27 and cholesterol efflux determined as the percent at baseline versus after incubation with HDL.28 Cell protein content was measured by bicinchoninic acid assay.
2.4 Endothelial cell adhesion assays
Assessment of endothelial cell adhesion molecules was performed in HUVEC (American Type Culture Collection, Manassas, VA) grown on 0.1% gelatin coated plates and exposed to HDL (50 μg/ml) and LPS (1μg/ml) for 5 h.29 Quantification of VCAM-1, ICAM-1, E-selectin and endogenous control human GAPDH rRNA levels was performed by real-time PCR. Probes for ICAM-1(forward: 5’-CCACAGTCACCTATGGCAAC, Reverse: 5’-AGTGTCTCCTGGCTCTGGTT), VCAM-1(forward: 5’-GCTTCAGGAGCTGAATACCC, Reverse: 5’-AAGGATCACGACCATCTTCC), E selectin (forward: 5’-TGAACCCAACAATAGGCAAA, Reverse: 5’-CCTCTCATCATTCCACATGC) GAPDH (forward:5’-GAAGGTGAAGGTCGG AGTC Reverse:5’-GAAGATGGTGATGGGATTTC) were obtained from Integrated DNA technologies (Coralville, IA).
Monocyte adhesion to endothelial cells was studied using HUVEC grown on coated plates.30 THP-1 cells were labeled with fluorescent dye calcein acetoxymethyl ester (4 μg/ml)(Life Technologies, Carlsbad, CA). HUVEC were exposed to HDL (50–300μg/ml) and TNF-α (1 ng/ml x 5h) (Peprotech, Rocky Hill, NJ) then calcein-labeled THP-1 cells. Fluorescence was measured at 494/520 nm (Abs/Em) by SpectraMax M5 Multi-Mode Microplate Readers (Molecular Devices, Sunnyvale, CA). Each experimental condition was done in triplicate and adhesion compared to the value obtained with TNFα only. In complementary microscopic studies, HUVEC grown on 0.1% gelatin coated glass culture slides (BD biosciences, San Jose, CA) were exposed to the fluorescence labeled THP-1 cells then fixed with 4% paraformaldehyde in phosphate buffered saline (Boston BioProducts Inc., Ashland, MA), mounted with VECTASHIELD Hard Set Mounting Medium with DAPI (Vector Laboratories Inc., Burlingame CA).
2.5 Endothelial cell proliferation, apoptosis, and necrosis assays
HUVECs were exposed to 50μg/ml of HDL and 10 ng/ml of TNF-α x 48 h. Cellular proliferation was measured with an MTS/PMS reagent based kit (Promega, Madison, WI). The quantity of formazan product was measured by 490nm absorbance using CellTiter 96 Aqueous One Solution Cell Proliferation Assay following the manufacturer’s instructions.31
Apoptotic cells were determined by staining with the Annexin V and 7-AAD using FITC Annexin V Apoptosis Detection Kit with 7-AAD (BioLegend San Diego, CA). HUVEC were exposed TNF-α (10ng/ml) and HDL (50 μg/ml) x 16 h. Cells were resuspended in Annexin binding buffer at 1 × 106 cells/ml and incubated with Annexin V and 7-AAD for 15 min at room temperature in the dark. The cells were analyzed using a LSR II flow cytometer (BD Bioscience, San Jose, CA).32
Caspase 3 (Cleaved-Asp-175) was measured by ELISA (Assay Biotech, Sunnyvale, CA). HUVEC were exposed to HDL 50–300 μg/ml and 10 ng/ml of TNF-α x 48 h. After removing the supernatant, cells were fixed with 4% formaldehyde for 20 min at room temperature and incubated with quenching buffer for 20 min and blocking buffer for 1 h. Primary antibodies (Anti caspase 3 (Cleaved-Asp-175) rabbit polyclonal antibody) were added for overnight at 4C and secondary antibodies (HRP conjugated anti rabbit IgG antibody) were added for 1.5 h at room temperature. Cells were incubated with substrate for 30min before adding stop solution. Absorbance was measured at 450nm and results adjusted by cell numbers by staining with crystal violet measuring absorbance at 595 nm.
2.6 Statistics
Results are expressed as a mean of triplicate assays in endothelial cell adhesion, efflux and apoptosis assays, and duplicate assays in mRNA of inflammation cytokine response and adhesion molecules. Descriptive statistics are presented as frequencies and percentages for categorical variables and mean ± SD or median (interquartile range [IQR]) according to the distribution of the continuous variables. Demographic and clinical factors were compared between HDLs among control, patients with CKD, ESRD using Kruskal-Wallis test and unpaired t-test, as appropriate. Statistical analyses were performed using R version 2.10.0. A 2-sided significance level of 5% was required for consideration as statistically significant.
3. RESULTS
3.1 Characteristics of study participants
Demographic and clinical characteristics of study participants are summarized in Table 1. The groups were comparable in terms of age, gender and race. Because the study included children over a range of ages, the body mass index (BMI), systolic and diastolic blood pressure measurements are expressed as percentile normalized by age and height. There were no differences in BMI. The systolic and diastolic blood pressure values in the CKD and ESRD groups were not different than controls. Many children in CKD (9/16) and ESRD (5/15) groups were taking antihypertensive medications, including angiotensin converting enzyme inhibitor (ACEI) and/or an angiotensin receptor blocker (ARB) (16/31) or calcium channel blockers (1/31). Compared with controls, plasma levels of total cholesterol, LDL cholesterol and triglycerides were higher in CKD and ESRD, whereas HDL levels were not different among the groups. High sensitivity CRP was higher in CKD and ESRD subjects than controls.33 Compositional analysis of HDL revealed that compared to children with normal kidney function, HDL of children with ESRD-PD have reduced phospholipid content (23%) and increased triglycerides (136%). Total cholesterol, free cholesterol, and cholesteryl ester levels were similar amongst ESRD-PD, CKD and Control groups (Table 1).
