Abstract
The newly described green-pigmented bacterium Pseudoalteromonas tunicata (D2) produces target-specific inhibitory compounds against bacteria, algae, fungi, and invertebrate larvae and is frequently found in association with living surfaces in the marine environment. As part of our studies on the ecology of P. tunicata and its interaction with marine surfaces, we examined the ability of P. tunicata to form biofilms under continuous culture conditions within the laboratory. P. tunicata biofilms exhibited a characteristic architecture consisting of differentiated microcolonies surrounded by water channels. Remarkably, we observed a repeatable pattern of cell death during biofilm development of P. tunicata, similar to that recently reported for biofilms of Pseudomonas aeruginosa (J. S. Webb et al., J. Bacteriol. 185:4585-4595, 2003). Killing and lysis occurred inside microcolonies, apparently resulting in the formation of voids within these structures. A subpopulation of viable cells was always observed within the regions of killing in the biofilm. Moreover, extensive killing in mature biofilms appeared to result in detachment of the biofilm from the substratum. A novel 190-kDa autotoxic protein produced by P. tunicata, designated AlpP, was found to be involved in this biofilm killing and detachment. A ΔalpP mutant derivative of P. tunicata was generated, and this mutant did not show cell death during biofilm development. We propose that AlpP-mediated cell death plays an important role in the multicellular biofilm development of P. tunicata and subsequent dispersal of surviving cells within the marine environment.
The newly designated genus Pseudoalteromonas resulted from the division of the genus Alteromonas into the two genera Alteromonas and Pseudoalteromonas, based on phylogenetic comparisons by Gauthier et al. (14). Pseudoalteromonas spp. have been isolated from diverse marine habitats globally (13, 18, 36, 49) and are frequently found in association with the surfaces of eukaryotic hosts. Species have been isolated from various animals, such as mussels (23, 25), puffer fish (47), tunicates (20), and sponges (26), as well as from a range of marine plants (13, 24, 56).
Pseudoalteromonas spp. are also known to produce a variety of extracellular compounds which inhibit or control adaptive and behavioral responses in many target organisms (19-21, 34). For example, the dark-green pigmented species Pseudoalteromonas tunicata, originally isolated from the tunicate Ciona intestinales (20), produces at least six novel extracellular compounds, each with inhibitory activity against a specific group of marine fouling organisms, including bacteria, invertebrate larvae, algal spores, diatoms, heterotrophic flagellates, and fungi (10-13, 19-21, 28). These inhibitory compounds are hypothesized to provide an advantage to P. tunicata during the competitive colonization of living marine surfaces (10, 11, 19-21).
One of the inhibitory compounds produced by P. tunicata is a novel 190-kDa antibacterial protein (AlpP) (28). AlpP inhibits the growth of both gram-positive and gram-negative bacteria, including terrestrial, medical, and marine isolates. Interestingly, logarithmic-phase growing cells of P. tunicata were found to be among the most sensitive to AlpP of the broad range of organisms tested, although stationary-phase cells become resistant (28). The role of this autocidal activity in P. tunicata is unclear. However, there are examples where autolysis plays an important in bacterial developmental processes (35). For example, in Myxococcus xanthus, a number of autocidal compounds are responsible for killing 80 to 90% of the cell population before fruiting body formation can occur (44, 55). More recently, a cell-cell signaling-mediated genetic mechanism was characterized whereby a subpopulation of Bacillus subtilis cells delay sporulation by killing sister cells and feeding on the nutrients that are released (15). Thus, autolysis, which appears undesirable to a single-cell organism, may be advantageous to a bacterial population at the multicellular level. In this study, we hypothesized that the autotoxic protein AlpP produced by P. tunicata may play a role in multicellular biofilm development.
Bacteria in biofilms are physiologically and morphologically different from their planktonic growing counterparts (3). In laboratory systems, biofilm bacteria often form highly differentiated three-dimensional structures (microcolonies) which become surrounded by a network of water channels. Microcolony formation has been demonstrated for most model biofilm-forming bacteria, e.g., Escherichia coli, Vibrio cholerae, and Pseudomonas aeruginosa (6, 8, 33, 52). Mature microcolonies can undergo complex differentiation. Dispersal of bacteria from the interior regions of microcolonies has been observed, apparently resulting in the formation of transparent voids inside the microcolonies (29, 45, 51). Furthermore, it was recently shown that killing and lysis occur reproducibly in localized regions in wild-type P. aeruginosa biofilms, inside microcolonies, by a mechanism that involves a genomic prophage of P. aeruginosa (54). It was proposed that cell death plays an important role in subsequent biofilm differentiation and dispersal, as surviving cells may benefit from the nutrients released during bacterial lysis (54). However, other roles for the death of a subpopulation of biofilm cells may be envisaged. For example, P. tunicata is thought to defend eukaryotic surfaces against further colonization and biofouling via production of its extracellular inhibitory compounds (20, 21). In this situation, autoregulation of biofilm formation by P. tunicata may be required to prevent detrimental overgrowth on the host surface.
In this study, we report that P. tunicata undergoes a highly reproducible pattern of cell death during normal development in a biofilm. We also demonstrate the involvement of a novel autotoxic protein, AlpP, in the killing process through the identification, sequencing, and subsequent site-directed mutagenesis of the corresponding gene alpP. We propose that AlpP-mediated killing may benefit a subpopulation of surviving cells and has an important role in subsequent biofilm development and dispersal in P. tunicata.
MATERIALS AND METHODS
Bacterial strains and media.
