Abstract
Although age-related differences in white matter have been well documented, the degree to which regional, tract-specific effects can be distinguished from global, brain-general effects is not yet clear. Similarly, the manner in which global and regional differences in white matter integrity contribute to age-related differences in cognition has not been well established. To address these issues, we analyzed diffusion tensor imaging measures from 52 younger adults (18–28) and 64 older adults (60–85). We conducted principal component analysis on each diffusion measure, using data from eight individual tracts. Two components were observed for fractional anisotropy: The first comprised high loadings from the superior longitudinal fasciculi and corticospinal tracts, and the second comprised high loadings from the optic radiations. In contrast, variation in axial, radial, and mean diffusivities yielded a single-component solution in each case, with high loadings from most or all tracts. For fractional anisotropy, the complementary results of multiple components and variability in component loadings across tracts suggest regional variation. However, for the diffusivity indices, the single component with high loadings from most or all of the tracts suggests primarily global, brain-general variation. Further analyses indicated that age was a significant mediator of the relation between each component and perceptual-motor speed. These data suggest that individual differences in white matter integrity, and their relation to age-related differences in perceptual-motor speed, represent influences that are beyond the level of individual tracts, but the extent to which regional or global effects predominate may differ between anisotropy and diffusivity measures.
Keywords: aging, diffusion tensor imaging, fractional anisotropy, cognition, mediation
Introduction
Cognitive functions such as perception and attention rely on widely distributed networks in the brain (McIntosh 2000; Mesulam 1990), and these networks critically depend on white matter for their efficiency (Filley 2005; Schmahmann et al. 2008). Age-related decline, even in the absence of disease, occurs in some aspects of cognition, such as perceptual-motor speed (Salthouse 1996, 2000; Salthouse and Madden 2007), attention (Kramer and Madden 2008; Verhaeghen and Cerella 2002), and episodic memory (Burke and Mackay 1997; Zacks et al. 2000; Park et al. 2002). Age-related declines have also been observed in the integrity of cerebral white matter, and these changes in white matter, by disrupting the networks critical for cognitive functioning, may contribute to the changes in cognitive performance associated with normal aging (Bartzokis et al. 2004; Bennett and Madden 2013; Carmichael and Lockhart 2012; Madden et al. 2012; Madden et al. 2009a; Sullivan and Pfefferbaum 2006, 2011).
Diffusion tensor imaging (DTI) is a widely used method for investigating white matter. DTI provides in vivo measures of the rate (e.g., mean diffusivity - MD) and directionality (i.e., fractional anisotropy - FA) of the diffusion of water molecules across tissue, and thus can be used to characterize tissue integrity (Basser 1995; Mori 2007; Jones 2008; Beaulieu 2002; Le Bihan 2003). In general, higher FA and lower MD values indicate higher levels of tissue integrity. DTI measures of the components of diffusivity provide additional information. Axial diffusivity (AD), the rate of diffusion along the principal direction, has been related to axonal integrity (Budde et al. 2007; Song et al. 2003); and radial diffusivity (RD), the average rate of diffusion orthogonal to the principal direction, has been related to the degree of myelination (Klawiter et al. 2011; Song et al. 2003; Song et al. 2002). However, while DTI-based estimation of white matter tract organization exhibits a high degree of anatomic validity (Catani et al. 2002; Miller et al. 2011), the constituent measures of diffusivity and anisotropy must be interpreted with caution, because these measures are sensitive to the mathematical and geometrical properties of the associated data, and the neurophysiological basis for specific diffusivity measures is not as yet completely defined (Jones et al. 2012; Wheeler-Kingshott and Cercignani 2009).
Neuroimaging studies commonly report decreases in FA and increases in MD as a function of increasing adult age, suggesting age-related decline in white matter tissue integrity (Kochunov et al. 2012; Lebel et al. 2012; Voineskos et al. 2012; Westlye et al. 2010; Stadlbauer et al. 2012). The pattern of age-related differences in AD and RD is complex but tends to be more pronounced for RD, possibly indicating greater age-related differences in myelin integrity (Bennett et al. 2010; Burzynska et al. 2010). Although age-related differences in white matter have been well documented, the regional pattern of age-related change has not been completely specified. Age-related effects have been observed more prominently in frontal brain regions than in more posterior regions (Davis et al. 2009; Sullivan et al. 2006; Zahr et al. 2009), and in superior compared to inferior regions such as within the internal capsule (Sullivan et al. 2010). These regional age-related trends may reflect the vulnerability of later myelinating tracts to the effects of aging.