Table 1.
Characteristics of Study Subjects
| Control (N=10) | CKD (N=16) | ESRD-PD N=15 | P Value | |
|---|---|---|---|---|
| Age, years | 9.3±2.0 | 9.8±5.5 | 9.6±5.0 | P=0.72 |
|
| ||||
| Body mass index (percentile) | 59±23 | 54±27 | 54±27 | P=0.93 |
|
| ||||
| Systolic blood pressure (percentile) | 70±24 | 72±20 | 76±22 | P=0.64 |
|
| ||||
| Diastolic blood pressure (percentile) | 62±17 | 59±27 | 72±28 | P=0.26 |
|
| ||||
| Total cholesterol (mg/dl) | 123±16 | 149±28 | 171±46 | P=0.037 |
|
| ||||
| TG (mg/dl) | 82±22 | 143±89 | 269±161 | P=0.004 |
|
| ||||
| HDL, mg/dl | 41.2±12.3 | 39.8±7.2 | 33.8±9.3 | P=0.14 |
|
| ||||
| LDL, mg/dl | 65±11 | 83±25 | 91±33 | P=0.096 |
|
| ||||
| hsCRP (mg/dl) | 0.40±0.20 | 0.76±0.76 | 1.65±1.40 | P=0.002 |
|
| ||||
| HDL composition (μg/mg protein) | ||||
| Total cholesterol | 0.291±0.071 | 0.264±0.052 | 0.293±0.098 | P=0.87 |
| Cholesteryl ester | 0.225±0.051 | 0.204±0.041 | 0.228±0.091 | P=0.85 |
| Free cholesterol | 0.065±0.027 | 0.060±0.014 | 0.065±0.007 | P=0.36 |
| Phospholipids | 0.549±0.129 | 0.424±0.045 | 0.493±0.068 | P=0.17 |
| Triglycerides | 0.065±0.023 | 0.087±0.036 | 0.154±0.108 | P=0.06 |
Continuous variables are presented as mean±SD. T test used: Kruskal-Wallis. TG=triglyceride; HDL=high density lipoprotein; LDL=low density lipoprotein; hsCRP=high - sensitivity C reactive protein.
3.2 HDL effects on macrophage inflammatory and chemotactic responses
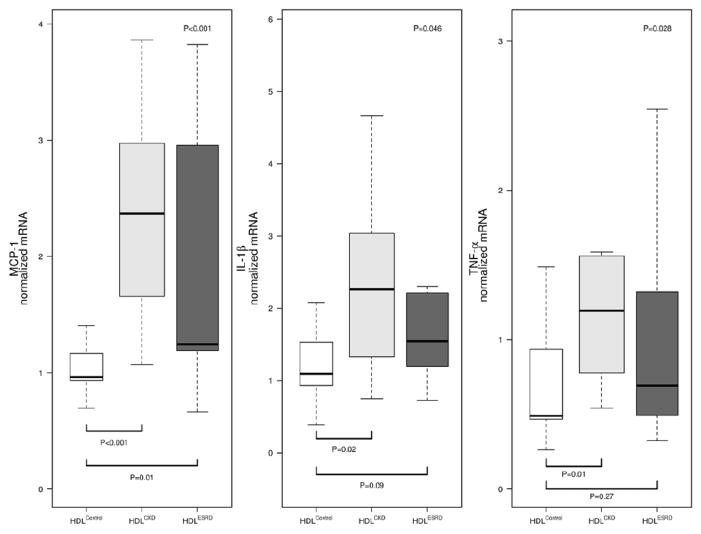
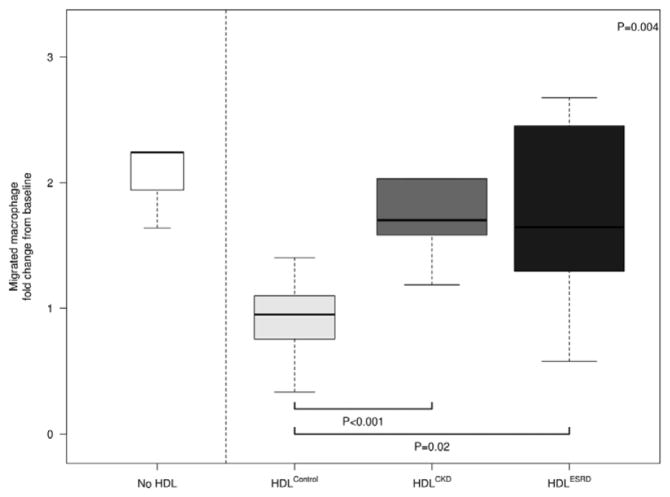
HDL from children with CKD and ESRD elicited an inflammatory cytokine response from THP-1 macrophages (Figure 1). Expression of MCP-1 in response to HDLCKD was increased 2-fold compared to HDLControl. Similarly, HDLESRD caused >80% greater cytokine response than elicited by HDLControl. Both HDLCKD and HDLESRD enhanced cellular expression of TNF-α, 90% and 64% greater than HDLControl, respectively, and IL-1β, 80% and 61% greater than HDLControl, respectively. In addition, HDLCKD and HDLESRD were found to have impaired anti-chemotactic functions. Whereas HDLControl dramatically inhibited MCP-1-induced THP-1 migration, neither HDLCKD nor HDLESRD had anti-chemotactic effects (Figure 2). Indeed, the migratory response of HDLCKD and HDLESRD was indistinguishable from chemotactic response elicited by MCP-1 alone.
Figure 1.