P. tunicata was routinely cultivated at room temperature in Väätänen nine-salt solution (VNSS) (37). The P. tunicata alpP mutant was maintained on VNSS medium containing the antibiotics streptomycin (100 μg ml−1) and kanamycin (50 μg ml−1). Biofilms were grown in marine minimal medium (42) containing 0.01% trehalose.
Biofilm experiments.
P. tunicata wild-type and ΔalpP mutant strains were grown in continuous culture flow cells (channel dimensions, 1 by 4 by 40 mm) at room temperature as previously described (40). Channels were inoculated with 0.5 ml of early-stationary-phase cultures containing approximately 109 cells ml−1 and incubated without flow for 1 h at room temperature. Flow was then started with a mean flow velocity in the flow cells of 0.2 mm s−1, corresponding to laminar flow with a Reynolds number of 0.02. To investigate cell death during biofilm development, biofilms were stained with the LIVE/DEAD BacLight bacterial viability kit (Molecular Probes Inc., Eugene, Oreg.). The two stock solutions of the stain (SYTO 9 and propidium iodide) were diluted to 3 μl ml−1 in biofilm medium and injected into the flow channels. Live SYTO 9-stained cells and dead propidium iodide-stained cells were visualized with a confocal laser scanning microscope (CLSM) (Olympus) with fluorescein isothiocyanate and tetramethyl rhodamine isocyanate optical filters, respectively.
To provide statistically based, quantitative measurements during biofilm development, we characterized biofilm morphology with the COMSTAT program (17). Biofilms were stained with acridine orange (ProSciTech, Kelso, Australia), and 5 image stacks were recorded for 3 biofilms, resulting in 15 image stacks per time point. Images were acquired at 2-μm intervals through the biofilm at random positions in the flow cell at 3 time points (24, 72, and 120 h) as previously described (16) with the CLSM. The following parameters were assessed: maximum thickness (μm), average thickness (μm), total biomass (μm3 μm−2), roughness coefficient, and surface-to-volume ratio (μm2 μm−3).
Identification and sequencing of alpP in P. tunicata.
Purification and characterization of the AlpP protein has previously been described (28). In the previous study, the multiple sodium dodecyl sulfate-polyacrylamide gel electrophoresis (PAGE) subunits (60 and 80 kDa) of AlpP were found to share an identical N-terminal sequence for the first 27 amino acids. This sequence was used to design the following oligonucleotide probe (P1) based on codons most frequently used by the closely related genus Vibrio (41): 5′-ATG AAT CTG AAA ATT CAT CCA TCT GTT GGT GTT GCA CGT CTG GGT AAT TCT GAA ATG GAG GAT AAA ATT CTG TCT CGT GA-3′. The P1 probe was used to screen a P. tunicata genomic library constructed by using the phagemid vector λ Zap Express (Stratagene) and the enhanced chemiluminescence 3′ oligolabeling and detection kit (Amersham). The phagemid vector (pBK-CMV; Stratagene) was then excised from the phage into E. coli strain XLOLR (Stratagene) according to manufacturer's instructions. P. tunicata genomic DNA fragments extracted from the genomic library with probe P1 were sequenced by primer walking with a BigDye terminator cycle sequencing reaction mix (Applied Biosystems) and analyzed on an ABI 377 DNA sequencing system. To obtain sequence information further downstream in the antibacterial protein gene, the same genomic library was subsequently screened with a second oligonucleotide probe (P2, 5′-GAT CCG GTT TTC TAA AGT AAT CAT AAT AAG CCT GTC TTT TGC-3′) which targeted a region of the gene 994 bp downstream from the site corresponding to the N terminus of AlpP.
To determine whether both subunits of the AlpP protein originate from a single copy of the alpP open reading frame, we designed the oligonucleotide probe P3 (5′-ATC CAT CCC TCA GTC GGT GTT GCC-3′) by using the sequence obtained for the N-terminal region shared by the two subunits. P. tunicata genomic DNA digests were prepared by using a range of restriction enzymes (PstI, KpnI, EcoRI, BamHI, HaeII, ScaI, HindIII, and XbaI), resolved on a 0.75% agarose gel and transferred via Southern blotting to a polyvinylidene difluoride membrane (Bio-Rad) prior to probing.
Site-directed mutagenesis of alpP.
To investigate the influence of AlpP on biofilm development, a ΔalpP mutant derivative of P. tunicata was generated by allelic displacement. The alpP gene (2.3 kb) was amplified with the following primers: forward, 5′-GAG AAT TCC ATA TGA ATT TAA AAA TCC ATC C-3′; reverse, 5′-AGT CTA AGC ATA TGG GAT CCT GCG TAA GTG ATA TCC C-3′. A kanamycin resistance (Kmr) cassette (1.2 kb) was amplified from the plasmid pUCR4K (Amersham) by using the following primers: forward, 5′-TAC TAG ATC TCA CGT GCG TCG ACC TGC AGG G-3′; reverse, 5′-GTG AAG ATC TCA CGT GCC GGA TCC GTC GAC C-3′. The alpP gene and Kmr cassette were each cloned separately into pGEM T-Easy vectors according to the manufacture's instructions (Invitrogen). The Kmr cassette was then inserted into alpP by using the PmlI restriction enzyme to create the plasmid pGEM alpP::Kmr.