Previous research has focused on specific regions of white matter or individual tracts, typically without consideration of the degree to which effects are shared across tracts. That is, variation in the properties of white matter may not be entirely specific to individual tracts but instead may represent more global effects, common to the brain as a whole. In fact, recent evidence suggests that measures of white matter are highly correlated across white matter tracts, particularly within homologous tracts (Lövdén et al. 2012; Penke et al. 2010; Wahl et al. 2010; Li et al. 2012). A critical finding is that of Penke et al. (2010), who focused on a sample of 132 older adults (71 – 73 years of age). Conducting principal component analysis (PCA) on each of several DTI measures (FA, AD, RD, MD) from eight white matter tracts, Penke et al. found that a single principal component explained approximately 45% of the variance in each measure, and that many or most of the individual tracts loaded highly on each principal component. Further, two of these global components (those for FA and RD) were correlated with information processing speed, but not with general intelligence or memory. Their results suggest that, among older adults, individual differences in white matter integrity are shared globally throughout the brain and are related to a specific aspect of cognition.
Lövdén et al. (2012) also investigated DTI data from older adults (60–87 years of age) to investigate global and regional variation in white matter integrity. Using structural equation modeling, they compared two models: one positing a general factor (i.e., similar cross-tract correlations) and the other positing tract-specific effects (i.e., each tract-tract correlation as a separate factor). Although a global factor was evident in the DTI data, in contrast to Penke et al. the model that explained the most variance contained factors for each specific bilateral tract. Moreover, age-related differences in inter-tract correlations occurred in only a subset of regions, further indicating that age-related effects were not homogeneous across all the tracts. Also supportive of a regional account, Li et al. (2012) conducted an independent component analysis (ICA) of whole-brain FA maps (for adults 20–50 years of age) and found that multiple independent components emerged, which corresponded to anatomically defined white matter pathways. The Li et al. investigation, however, did not address age-related differences, and the Penke et al. and Lövdén et al. studies, while providing analyses of age-related effects, limited their participant samples to older adults. Additionally, none of these previous studies investigated the potential role of white matter anisotropy or diffusivity as a mediator (Baron and Kenny 1986; Judd and Kenny 1981) of cognition.
Building on these previous findings, in the present study we used PCA to identify brain-general and regionally specific components of individual differences in DTI variables (FA, AD, RD, and MD) across regions of interest defined to capture white matter pathways. Our analyses extend the previous age-related studies (Li et al. 2012; Lövdén et al. 2012; Penke et al. 2010) by including younger as well as older adults. We selected pathways critical for inter-hemispheric communication (genu and splenium of the corpus callosum), language and attention (superior longitudinal fasciculus; SLF), motor functioning (corticospinal tract; CST), and visual perception (optic radiations; OR). We hypothesized that if a global, brain-general effect accounts for individual differences in cerebral white matter, then, for each of the DTI variables, PCA should yield a single component with high loadings from most or all of the tracts. Alternatively, if regional or tract-specific effects account for the individual differences, then we would expect a different PCA pattern: for each DTI variable, PCA should yield either multiple components (corresponding to individual tracts or tract groups) or a single-component driven by a small number of tracts. Either of these patterns would reflect regional variability in white matter integrity. Additionally, because age-related differences in raw DTI measures have been reported previously, such as a decline in anisotropy and increase in diffusivity (Kochunov et al. 2012; Lebel et al. 2012; Voineskos et al. 2012; Westlye et al. 2010; Stadlbauer et al. 2012), we examined the relationship between the PCA-derived components and age.
We also investigated the relation between the PCA components of the DTI variables and behavioral measures of cognition. Previous research suggests that individual differences in white matter contribute to age-related differences in cognition (Madden et al. 2009b; Gold et al. 2010; Salami et al. 2012; Perry et al. 2009; Borghesani et al. 2013) but the pattern of this relationship is still unclear. The Penke et al. (2010) and other data (Lu et al. 2013) suggest that white matter integrity is particularly important for speed-dependent measures of cognition, but substantial variability exists in the brain regions and behavioral tasks that covary with adult age (Bennett et al. 2011; Bennett et al. 2012; Kennedy and Raz 2009). Here we examined age-related differences in measures of perceptual-motor speed (digit-symbol performance) and vocabulary. Because it is well established that age-related decline is more prominent in perceptual-motor speed (and other measures of fluid intelligence and executive function) than in semantic memory (and other measures of crystallized intelligence and linguistic ability; (Burke and Mackay 1997; Park et al. 2002; Salthouse 1991b), we hypothesized that the DTI components (whether global or tract-specific) would mediate the age-related variance in perceptual-motor speed but not vocabulary.