Cytokine response of THP-1 macrophages to HDLControl, HDLCKD, and HDLESRD relative to cells exposed to LPS alone. mRNA expression for monocyte chemoattractant protein (MCP)-1, interleukin-1β (IL-1β), and tumor necrosis factor (TNF)-α measured by real-time PCR. N=9, 16, 14 for HDLControl, HDLCKD and HDLESRD, respectively. Comparison of HDL effects among control, CKD, ESRD subjects used Kruskal-Wallis test (p value shown in upper right corner of each panel) and unpaired t-test (shown as bars between individual groups).
Figure 2.
Chemotactic effects of HDLControl, HDLCKD and HDLESRD. THP-1 cells in the upper compartment of a microchemotaxis chamber are separated by a filter from the lower compartment containing MCP-1. N=7, 13, 13 for HDLControl, HDLCKD and HDLESRD, respectively. Bars show median and interquartile range values. Comparison of HDL effects among control, CKD, ESRD subjects used Kruskal-Wallis test (p value shown in upper right corner of each panel) and unpaired t-test (shown as bars between individual groups).
3.3 HDL effect to vascular endothelial cells
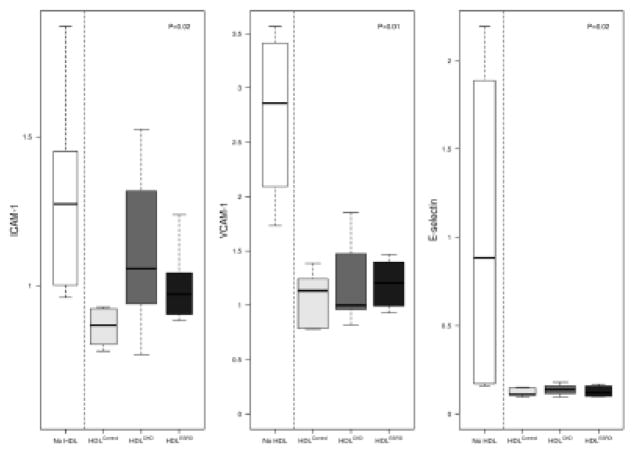
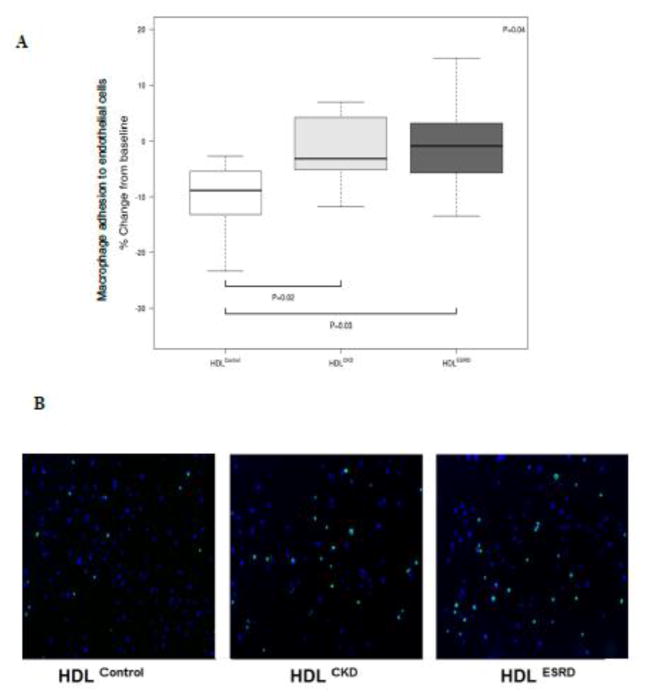
A key vasoprotective feature of HDL is support of endothelial function.10,14 To determine whether HDL from children with CKD and ESRD affect endothelial adhesiveness, we tested HDL’s ability to suppress adhesion molecule expression, and found that compared to HDLControl, both HDLCKD and HDLESRD had blunted ability to suppress endothelial activation (Figure 3). HDLControl was found to dramatically reduced endothelial gene expression of ICAM-1, VCAM, and E-selectin. By contrast, HDLCKD and HDLESRD resulted in significantly less suppression of ICAM-1 expression. VCAM-1 and E-selectin were similarly affected by HDL of all groups. These expression studies were complemented by functional experiments. HDLControl caused a 10.4% reduction in monocyte adhesion to endothelial cells compared to only 1.9 and 1.3% reduction following exposure to HDLCKD or HDLESRD, respectively (Figure 4A). The extent of this impairment is visually illustrated in Figure 4B. Calcein-labeled THP-1 cells (green) were found to have greater adhesion to HUVECs (blue-DAPI) exposed to HDLCKD or HDLESRD than cells exposed to HDLControl.
Figure 3.
Adhesion molecule response of endothelial cells to HDLControl, HDLCKD, and HDLESRD. mRNA expression for adhesion molecule (ICAM)-1; vascular cell adhesion molecule (VCAM)-1; and E-selectin measured by real-time PCR. N=6, 8, 6 for HDLControl, HDLCKD and HDLESRD, respectively.
Figure 4.
Quantitated adhesion of THP-1 cells and HUVECs. (A) Cellular fluorescence was measured by SpectraMax M5 Multi-Mode Microplate Reader with each experimental condition done in triplicate and adhesion compared to the value obtained with TNF-α only. N=7, 13, 13 for HDLControl, HDLCKD and HDLESRD, respectively. Bars show median and interquartile range values. Comparison of HDL effects among control, CKD, ESRD subjects used Kruskal-Wallis test (p value shown in upper right corner of each panel) and unpaired t-test (shown as bars between individual groups). (B) Microscopic appearance of adhesion of calcein-labeled THP-1 to TNF-α-exposed HUVEC (Green: THP-1; Blue: DAPI).