The alpP knockout plasmid, pAP704, was constructed by inserting the alpP::Kmr construct into the SmaI site of the suicide vector pGP704 (39), which was then transformed into E. coli SM10. The P. tunicata ΔalpP mutant was constructed by site-directed mutagenesis by conjugation with P. tunicata Smr and E. coli SM10 containing the vector pAP704. Exconjugants with the alpP::Kmr constructs inserted into the chromosome were selected by using VNSS plates supplemented with streptomycin (200 μg ml−1) and kanamycin (85 μg ml−1), and confirmation that the alpP::Kmr cassette has been inserted into the genome of P. tunicata was obtained by PCR. Loss of autocidal activity in the concentrated supernatants of P. tunicata exconjugants was confirmed by using the drop plate assay for the detection of AlpP activity as previously described (28). Biofilm development of the alpP mutant was compared with that of wild-type P. tunicata. Flow cell experiments were carried out, and biofilms were stained with the LIVE/DEAD BacLight bacterial viability kit as described above.
Add-back of purified AlpP to P. tunicata ΔalpP mutant and wild-type biofilms.
To determine whether AlpP could restore killing in P. tunicata ΔalpP mutant biofilms, we added purified AlpP into flow cell channels containing P. tunicata ΔalpP mutant biofilms. We also added purified AlpP to young wild-type P. tunicata biofilms, before the normal onset of cell death, to determine whether AlpP could induce early killing in the wild-type strain. AlpP was prepared and purified from the supernatant as previously described (28). AlpP (10 to 12 μg in dialysis buffer [20 mM Tris, 0.3 M NaCl]) was injected into the flow cells with a syringe needle. Silicone tubing on either side of the flow cell was then blocked off by using tubing clamps. As a control, dialysis buffer was inoculated into separate flow cell channels. Biofilms were incubated at room temperature for 5 h without flow before staining with the LIVE/DEAD BacLight bacterial viability kit and visualizing with the CLSM.
TEM examination of AlpP.
Recently, cell death during development of P. aeruginosa biofilms was linked to the activity of a P. aeruginosa prophage (54). To investigate the possibility that AlpP is also a protein component of a bacteriophage or phage-like bacteriocin, we examined purified native AlpP by transmission electron microscopy (TEM). An AlpP-containing ion-exchange fraction was run on a native PAGE gel as previously described (28). The AlpP protein band was extracted from the gel (46) and eluted into 20 mM Tris (pH 7.4). Samples were placed onto a carbon- and Formvar-coated copper grid and fixed with 0.1% (vol/vol) glutaraldehyde in phosphate-buffered saline for 5 min. The grid was washed several times with phosphate-buffered saline (pH 7.0) and stained with 2% phosphotungstic acid for 30 s. The stained grid was examined on a Hitachi H700 TEM at an accelerating voltage of 75 kV.
Nucleotide sequence accession number.
The primary nucleotide sequence data for the alpP gene has been deposited in the GenBank database under accession number AY 295768.
RESULTS AND DISCUSSION
P. tunicata biofilm development and cell death.
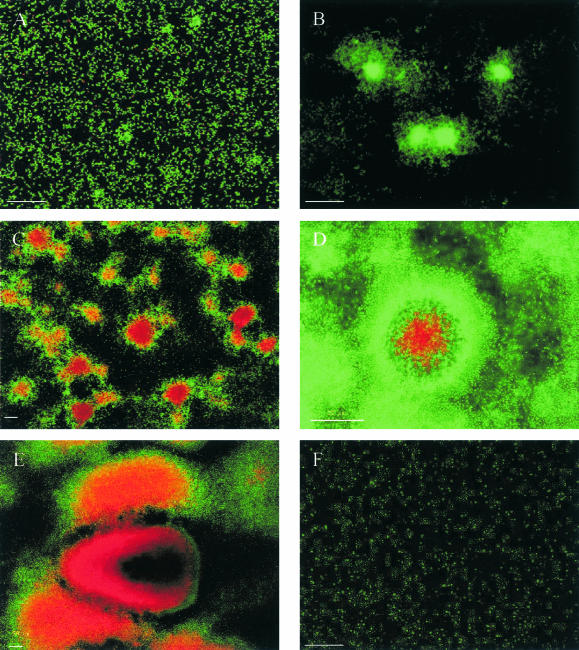
We characterized P. tunicata wild-type biofilm development and cell death in glass flow cells by using the LIVE/DEAD BacLight bacterial viability kit. Immediately after inoculation, single viable cells were observed attached to the substratum (Fig. 1A). After 24 h, microcolonies had developed (Fig. 1B) and no dead cells were visible within the biofilm. Between 48 and 96 h postinoculation, dead cells occurred in the interior portions of microcolonies and were surrounded by an outer layer of live cells (Fig. 1C and D). We observed dead cells and partially lysed cells as well as amorphous red propidium iodide-stained material, which was possibly DNA-containing debris from lysed cells. A subpopulation of cells in the region of killing remained viable. At this stage of biofilm development, the substratum was completely covered by bacteria. After 96 h, when killing had occurred inside all microcolonies, open voids within the regions of killing inside the microcolonies were observed (Fig. 1E). These open voids were similar to the voids and hollow microcolonies recently described for P. aeruginosa (45, 51). Regions of extensive killing subsequently occurred within and around microcolonies, and the biofilm structure started to disrupt and detach. Once the biofilm had dispersed, no microcolonies could be observed while only single live cells remained attached within the flow cell (Fig. 1F). Similar results were observed in five additional sets of experiments.
FIG. 1.
Biofilm development and cell death of the P. tunicata wild-type strain. Biofilms were stained with the BacLight LIVE/DEAD bacterial viability kit. Red propidium iodide-stained cells have a compromised cell membrane and are dead. Time points after inoculation are shown as follows: (A) 1 h; (B) 24 h; (C) 48 h; (D) 72 h; (E) 144 h; (F) 168 h. Bars, 50 μm.