Methods
Participants
The analyses were conducted on data sets from previously published studies (Bucur et al. 2008; Madden et al. 2009b), as well as previously unpublished data. The initial sample included 123 participants, who were healthy, right-handed, community-dwelling adults with normal or corrected to normal vision and no history of neurological or psychological disorders. From a screening questionnaire, all participants were free from major medical conditions (including diabetes, atherosclerosis, and neurological and psychiatric disorders), other than mild essential hypertension (Christiansen and MacDonald 2009). One participant in the younger group was hypertensive and untreated. Twenty-one of the 64 older participants were hypertensive, 14 of these individuals were treated. Because this is a variable known to influence the brain and MRI measurements, the presence of hypertension was partialed out from the PCA component scores and subsequent analyses. None reported taking other medications that might affect the brain. All participants were screened for good near vision (corrected, < 20/40 acuity), and visual acuity was not significantly correlated with any of the component scores or with the FA and diffusivity values. Data for seven participants were eliminated due to missing data in one or more critical variable(s) of interest. The final data set thus contained 116 individuals, 52 younger adults (24 females) between 18 and 28 years of age (M = 23.72, SD = 2.66) and 64 older adults (35 females) between 60 and 85 years of age (M = 68.82, SD = 4.94). Demographic and psychometric data are presented in Table 1. All participants scored 27 or above on the Mini-Mental State Exam (Folstein et al. 1975). Age groups did not differ in years of education (Table 1). Each participant provided informed consent and was paid for his or her participation. All experimental procedures were approved by the Duke University Medical Center Institutional Review Board.
Table 1.
Participant Characteristics by Age Group
| Younger Adults | Older Adults | |
|---|---|---|
| Age (years) | 23.72 (2.66) | 68.82 (4.94) *** |
| Education (years) | 15.96 (2.23) | 16.45 (2.46) |
| Semantic Memory (WAIS Vocabulary) |
65.31 (3.63) | 64.13 (4.54) |
| Perceptual-Motor Speed (WAIS Digit Symbol) |
1311.83 (206.13) | 1809.28 (285.98) *** |
| 3T scanner (n) | 28 | 59 |
| 4T scanner (n) | 24 | 5 |
Note. n = 52 younger adults and 64 older adults. Values are means with standard deviations in parentheses. Semantic Memory = vocabulary subtest of the Wechsler Adult Intelligence Scale-Revised [WAIS, (Wechsler 1981)]; Perceptual-Motor Speed = reaction time in ms on a computer test of digit-symbol coding similar to the Digit Symbol Substitution subtest test of the WAIS.
p < .0001
Neuroimaging
Image acquisition
Data were collected on two different magnetic resonance imaging (MRI) scanners at the Brain Imaging and Analysis Center, at the Duke University Medical Center. For all participants, we collected a sagittal localizer scan to identify the anterior and posterior commissures for slice selection, a high resolution T1 weighted series, and DTI scans. In addition, for all sessions a semi-automated high-order shimming program was used to ensure global field homogeneity. The exact parameters of each series varied slightly depending on the scanner and are reported below.
Twenty-nine participants were scanned on a 4.0 Tesla GE LX Nvi MRI scanner equipped with a 41 mT/m gradient coil. Radio frequency (RF) transmission and reception was achieved with a birdcage RF head coil (General Electric, Milwaukee, Wisconsin, USA). High-resolution T1 images were acquired using a 3D fSPGR pulse sequence (TR = 12.3ms; TE = 5.4ms; ti = 300ms; FOV = 24.0cm2; flip angle = 20° voxel size = 1 × 1 x 2 mm; 60 contiguous oblique-axial slices parallel to the AC-PC plane). Diffusion MR scans were acquired for each participant (TR = 30,000ms; TE = 138.8ms; FOV = 24cm2; flip angle = 90° voxel size = 1.875 × 1.875 × 3.8 mm; 30 contiguous oblique-axial slices parallel to the AC-PC plane; 6 diffusion-weighted directions; b = 1000s/mm2; 1 non-diffusion-weighted reference image).