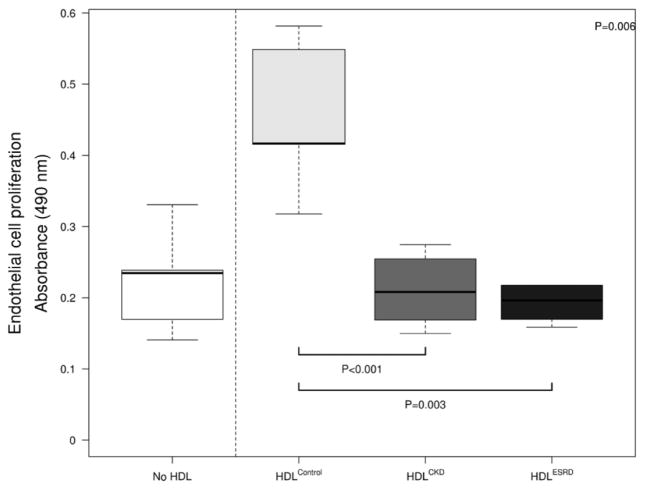
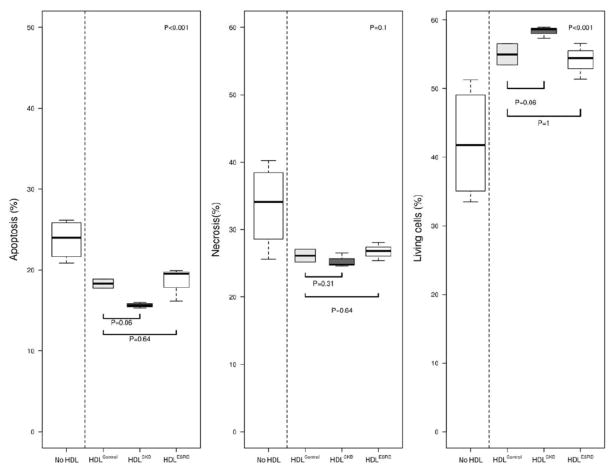
We next examined the impact of CKD on vasoprotective properties of HDL that involve endothelial cell proliferation and death. Due to limited sample volumes, HDL samples were pooled in each group using the same quantity of HDL total protein concentration. HDLControl more than doubled HUVECs proliferation capacity following TNF-α activation (Figure 5). By contrast, neither HDLCKD nor HDLESRD affected on cellular proliferation. Although HDL had a dramatic effect on endothelial cell proliferation, there was little difference in cell death, as HDL from all three groups repressed apoptosis. We also found little difference among HDLControl, HDLCKD, and HDLESRD on cell survival and necrosis over a range of HDL concentrations (50–300μg/ml) (Figure 6). Complementing the findings are our observations of cleaved caspase 3 activity in HDL-exposed HUVECs. Thus, similar to our flow cytometry results, HDL from the three groups reduced apoptosis, but there was no difference among HDLControl, HDLCKD and HDLESRD on cleaved caspase 3 activity (medium 26.3±1.2, 29.7±1.5, 29.6±1.2, 28.7±0.7, respectively, P=0.111). The lack of effect was observed over a range of HDL concentrations (50–300μg/ml).
Figure 5.
Endothelial cell proliferation showing impaired proliferation effects of HDLCKD and HDLESRD. Due to limited sample volumes, HDL samples were pooled in each group using the same quantity of HDL protein concentration; each experiment was done in triplicate and repeated 2–4 times. Bars show median and interquartile range values. Comparison of HDL effects among control, CKD, ESRD subjects used Kruskal-Wallis test (p value shown in upper right corner) and unpaired t-test (shown as bars between individual groups).
Figure 6.
HDL effects on endothelial cells survival, apoptosis, and necrosis was measured by flow cytometry using FITC Annexin V Apoptosis Detection Kit with 7-AAD showing similar effects among HDLControl, HDLCKD, and HDLESRD. HDL samples pooled in each group using the same quantity of HDL protein concentration and each experiment was done in triplicate and repeated 2–4 times. Bars show median and interquartile range values. Comparison of HDL effects among control, CKD, ESRD subjects used Kruskal-Wallis test (p value shown in upper right corner of each panel) and unpaired t-test (shown as bars between individual groups).
3.4 HDL effects on cholesterol acceptor function
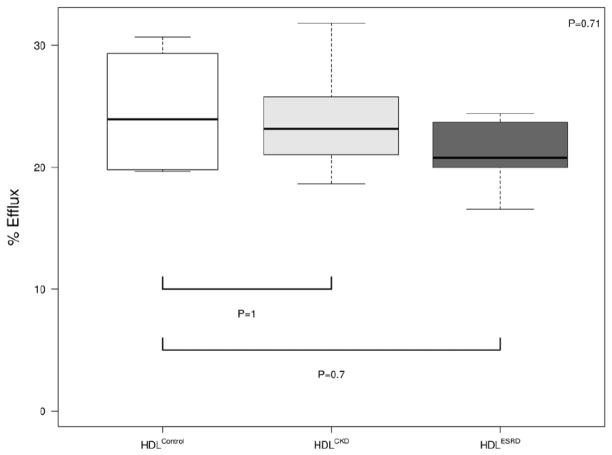
We and others have reported that the cholesterol acceptor function of HDL from adult ESRD patients on hemodialysis is impaired.10,14,15,19 We therefore also analyzed the cholesterol efflux capacity of HDL in children with CKD. Both HDLCKD and HDLESRD showed a non-significant trend toward reduced capacity to accept cholesterol from lipid-laden macrophages (Figure 7).
Figure 7.