Comparable cell death within mature microcolonies has recently been demonstrated in the opportunistic human pathogen P. aeruginosa (54), and other observations have shown that loss of viability also occurs inside microcolonies in biofilms formed by oral bacteria (2, 22). Thus, cell death inside microcolonies may be widespread among biofilm-forming bacteria, and we hypothesize that these killing events play an important role in biofilm development and dispersal. For example, nutrients released during cell death may benefit other bacteria within the biofilm because they provide an energy source for continued development and dispersal. However, little is known about the mechanism(s) by which hollow microcolonies are formed or by which cells disperse from the internal regions inside microcolonies. Generally, large-scale biofilm detachment and sloughing is known to be influenced by both physical and physiological properties. Hydrodynamic conditions such as shear stress and velocity of fluids can greatly affect the biofilm matrix and detachment of biofilms (5, 43). In addition to hydrodynamics, biofilm detachment is also influenced by environmental sensors and nutritional conditions. For example, it was shown that the global regulatory protein CsrA (carbon storage regulator) serves as an activator of biofilm dispersal through the regulation of intracellular glycogen biosynthesis and catabolism in E. coli (27). In the present study, we investigated whether the autotoxic protein AlpP of P. tunicata plays a role in biofilm killing and dispersal.
Identification and sequencing of alpP.
P. tunicata was previously found to produce a multisubunit (60 and 80 kDa) protein with autotoxic activity (28), which it releases into its surrounding environment when grown both as colonies on plates and in liquid culture (28). The complete sequence of the gene for the P. tunicata autotoxic protein was determined from DNA fragments drawn from a genomic library of this bacterium with the probes P1 and P2. The open reading frame of this gene encodes a 748-residue protein with a calculated molecular mass of 80.9 kDa, in close agreement with the large 80-kDa subunit observed in sodium dodecyl sulfate-PAGE. The gene was designated alpP (for “autolytic protein, Pseudoalteromonas”), and the primary nucleotide sequence data for the alpP gene has been deposited in the GenBank database (see above).
Because both the 80- and 60-kDa subunits of AlpP share an identical N-terminal sequence (28), we examined whether these subunits originate from the same gene. Southern analysis of P. tunicata genomic digests was carried out with the DNA probe P3 (specific for the sequence of the shared N-terminal region). The P3 probe hybridized to a single band in all digests (data not shown), indicating that both the 60- and 80-kDa protein subunits originate from a single gene. The mechanism by which the 60-kDa subunit is expressed from alpP is not clear; however, possibilities include the posttranslational proteolytic cleavage of the larger 80-kDa subunit or early termination of transcription of alpP.
The sequence obtained contains the elements necessary for transcription, with promoter prediction analysis revealing a putative promoter region upstream of the start codon and a ribosome-binding site, 5′-AGGA-3′, preceding the initiation codon. The AT content is also high (70%) where strand-opening is predicted to occur, from the −10 region through to the first three codons of the open reading frame. The original publication of the first 18 N-terminal amino acids of AlpP indicated that it was a previously undiscovered protein (28). The entire predicted amino acid sequence of AlpP presented here shows identity with only three hypothetical proteins, which were more-recently entered into GenBank. These matches were found within the genomes of Chromobacterium violaceum ATCC 12472 (GenBank accession number NP_902938), Magnetococcus sp. strain MC-1 (GenBank accession number ZP_00044814), and Caulobacter crescentus CB15 (GenBank accession number NP_419374), with identities of 35, 32, and 27%, respectively, each being hypothetical proteins of no known function.
Previously, killing in P. aeruginosa biofilms has been linked to the activity of a prophage that is encoded within the genome of P. aeruginosa (54). We therefore examined native PAGE-purified AlpP by using TEM to investigate whether AlpP may be a component of a bacteriophage or bacteriophage-like bacteriocin. However, images obtained from the electron microscope clearly showed no phage or phage-like particles in the purified AlpP band (data not shown). Although investigations by Kivelä et al. (30, 31) have shown that phage are present in at least two Pseudoalteromonas species, our results indicate that cell death in P. tunicata biofilms does not involve bacteriophage. P. tunicata and P. aeruginosa therefore appear to have acquired different mechanisms that cause cell death during biofilm development.
Biofilm development of a P. tunicata alpP mutant.
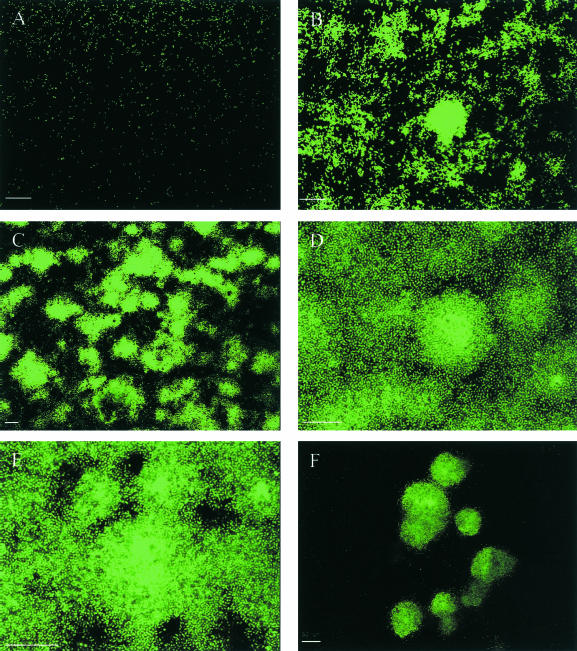
To investigate the role of alpP during P. tunicata biofilm growth, a ΔalpP mutant derivative of P. tunicata was generated. Biofilm development of the ΔalpP strain was investigated in comparison to wild-type P. tunicata. In a similar fashion to the wild-type, the alpP mutant formed a differentiated biofilm with a three-dimensional structure consisting of microcolonies (Fig. 2). We also characterized biofilms of the wild-type and the ΔalpP mutant strain with the biofilm quantification software COMSTAT (17). The results are summarized in Table 1. All assessed parameters showed no significant differences between the wild-type and the mutant strain after 1, 3, and 5 days of biofilm development. Thus, our data show that the alpP mutant is still capable of forming the normal biofilm architecture characteristic of wild-type P. tunicata.