Eighty-seven participants were scanned on a 3.0 Tesla GE Signa Excite HD MRI scanner equipped with 50 mT/m gradients. An eight-channel head coil was used for RF transmission and reception (General Electric, Milwaukee, Wisconsin, USA). High-resolution T1 images were acquired using a 3D fSPGR pulse sequence (TR = 7.4ms; TE = 3ms; ti = 450ms; FOV = 25.6 cm2; flip angle = 12° voxel size = 1 × 1 x 1.2 mm; 104 contiguous slices). Diffusion MR scans were acquired for each participant (TR = 17,000ms; TE = 86.7 msec; FOV = 25.6 cm2; flip angle = 90°; voxel size = 1 × 1 x 2.4 mm; 52 contiguous oblique-axial slices parallel to the AC-PC plane; 15 diffusion-weighted directions; b = 1000s/mm2; 1 non-diffusion-weighted reference image).
Because there were scanner and sequence variations, signal fluctuation to noise ratios (SFNR), derived from an agar phantom, were included as statistical controls in the analyses. Different field strengths and number of diffusion-weighted gradients are known to affect SNR and diffusion measurements (Jones 2004; Polders et al. 2011; Reischauer et al. 2009; Vollmar et al. 2010; Zhang et al. 2009; Zhu et al. 2011); thus statistically controlling for SFNR can be a viable method to address scanner and sequence variability. We partialed out SFNR during the PCAs, thereby removing the associated variance from the extracted components and from subsequent analyses which utilized those components (i.e., age group tests and mediation tests). We combined data sets across scanners to maximize our sample size and to ensure a sufficient sample size for a reliable PCA. Details on the distribution of participants across scanners can be found in Table 1. Examples of raw diffusion-weighted gradient and FA images are shown in Supplementary Figure 1.
White matter tract definition and DTI preprocessing
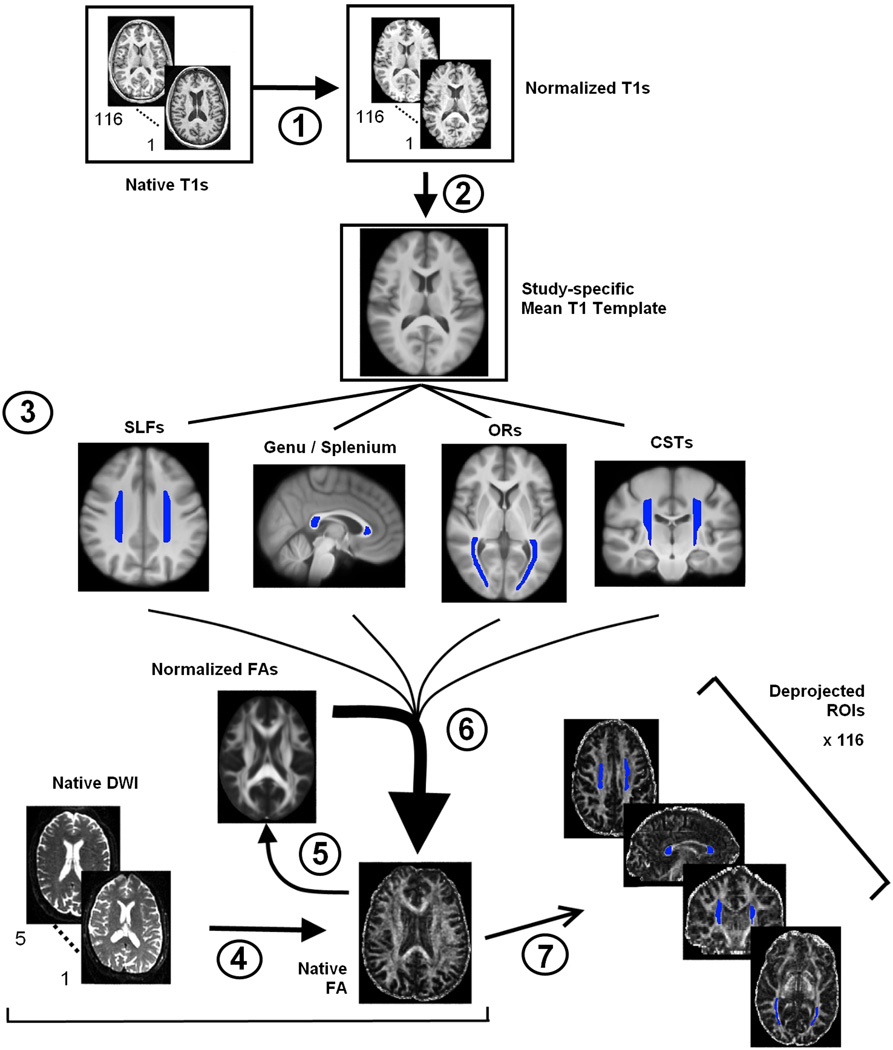
An overview of our data analysis procedure is provided in Figure 1, which illustrates seven general stages. Following visual inspection of each image for sufficient quality and orientation, we used brain extraction tools (Smith 2002) to create a normalized T1 image for each participant, using the Montreal Neurological Institute (MNI) standard 1 mm template (Stage 1). We concatenated the 116 images into a 4D volume, and with voxel-wise averaging combined the images into a study-specific structural template in MNI space (Stage 2).