Cholesterol acceptor capacity of HDLControl, HDLCKD and HDLESRD measured by gas chromatography. Cholesterol efflux was determined as the percent at baseline versus after incubation with HDL. N=4, 6, 6 for HDLControl, HDLCKD and HDLESRD, respectively. Bars show median and interquartile range values. Comparison of HDL effects among control, CKD, ESRD subjects used Kruskal-Wallis test.
4. DISCUSSION
Our results suggest that children with moderate CKD and ESRD requiring dialysis have dysfunctional HDL and the dysfunction is not dependent on the level of plasma HDL cholesterol. Importantly, HDL dysfunction occurs in the absence of long-standing comorbidities and risk factors that plague adult CKD patients. Compared with HDLControl, HDLCKD and HDLESRD potentiate the macrophage inflammatory response and impair protective functions responsible for endothelial cell integrity, including cellular adhesion and proliferation. Notably, not all HDL functions are equally disrupted, as there were no significant differences among the HDL’s on cholesterol efflux capacity or endothelial cell survival. The study suggests that CKD alone is sufficient to cause HDL dysfunction and that even moderate loss of renal function causes specific changes in HDL function that may be linked to early CVD in this population.
CKD is characterized by complex vasculopathy that includes endothelial dysfunction linked to inflammation.33–36 HDL have important vasoprotective properties by providing anti-inflammatory actions and protecting the vascular endothelium.10–14,37 The current study demonstrates that children with CKD and ESRD have dysfunctional HDL. Our data show that HDLCKD and HDLESRD increase macrophage expression of IL-1β, TNF-α and MCP-1. Moreover, HDLCKD and HDLESRD were not found to reduce MCP-1-induced chemotaxis, an effect clearly apparent with HDLControl. These results significantly add to previous reports in adults with chronic disorders such as diabetes, coronary artery disease, metabolic syndrome, rheumatoid arthritis, showing that HDL loses its anti-inflammatory capacity.38–40 Adults with CKD also have HDL with pro-inflammatory effects which in turn correlate with poor outcome.38 Importantly, however, adults with CKD have a host of other potential confounders of HDL activity. Thus, more than half of the CKD subjects have diabetes mellitus, underlying cardiac disease, obesity, long-standing hypertension.15–19 The current results are the first to describe enhanced macrophage inflammatory cytokine response to HDL from children with CKD, in the absence of concomitant conditions and risk factors. The results also reveal that this impairment occurs before progression to kidney failure since even children with stage 3–4 CKD have HDL that amplified macrophage cytokine response and chemotaxis to a degree indistinguishable from HDLESRD.
CKD-associated vasculopathy is critically dependent on endothelial cell function.4,36,41,42 Our study suggests that the effect of CKD on HDL contributes to endothelial dysfunction, as both HDLCKD and HDLESRD were ineffective in suppressing endothelial cells expression of adhesion molecules, specifically, endothelial ICAM-1. These results complement previous observations that vascular expression and plasma levels of ICAM-1 predict CVD events among ESRD patients.43,44 Our expression studies are supported by functional results showing HDLCKD and HDLESRD have significantly reduced ability to prevent monocyte adhesion to endothelial cells.
Our results reveal a more pronounced inhibitory effect of HDL of children with CKD on cellular proliferation than on protection against cell death. Compared with HDLControl, HDLCKD and HDLESRD were significantly less effective in restoring endothelial cell proliferation following TNF-α stimulus. The results are in line with previous observations that uremic serum impairs endothelial cell proliferation.45,46 Our novel results suggest that the endothelial dysfunction commonly seen across the spectrum of CKD may be influenced by dysfunctional HDL. Conversely, HDL from all three groups provided similar degree of protection against apoptosis and necrosis of endothelial cells. The divergent effects of HDLCKD and HDLESRD on cellular proliferation versus death echo observations that uremic serum regulates endothelial cell proliferation, but has little impact on endothelial apoptosis.46 The variable effect of HDL on proliferation versus survival has been documented in different cells, including, hematopoietic, adipocytes, cardiomyocytes, and cancer cells.47,48 Taken together, these results suggest that HDL of CKD patients retain its ability to protect against apoptosis and necrosis; however, HDL-directed proliferation of endothelial cells in response to injurious stimuli occurring in CKD (toxic metabolites, oxidant stress, and inflammation) is markedly compromised.
Although we and others have reported that HDL from adults with ESRD on dialysis have impaired cholesterol efflux capacity,15 ,19 we now report that HDL from children with CKD and ESRD showed only a non-significant trend for reduced efflux. It is possible that CKD may preferentially disrupt cholesterol efflux to phospholipid-poor apolipoprotein A-I. However, in our system of cholesterol-loaded macrophages, cholesterol is mobilized through scavenger receptor class-BI (SR-BI), ATP-binding cassette transporter G1 (ABCG1), and in particular ATP-binding cassette transporter A1 (ABCA1).49–51 Further, density-gradient ultracentrifugation-isolated HDL fractions have been reported to stimulate ABCA1 cholesterol efflux due to the presence of small phospholipid-poor/apoA-1 particles (pre-β-1 HDL) and the ability of apoA-I to dissociate from mature HDL.52–55 Using these HDL fractions, we did not observe a difference in efflux with HDL from control versus CKD or ESRD-PD subjects suggesting that ABCA1-mediated efflux is not affected. It is possible that other lipid-poor apoproteins/particles (d >1.22g/ml) could be impaired in their ability to stimulate ABCA1 efflux. However, using serum fractions depleted of apoB lipoproteins by PEG precipitation, i.e. whereby all of the HDL acceptors for ABCA1 would be present, revealed that, similar to the gradient ultracentrifugation-isolated HDL fractions, there were no difference in cholesterol efflux using the serum fractions. Interestingly, while the composition of HDL from ESRD-PD group were directionally similar to those reported in adult ESRD hemodialysis patients (i.e. reduction in in phospholipids and increase in triglycerides) they were not statistically significant and suggest that the more profound alterations in composition of HDL particles are linked to impaired cholesterol acceptor capacity. Indeed, in adults, changes in the phospholipid and triglyceride content of HDL particles were shown to correlate with impairment in cholesterol efflux.15 It is therefore possible that alterations in HDL composition observed in children in the current study are insufficiently disturbed to impair efflux capacity. It is also possible that duration and the associated conditions that characterize adult CKD provoke the disruption in HDL efflux capacity. Furthermore, the children in our study were on peritoneal dialysis while adult patients in the published reports were on hemodialysis. Whether the mode of dialysis affects cholesterol acceptor capacity remains to be determined and requires further investigation. Recently, Khera et al. reported that cholesterol efflux capacity was inversely associated with CVD56; however, it is possible that reduced efflux is a consequence and not a predictor of future development in atherosclerosis. The median age of our population was about 9 years of age, which may not be sufficient duration to establish atherosclerotic vasculopathy linked to impairment in cholesterol efflux. In this regard, Li et al. have reported that increased, rather than decreased, cholesterol efflux in adults with CKD is associated with risk of myocardial infarction, stroke, and death, while confirming the inverse association between cholesterol efflux and prevalent coronary artery disease.57
In summary, even in the absence of long-standing CKD and concomitant risk factors, CKD in childhood causes specific HDL abnormalities linked to maintenance of normal vascular function, including inflammatory responses in macrophages and production of adhesion molecules by endothelial cells.