FIG. 2.
The P. tunicata AlpP mutant does not show cell death during biofilm development. Biofilms were stained with the LIVE/DEAD BacLight bacterial viability kit. Time points after inoculation are shown as follows: (A) 1 h; (B) 24 h; (C) 48 h; (D) 72 h; (E) 144 h; (F) 168 h. Bars, 50 μm.
TABLE 1.
Analysis of P. tunicata wild-type and ΔalpP biofilms with COMSTAT
| Parameter (units) | Time (h) | Result for:
|
|
|---|---|---|---|
| Wild type | ΔalpP | ||
| Maximum thickness (μm) | 24 | 10.57 ± 3.21 | 16.67 ± 4.68 |
| 72 | 28.29 ± 5.59 | 24.00 ± 4.62 | |
| 120 | 38.00 ± 5.29 | 37.60 ± 2.19 | |
| Avg thickness (μm) | 24 | 2.01 ± 0.48 | 2.15 ± 1.98 |
| 72 | 14.45 ± 1.96 | 10.63 ± 3.62 | |
| 120 | 31.64 ± 6.7 | 24.95 ± 1.44 | |
| Total biomass (μm3 μm−2) | 24 | 2.12 ± 0.46 | 2.00 ± 1.78 |
| 72 | 11.77 ± 1.41 | 10.49 ± 4.87 | |
| 120 | 24.79 ± 5.64 | 27.38 ± 1.35 | |
| Roughness coefficient | 24 | 1.08 ± 0.21 | 1.38 ± 0.48 |
| 72 | 0.25 ± 0.07 | 0.49 ± 0.36 | |
| 120 | 0.11 ± 0.04 | 0.14 ± 0.02 | |
| Surface-to-biovolume ratio (μm2 μm−3) | 24 | 3.56 ± 0.46 | 2.12 ± 0.36 |
| 72 | 2.80 ± 0.58 | 1.36 ± 0.43 | |
| 120 | 2.43 ± 0.66 | 0.52 ± 0.09 | |
However, unlike wild-type P. tunicata, the alpP mutant did not undergo cell death at any stage of biofilm development, and no hollow cavities within microcolonies were observed. Moreover, detachment of the biofilm was considerably delayed, with the majority of microcolonies persisting after 9 days (wild-type biofilms were completely dispersed after 7 days) (Fig. 2F). These results suggest that AlpP-mediated killing affects both dispersal from within the interior portions of attached microcolonies as well as large-scale detachment of the whole biofilm.
Add-back of purified AlpP to P. tunicata biofilms.
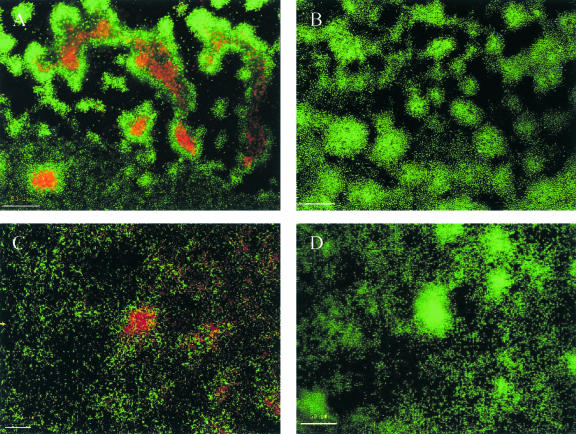
To provide direct evidence that AlpP is involved in cell death and subsequent detachment in P. tunicata biofilms, we added purified AlpP to P. tunicata ΔalpP 48-h biofilms in an attempt to restore AlpP-mediated killing in the biofilm. Similar to wild-type P. tunicata, the autotoxic protein caused cell death in the centers of microcolonies and also killed other cells throughout the biofilm (Fig. 3A). We also added AlpP protein to young wild-type biofilms (30 h) before the normal onset of killing within the microcolonies. Add-back of AlpP induced early killing in P. tunicata wild-type biofilms (Fig. 3C). Cell death occurred within microcolonies as well as in other regions of the biofilm. Interestingly, cells on the exterior of microcolonies were not killed by add-back of AlpP. While we understand that AlpP-mediated cell death is growth-phase-regulated in P. tunicata (28), the mode of action of AlpP is not fully understood. Thus, the means by which there is differential killing of subpopulations of cells within the biofilm remains to be fully explored.
FIG. 3.
Addition of purified of AlpP to P. tunicata biofilms. Biofilms were stained with the LIVE/DEAD BacLight bacterial viability kit. (A) Add-back of AlpP to P. tunicata ΔalpP 48-h biofilms; (B) P. tunicata ΔalpP mutant biofilm plus buffer control (20 mM Tris, 0.3 M NaCl); (C) AlpP added back to young (30 h) P. tunicata wild-type biofilms before the normal onset of killing within the biofilm; (D) P. tunicata wild-type biofilm plus buffer control (20 mM Tris, 0.3 M NaCl). Bars, 50 μm. Similar results were obtained in four replicate experiments.