Acknowledgments
6. FUNDING.
This work was supported in part by grants NIH HL087061, DK44757, HL65709 and HL57986 and the Lipid, Lipoprotein, and Atherosclerosis Core of the Vanderbilt Mouse Metabolic Phenotyping Center (NIH DK59637-01) and NIH 1P01HL116263.
The authors do not have any relevant affiliations or financial involvement with any entity with financial interest or conflict related to these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fox CS, Matsushita K, Woodward M, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380(9854):1662–73. doi: 10.1016/S0140-6736(12)61350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahmoodi BK, Matsushita K, Woodward M, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without hypertension: a meta-analysis. Lancet. 2012;380(9854):1649–61. doi: 10.1016/S0140-6736(12)61272-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. 2003;42(5):1050–1065. doi: 10.1161/01.HYP.0000102971.85504.7c. [DOI] [PubMed] [Google Scholar]
- 4.Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116(1):85–97. doi: 10.1161/CIRCULATIONAHA.106.678342. [DOI] [PubMed] [Google Scholar]
- 5.Tonelli M, Muntner P, Lloyd A, et al. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet. 2012;380(9844):807–814. doi: 10.1016/S0140-6736(12)60572-8. [DOI] [PubMed] [Google Scholar]
- 6.Weiner DE, Tighiouart H, Elsayed EF, et al. The relationship between nontraditional risk factors and outcomes in individuals with stage 3 to 4 CKD. Am J Kidney Dis. 2008;51(2):212–223. doi: 10.1053/j.ajkd.2007.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitsnefes MM. Cardiovascular disease in children with chronic kidney disease. JASN. 2012;23(4):578–585. doi: 10.1681/ASN.2011111115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitsnefes MM, Laskin BL, Dahhou M, Zhang X, Foster BJ. Mortality risk among children initially treated with dialysis for end-stage kidney disease, 1990–2010. JAMA. 2013;309(18):1921–1929. doi: 10.1001/jama.2013.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US Renal Data System U. Atlas of end-stage renal disease in the United States: Annual Data Report. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2009. [Google Scholar]
- 10.Besler C, Heinrich K, Rohrer L, et al. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J Clin Invest. 2011;121(7):2693–2708. doi: 10.1172/JCI42946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de la Llera Moya M, McGillicuddy FC, Hinkle CC, et al. Inflammation modulates human HDL composition and function in vivo. Atherosclerosis. 2012;222(2):390–394. doi: 10.1016/j.atherosclerosis.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soran H, Hama S, Yadav R, Durrington PN. HDL functionality. Curr Opin Lipidol. 2012;23(4):353–366. doi: 10.1097/MOL.0b013e328355ca25. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Rader DJ. Molecular regulation of macrophage reverse cholesterol transport. Curr Opin Cardiol. 2007;22(4):368–372. doi: 10.1097/HCO.0b013e3281ec5113. [DOI] [PubMed] [Google Scholar]
- 14.Riwanto M, Landmesser U. High density lipoproteins and endothelial functions: mechanistic insights and alterations in cardiovascular disease. Journal of Lipid Research. 2013;54(12):3227–3243. doi: 10.1194/jlr.R037762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holzer M, Birner-Gruenberger R, Stojakovic T, et al. Uremia alters HDL composition and function. Journal of the American Society of Nephrology:JASN. 2011;22(9):1631–1641. doi: 10.1681/ASN.2010111144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Speer T, Rohrer L, Blyszczuk P, et al. Abnormal high-density lipoprotein induces endothelial dysfunction via activation of Toll-like receptor-2. Immunity. 2013;38(4):754–768. doi: 10.1016/j.immuni.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Tolle M, Huang T, Schuchardt M, et al. High-density lipoprotein loses its anti-inflammatory capacity by accumulation of pro-inflammatory-serum amyloid A. Cardiovasc Res. 2012;94(1):154–162. doi: 10.1093/cvr/cvs089. [DOI] [PubMed] [Google Scholar]
- 18.Weichhart T, Kopecky C, Kubicek M, et al. Serum amyloid A in uremic HDL promotes inflammation. Journal of the American Society of Nephrology:JASN. 2012;23(5):934–947. doi: 10.1681/ASN.2011070668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto S, Yancey PG, Ikizler TA, et al. Dysfunctional high-density lipoprotein in patients on chronic hemodialysis. J Am Coll Cardiol. 2012;60(23):2372–2379. doi: 10.1016/j.jacc.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kennedy DJ, Tang WH, Fan Y, et al. Diminished antioxidant activity of high-density lipoprotein-associated proteins in chronic kidney disease. J Am Heart Assoc. 2013;2(2):e000104. doi: 10.1161/JAHA.112.000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moradi H, Streja E, Kashyap ML, Vaziri ND, Fonarow GC, Kalantar-Zadeh K. Elevated high-density lipoprotein cholesterol and cardiovascular mortality in maintenance hemodialysis patients. Nephrology, Dialysis, Transplantation. 2014 doi: 10.1093/ndt/gfu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaziri ND, Navab M, Fogelman AM. HDL metabolism and activity in chronic kidney disease. Nat Rev Nephrol. 2010;6(5):287–296. doi: 10.1038/nrneph.2010.36. [DOI] [PubMed] [Google Scholar]