Proposed ecological and developmental roles of AlpP-mediated cell death.
In this study, the inhibitory marine bacterium P. tunicata demonstrated a repeatable pattern of cell death during normal biofilm development. The involvement of the autotoxic protein AlpP suggests that this process is an evolved capacity and may benefit the bacteria at the multicellular level in a similar manner to that previously described for autolysis in M. xanthus and B. subtilis (15, 44, 55). Although the experiments presented here were carried out in an artificial flow cell environment, differentiated microcolony structures, similar to those reported here for P. tunicata biofilms, are commonly observed in natural and medical situations (1, 4, 48, 53). Moreover, several recent publications suggest that similar processes of cell death occur in microcolonies in more complex biofilms, such as those of dental plaque (2, 22) and within the biofilm flocs of wastewater treatment processes (38). Future experimentation will address the possible benefits that P. tunicata and other bacteria derive from processes of cell death during biofilm development.
One possibility is that cell death may function in P. tunicata biofilm dispersal. We observed that cell death occurred in localized regions within microcolonies of P. tunicata biofilms but that a subpopulation of cells remained viable in these regions. We propose that killing and lysis benefit this subpopulation of cells (through the release of nutrients and DNA) which then undergo continued differentiation and dispersal.
Another possible function of AlpP-mediated cell death in P. tunicata is that it may regulate biofilm growth on the surfaces of living hosts in the marine environment. Many sessile algae and animals have evolved defense mechanisms against fouling by producing metabolites that can influence the settlement, growth and survival of other organisms (for examples, see references 7 and 9). However, algae and animals lacking chemical and nonchemical defenses are thought to rely on secondary metabolites produced by associated surface bacteria, such as P. tunicata, as their defense against fouling (19, 32, 50). Cell death and dispersal of a subpopulation of cells within P. tunicata biofilms may protect its host against uncontrolled biofilm formation and fouling by P. tunicata itself.
Acknowledgments
We thank our colleagues at the University of New South Wales for their support.
This research was supported by grants from the Australian Research Council, Canberra, Australia, and the Leverhulme Trust, London, United Kingdom, and by the Centre for Marine Biofouling and Bio-Innovation at the University of New South Wales, Sydney, Australia.
REFERENCES
- 1.Anderson, G. G., J. J. Palermo, J. D. Schilling, R. Roth, J. Heuser, and S. J. Hultgren. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105-107. [DOI] [PubMed] [Google Scholar]
- 2.Auschill, T. M., N. B. Arweiler, L. Netuschil, M. Brecx, E. Reich, A. Sculean, and N. B. Artweiler. 2001. Spatial distribution of vital and dead microorganisms in dental biofilms. Arch. Oral Biol. 46:471-476. [DOI] [PubMed] [Google Scholar]
- 3.Costerton, J. W., Z. Lewandowski, D. DeBeer, D. Caldwell, D. Korber, and G. James. 1994. Biofilms, the customized microniche. J. Bacteriol. 176:2137-2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 5.Cowan, S. E., E. Gilbert, D. Liepmann, and J. D. Keasling. 2000. Commensal interactions in a dual-species biofilm exposed to mixed organic compounds. Appl. Environ. Microbiol. 66:4481-4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davey, M. E., and A. G. O'Toole. 2000. Microbial biofilms: from ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 64:847-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis, A. R., and A. E. Wright. 1990. Inhibtion of larval settlement by natural products from ascidian, Eudistoma olivaceum (Van Name). J. Chem. Ecol. 16:1349-1357. [DOI] [PubMed] [Google Scholar]
- 8.Debeer, D., P. Stoodley, F. Roe, and Z. Lewandowski. 1994. Effects of biofilm structures on oxygen distribution and mass-transport. Biotechnol. Bioeng. 43:1131-1138. [DOI] [PubMed] [Google Scholar]
- 9.De Nys, R., P. D. Steinberg, P. Willemsen, S. A. Dworjanyn, C. L. Gabelish, and R. J. King. 1994. Broad spectrum affects of secondary metabolites from the red algae Delisea pulchra in antifouling assays. Biofouling 8:259-271. [Google Scholar]
- 10.Egan, S., S. James, C. Holmström, and S. Kjelleberg. 2001. Inhibition of algal spore germination by the marine bacterium Pseudoalteromonas tunicata. FEMS Microbiol. Ecol. 35:67-73. [DOI] [PubMed] [Google Scholar]
- 11.Egan, S., S. James, C. Hölmstrom, and S. Kjelleberg. 2002. Correlation between pigmentation and antifouling compounds produced by Pseudoalteromonas tunicata. Environ. Microbiol. 4:433-442. [DOI] [PubMed] [Google Scholar]
- 12.Egan, S., S. James, and S. Kjelleberg. 2002. Identification and characterization of a putative transcriptional regulator controlling the expression of fouling inhibitors in Pseudoalteromonas tunicata. Appl. Environ. Microbiol. 68:372-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egan, S., T. Thomas, C. Holmström, and S. Kjelleberg. 2000. Phylogenetic relationship and antifouling activity of bacterial epiphytes from the marine alga Ulva lactuca. Environ. Microbiol. 2:343-347. [DOI] [PubMed] [Google Scholar]