- 23.Jerome WG, Cox BE, Griffin EE, Ullery JC. Lysosomal cholesterol accumulation inhibits subsequent hydrolysis of lipoprotein cholesteryl ester. Microsc Microanal. 2008;14(2):138–149. doi: 10.1017/S1431927608080069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maugeais C, Perez A, von der Mark E, Magg C, Pflieger P, Niesor EJ. Evidence for a role of CETP in HDL remodeling and cholesterol efflux: role of cysteine 13 of CETP. Biochimica et Biophysica Acta. 2013;1831(11):1644–1650. doi: 10.1016/j.bbalip.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Hung A, Pupim L, Yu C, et al. Determinants of C-reactive protein in chronic hemodialysis patients: relevance of dialysis catheter utilization. Hemodial Int. 2008;12(2):236–243. doi: 10.1111/j.1542-4758.2008.00260.x. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz GJ, Schneider MF, Maier PS, et al. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney International. 2012;82(4):445–453. doi: 10.1038/ki.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klansek JJ, Yancey P, St Clair RW, Fischer RT, Johnson WJ, Glick JM. Cholesterol quantitation by GLC: artifactual formation of short-chain steryl esters. Journal of Lipid Research. 1995;36(10):2261–2266. [PubMed] [Google Scholar]
- 28.Yancey PG, Jerome WG. Lysosomal cholesterol derived from mildly oxidized low density lipoprotein is resistant to efflux. Journal of Lipid Research. 2001;42(3):317–327. [PubMed] [Google Scholar]
- 29.Norata GD, Cattaneo P, Poletti A, Catapano AL. The androgen derivative 5alpha-androstane-3beta,17beta-diol inhibits tumor necrosis factor alpha and lipopolysaccharide induced inflammatory response in human endothelial cells and in mice aorta. Atherosclerosis. 2010;212(1):100–106. doi: 10.1016/j.atherosclerosis.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 30.Ludwig A, Lorenz M, Grimbo N, et al. The tea flavonoid epigallocatechin-3-gallate reduces cytokine-induced VCAM-1 expression and monocyte adhesion to endothelial cells. Biochemical and Biophysical Research Communications. 2004;316(3):659–665. doi: 10.1016/j.bbrc.2004.02.099. [DOI] [PubMed] [Google Scholar]
- 31.Cozin M, Pinker BM, Solemani K, et al. Novel therapy to reverse the cellular effects of bisphosphonates on primary human oral fibroblasts. Journal of Oral and Maxillofacial Surgery. 2011;69(10):2564–2578. doi: 10.1016/j.joms.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lovren F, Pan Y, Quan A, et al. Adropin is a novel regulator of endothelial function. Circulation. 2010;122(11 Suppl):S185–192. doi: 10.1161/CIRCULATIONAHA.109.931782. [DOI] [PubMed] [Google Scholar]
- 33.Miyamoto T, Carrero JJ, Stenvinkel P. Inflammation as a risk factor and target for therapy in chronic kidney disease. Curr Opin Nephrol Hypertens. 2011;20(6):662–668. doi: 10.1097/MNH.0b013e32834ad504. [DOI] [PubMed] [Google Scholar]
- 34.Moody WE, Edwards NC, Madhani M, et al. Endothelial dysfunction and cardiovascular disease in early-stage chronic kidney disease: cause or association? Atherosclerosis. 2012;223(1):86–94. doi: 10.1016/j.atherosclerosis.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 35.Pecoits-Filho R, Heimburger O, Barany P, et al. Associations between circulating inflammatory markers and residual renal function in CRF patients. Am J Kidney Dis. 2003;41(6):1212–1218. doi: 10.1016/s0272-6386(03)00353-6. [DOI] [PubMed] [Google Scholar]
- 36.Recio-Mayoral A, Banerjee D, Streather C, Kaski JC. Endothelial dysfunction, inflammation and atherosclerosis in chronic kidney disease--a cross-sectional study of predialysis, dialysis and kidney-transplantation patients. Atherosclerosis. 2011;216(2):446–451. doi: 10.1016/j.atherosclerosis.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 37.Jurek A, Turyna B, Kubit P, Klein A. The ability of HDL to inhibit VCAM-1 expression and oxidized LDL uptake is impaired in renal patients. Clinical Biochemistry. 2008;41(12):1015–1018. doi: 10.1016/j.clinbiochem.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 38.Kalantar-Zadeh K, Kopple JD, Kamranpour N, Fogelman AM, Navab M. HDL-inflammatory index correlates with poor outcome in hemodialysis patients. Kidney International. 2007;72(9):1149–1156. doi: 10.1038/sj.ki.5002491. [DOI] [PubMed] [Google Scholar]