- 14.Gauthier, G., M. Gauthier, and R. Christen. 1995. Phylogenetic analysis of the genera Alteromonas, Shewanella, and Moritella using genes coding for small-subunit rRNA sequences and division of the genus Alteromonas into two genera, Alteromonas (emended) and Pseudoalteromonas gen. nov., and proposal of twelve new species combinations. Int. J. Syst. Bacteriol. 45:755-761. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez-Pastor, J. E., E. C. Hobbs, and R. Losick. 2003. Cannibalism by sporulating bacteria. Science 301:510-513. [DOI] [PubMed] [Google Scholar]
- 16.Heydorn, A., B. Ersboll, J. Kato, M. Hentzer, M. R. Parsek, T. Tolker-Nielsen, M. Givskov, and S. Molin. 2002. Statistical analysis of Pseudomonas aeruginosa biofilm development: impact of mutations in genes involved in twitching motility, cell-to-cell signaling, and stationary-phase sigma factor expression. Appl. Environ. Microbiol. 68:2008-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heydorn, A., A. T. Nielsen, M. Hentzer, C. Sternberg, M. Givskov, B. K. Ersboll, and S. Molin. 2000. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 146:2395-2407. [DOI] [PubMed] [Google Scholar]
- 18.Holmes, A. J., N. A. Tujula, M. Holley, A. Contos, J. M. James, P. Rogers, and M. R. Gillings. 2001. Phylogenetic structure of unusual aquatic microbial formations in Nullarbor caves, Australia. Environ. Microbiol. 3:256-264. [DOI] [PubMed] [Google Scholar]
- 19.Holmström, C., D. Rittschoff, and S. Kjelleberg. 1992. Inhibition of attachment of larval barnacles, Balanus amphitrite and Ciona intestinales by a surface colonizing marine bacterium. Appl. Environ. Microbiol. 58:2111-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holmström, C., S. James, B. A. Neilan, D. C. White, and S. Kjelleberg. 1998. Pseudoalteromonas tunicata sp. nov., a bacterium that produces antifouling agents. Int. J. Syst. Bacteriol. 48(Pt. 4):1205-1212. [DOI] [PubMed] [Google Scholar]
- 21.Holmström, C., and S. Kjelleberg. 1999. Marine Pseudoalteromonas species are associated with higher organisms and produce biologically active extracellular agents. FEMS Microbiol. Ecol. 30:285-293. [DOI] [PubMed] [Google Scholar]
- 22.Hope, C. K., D. Clements, and M. Wilson. 2002. Determining the spatial distribution of viable and nonviable bacteria in hydrated microcosm dental plaques by viability profiling. J. Appl. Microbiol. 93:448-455. [DOI] [PubMed] [Google Scholar]
- 23.Ivanova, E. P., E. A. Kiprianova, V. V. Mikhailov, G. F. Levanova, A. D. Garagulya, N. M. Gorshkova, M. V. Vysotskii, D. V. Nicolau, N. Yumoto, T. Taguchi, and S. Yoshikawa. 1998. Phenotypic diversity of Pseudoalteromonas citrea from different marine habitats and emendation of the description. Int. J. Syst. Bacteriol. 48:247-256. [DOI] [PubMed] [Google Scholar]
- 24.Ivanova, E. P., T. Sawabe, Y. V. Alexeeva, A. M. Lysenko, N. M. Gorshkova, K. Hayashi, N. V. Zukova, R. Christen, and V. V. Mikhailov. 2002. Pseudoalteromonas issachenkonii sp. nov., a bacterium that degrades the thallus of the brown alga Fucus evanescens. Int. J. Syst. Bacteriol. Evol. Microbiol. 52:229-234. [DOI] [PubMed] [Google Scholar]
- 25.Ivanova, E. P., T. Sawabe, A. M. Lysenko, N. M. Gorshkova, V. I. Svetashev, D. V. Nicolau, N. Yumoto, T. Taguchi, S. Yoshikawa, R. Christen, and V. V. Mikhailov. 2002. Pseudoalteromonas ruthenica sp. nov., isolated from marine invertebrates. Int. J. Syst. Bacteriol. Evol. Microbiol. 52:235-240. [DOI] [PubMed] [Google Scholar]
- 26.Ivanova, E. P., L. S. Shevchenko, T. Sawabe, A. M. Lysenko, V. I. Svetashev, N. M. Gorshkova, M. Satomi, R. Christen, and V. V. Mikhailov. 2002. Pseudoalteromonas maricaloris sp. nov., isolated from an Australian sponge, and reclassification of [Pseudoalteromonas aurantia] NCIMB 2033 as Pseudoalteromonas flavipulchra sp. nov. Int. J. Syst. Bacteriol. Evol. Microbiol. 52:263-271. [DOI] [PubMed] [Google Scholar]
- 27.Jackson, D. W., K. Suzuki, L. Oakford, J. W. Simecka, M. E. Hart, and T. Romeo. 2002. Biofilm formation and dispersal under the influence of the global regulator CsrA of Escherichia coli. J. Bacteriol. 184:290-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.James, S. G., C. Holmström, and S. Kjelleberg. 1996. Purification and characterization of a novel antibacterial protein from the marine bacterium D2. Appl. Environ. Microbiol. 62:2783-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaplan, J. B., M. F. Meyenhofer, and D. H. Fine. 2003. Biofilm growth and detachment of Actinobacillus actinomycetemcomitans. J. Bacteriol. 185:1399-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kivela, H. M., N. Kalkkinen, and D. H. Bamford. 2002. Bacteriophage PM2 has a protein capsid surrounding a spherical proteinaceous lipid core. J. Virol. 76:8169-8178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kivela, H. M., R. H. Mannisto, N. Kalkkinen, and D. H. Bamford. 1999. Purification and protein composition of PM2, the first lipid-containing bacterial virus to be isolated. Virology 262:364-374. [DOI] [PubMed] [Google Scholar]