- 39.McMahon M, Grossman J, Skaggs B, et al. Dysfunctional proinflammatory high-density lipoproteins confer increased risk of atherosclerosis in women with systemic lupus erythematosus. Arthritis Rheum. 2009;60(8):2428–2437. doi: 10.1002/art.24677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ragbir S, Farmer JA. Dysfunctional high-density lipoprotein and atherosclerosis. Curr Atheroscler Rep. 2010;12(5):343–348. doi: 10.1007/s11883-010-0091-x. [DOI] [PubMed] [Google Scholar]
- 41.Stam F, van Guldener C, Becker A, et al. Endothelial dysfunction contributes to renal function-associated cardiovascular mortality in a population with mild renal insufficiency: the Hoorn study. Journal of the American Society of Nephrology:JASN. 2006;17(2):537–545. doi: 10.1681/ASN.2005080834. [DOI] [PubMed] [Google Scholar]
- 42.Yilmaz MI, Stenvinkel P, Sonmez A, et al. Vascular health, systemic inflammation and progressive reduction in kidney function; clinical determinants and impact on cardiovascular outcomes. Nephrology, Dialysis, Transplantation. 2011;26(11):3537–3543. doi: 10.1093/ndt/gfr081. [DOI] [PubMed] [Google Scholar]
- 43.Suliman ME, Qureshi AR, Heimburger O, Lindholm B, Stenvinkel P. Soluble adhesion molecules in end-stage renal disease: a predictor of outcome. Nephrology, Dialysis, Transplantation. 2006;21(6):1603–1610. doi: 10.1093/ndt/gfl005. [DOI] [PubMed] [Google Scholar]
- 44.Upadhyay A, Larson MG, Guo CY, et al. Inflammation, kidney function and albuminuria in the Framingham Offspring cohort. Nephrology, Dialysis, Transplantation. 2011;26(3):920–926. doi: 10.1093/ndt/gfq471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chitalia VC, Murikipudi S, Indolfi L, et al. Matrix-embedded endothelial cells are protected from the uremic milieu. Nephrology, Dialysis, Transplantation. 2011;26(12):3858–3865. doi: 10.1093/ndt/gfr337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Serradell M, Diaz-Ricart M, Cases A, Petriz J, Ordinas A, Escolar G. Uraemic medium accelerates proliferation but does not induce apoptosis of endothelial cells in culture. Nephrology, Dialysis, Transplantation. 2003;18(6):1079–1085. doi: 10.1093/ndt/gfg161. [DOI] [PubMed] [Google Scholar]
- 47.Sayd S, Thirant C, El-Habr EA, et al. Sirtuin-2 activity is required for glioma stem cell proliferation arrest but not necrosis induced by resveratrol. Stem Cell Reviews. 2014;10(1):103–113. doi: 10.1007/s12015-013-9465-0. [DOI] [PubMed] [Google Scholar]
- 48.Song G, Xu G, Ji C, et al. The role of microRNA-26b in human adipocyte differentiation and proliferation. Gene. 2014;533(2):481–487. doi: 10.1016/j.gene.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 49.Yancey PG, Jerome WG, Yu H, Griffin EE, Cox BE, Babaev VR, Fazio S, Linton MF. Severely altered cholesterol homeostasis in macrophages lacking apoe and sr-bi. J Lipid Res. 2007 doi: 10.1194/jlr.M600539-JLR200. [DOI] [PubMed] [Google Scholar]
- 50.Kennedy M, Barrera G, Nakamura K, Baldan A, Tarr P, Fishbein M, Frank J, Francone O, Edwards P. Abcg1 has a critical role in mediating cholesterol efflux to hdl and preventing cellular lipid accumulation. Cell Metab. 2005;1:121–131. doi: 10.1016/j.cmet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 51.Yancey PG, Yu H, Linton MF, Fazio S. A pathway-dependent on apoe, apoai, and abca1 determines formation of buoyant high-density lipoprotein by macrophage foam cells. Arterioscler Thromb Vasc Biol. 2007;27:1123–1131. doi: 10.1161/ATVBAHA.107.139592. [DOI] [PubMed] [Google Scholar]
- 52.Remaley A, Schumacher U, Stonik J, Farsi B, Nazih H, Brewer H. Decreased reverse cholesterol transport from tangier disease fibroblasts. Arter Thromb Vasc Biol. 1997;17:1813–1821. doi: 10.1161/01.atv.17.9.1813. [DOI] [PubMed] [Google Scholar]
- 53.Francis GA, Knopp RH, Oram JF. Defective removal of cellular cholesterol and phospholipids by apolipoprotein a-i in tangier disease. J Clin Invest. 1995;96:78–87. doi: 10.1172/JCI118082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosenson RS, Brewer HB, Jr, Chapman MJ, Fazio S, Hussain MM, Kontush A, Krauss RM, Otvos JD, Remaley AT, Schaefer EJ. Hdl measures, particle heterogeneity, proposed nomenclature, and relation to atherosclerotic cardiovascular events. Clin Chem. 2011;57:392–410. doi: 10.1373/clinchem.2010.155333. [DOI] [PubMed] [Google Scholar]
- 55.Oram JF. Hdl apolipoproteins and abca1: Partners in the removal of excess cellular cholesterol. Arterioscler Thromb Vasc Biol. 2003;23:720–727. doi: 10.1161/01.ATV.0000054662.44688.9A. [DOI] [PubMed] [Google Scholar]
- 56.Khera AV, Cuchel M, de la Llera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. The New England Journal of Medicine. 2011;364(2):127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li XM, Tang WH, Mosior MK, et al. Paradoxical Association of Enhanced Cholesterol Efflux With Increased Incident Cardiovascular Risks. Arterioscler Thromb Vasc Biol. 2013;33(7):1696–705. doi: 10.1161/ATVBAHA.113.301373. [DOI] [PMC free article] [PubMed] [Google Scholar]