- 32.Kon-ya, K., N. Shimidzu, N. Otaki, A. Yokoyama, K. Adachi, and W. Miki. 1995. Inhibitory effect of bacterial ubiquinones on the settling of barnacle, Balanus amphitrite. Experientia 51:153-155. [Google Scholar]
- 33.Lawrence, J. R., D. R. Korber, B. D. Hoyle, J. W. Costerton, and D. E. Caldwell. 1991. Optical sectioning of microbial biofilms. J. Bacteriol. 173:6558-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee, S. O., J. Kato, N. Takiguchi, A. Kuroda, T. Ikeda, A. Mitsutani, and H. Ohtake. 2000. Involvement of an extracellular protease in algicidal activity of the marine bacterium Pseudoalteromonas sp. strain A28. Appl. Environ. Microbiol. 66:4334-4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis, K. 2000. Programmed death in bacteria. Microbiol. Mol. Biol. Rev. 64:503-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lovejoy, C., J. P. Bowman, and G. M. Hallegraeff. 1998. Algicidal effects of a novel marine Pseudoalteromonas isolate (class Proteobacteria, gamma subdivision) on harmful algal bloom species of the genera Chattonella, Gymnodinium, and Heterosigma. Appl. Environ. Microbiol. 64:2806-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marden, P., A. Tunlid, K. Malmcrona-Friberg, G. Odham, and S. Kjelleberg. 1985. Physiological and morphological changes during short term starvation of marine bacterial isolates. Arch. Microbiol. 142:326-332. [Google Scholar]
- 38.Meyer, R. L., A. M. Saunders, R. J. Zeng, J. Keller, and L. L. Blackall. 2003. Microscale structure and function of anaerobic-aerobic granules containing glycogen accumulating organisms. FEMS Microbiol. Ecol. 45:253-261. [DOI] [PubMed] [Google Scholar]
- 39.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moller, S., C. Sternberg, J. B. Andersen, B. B. Christensen, J. L. Ramos, M. Givskov, and S. Molin. 1998. In situ gene expression in mixed-culture biofilms: evidence of metabolic interactions between community members. Appl. Environ. Microbiol. 64:721-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamura, Y., T. Gojobori, and T. Ikemura. 1998. Codon usage tabulated from the international DNA sequence databases. Nucleic Acids Res. 26:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neidhardt, F. C., P. L. Bloch, and D. F. Smith. 1974. Culture medium for enterobacteria. J. Bacteriol. 119:736-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Purevdorj, B., J. W. Costerton, and P. Stoodley. 2002. Influence of hydrodynamics and cell signaling on the structure and behavior of Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 68:4457-4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenbluh, A., and E. Rosenberg. 1990. Role of autocide AMI in development of Myxococcus xanthus. J. Bacteriol. 172:4307-4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sauer, K., A. K. Camper, G. D. Ehrlich, J. W. Costerton, and D. G. Davies. 2002. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 184:1140-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scheer, J. M., and C. A. Ryan. 2001. A method for the quantitative recovery of proteins from polyacrylamide gels. Anal. Biochem. 298:130-132. [DOI] [PubMed] [Google Scholar]
- 47.Simidu, U., K. Kita-Tsukamoto, T. Yasumoto, and M. Yotsu. 1990. Taxonomy of four marine bacterial strains that produce tetrodotoxin. Int. J. Syst. Bacteriol. 40:331-336. [DOI] [PubMed] [Google Scholar]
- 48.Singh, P. K., A. L. Schaefer, M. R. Parsek, T. O. Moninger, M. J. Welsh, and E. P. Greenberg. 2000. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 407:762-764. [DOI] [PubMed] [Google Scholar]
- 49.Skovhus, L. T., N. Ramsing, C. Holmström, S. Kjelleberg, and I. Dhallof. 2004. Real-time quantitative PCR for assessment of abundance of Pseudoalteromonas species in marine samples. Appl. Environ. Microbiol. 70:2373-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas, R. W. S. P., and D. Allsopp. 1983. The effects of certain periphytic marine bacteria upon the settlement and growth of Enteromorpha, a fouling alga. Biodeterioration 5:348-357. [Google Scholar]
- 51.Tolker-Nielsen, T., U. C. Brinch, P. C. Ragas, J. B. Andersen, C. S. Jacobsen, and S. Molin. 2000. Development and dynamics of Pseudomonas sp. biofilms. J. Bacteriol. 182:6482-6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watnick, P. I., and R. Kolter. 1999. Steps in the development of a Vibrio cholerae El Tor biofilm. Mol. Microbiol. 34:586-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Webb, J. S., M. Givskov, and S. Kjelleberg. 2003. Bacterial biofilms: prokaryotic adventures in multicellularity. Curr. Opin. Microbiol. 6:578-585. [DOI] [PubMed] [Google Scholar]
- 54.Webb, J. S., L. S. Thompson, S. James, T. Charlton, T. Tolker-Nielsen, B. Koch, M. Givskov, and S. Kjelleberg. 2003. Cell death in Pseudomonas aeruginosa biofilm development. J. Bacteriol. 185:4585-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wireman, J. W., and M. Dworkin. 1977. Developmentally induced autolysis during fruiting body formation by Myxococcus xanthus. J. Bacteriol. 129:798-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoshikawa, K., T. Takadera, K. Adachi, M. Nishijima, and H. Sano. 1997. Korormicin, a novel antibiotic specifically active against marine gram-negative bacteria, produced by a marine bacterium. J. Antibiot. (Tokyo) 50:949-953. [DOI] [PubMed] [Google Scholar]