Figure 1.
Overview diagram of data processing steps. Stage 1. Normalization of structural images to standard MNI space. Stage 2. Creation of study-specific structural template in MNI space. Stage 3. ROI definition of the eight white matter tracts. Stage 4. Averaging and preprocessing of diffusion data and tensor-fitting to create FA maps. Stage 5. Normalization of FA space to MNI space. Stage 6. Deprojection of white matter ROIs using the inverse transformation parameters from the FA to MNI normalization. Stage 7. Quality assessment of deprojected ROIs and extraction of FA, AD, RD, and MD.
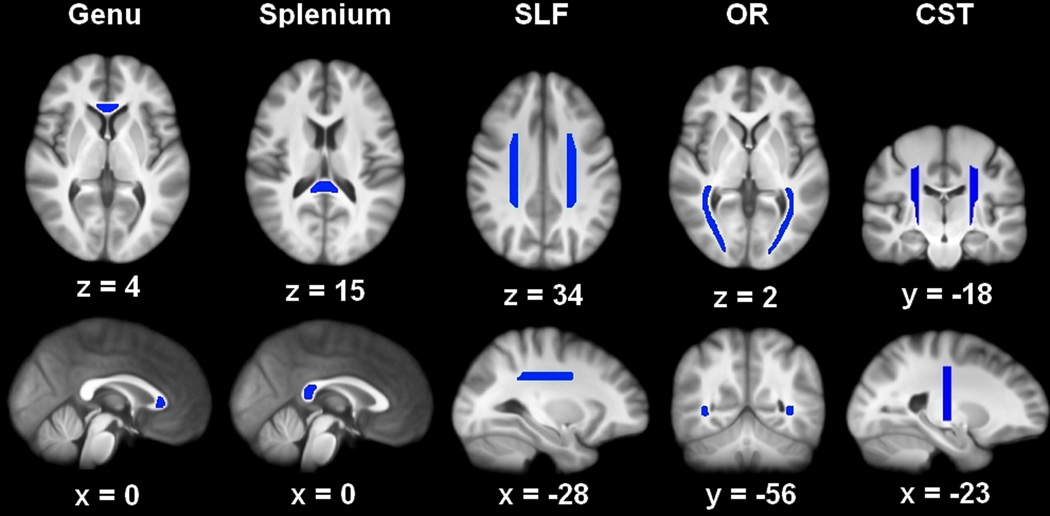
We then drew regions corresponding to eight white matter tracts on the averaged, study-specific template: the genu and splenium of the corpus callosum and, in both hemispheres: SLF, CST, and OR (Stage 3). These regions are illustrated in Figure 2. The genu and splenium were chosen for their role in inter-hemispheric communication, the SLF for its role in attention and language (Bartolomeo et al. 2007; Saur et al. 2008), the CST for its role in motor control (Taylor and Gandevia 2004), and the OR for its role in visual perception (Toosy et al. 2004). We created each of the eight white matter tracts once on the study-specific structural template, using Mango 2.5 (Multi-image Analysis GUI, http://ric.uthscsa.edu/mango/) and guided by a white matter atlas (Oishi et al. 2011). Anatomical guidelines used for tract definition are presented in the ROI Drawing section of the Supplementary Materials.
Figure 2.
The eight white matter tracts on which analyses were conducted: genu and splenium, bilateral corticospinal tract, CST (sagittal view is of the left hemisphere tract), bilateral optic radiation, OR, and bilateral superior longitudinal fasciculus, SLF (sagittal view is of left hemisphere tract).
We conducted tensor-fitting on the diffusion-weighted images (DWI) for each participant to create an FA map in native space (Stage 4). We normalized each participant’s native FA image to the MNI FA template (FMRIB58_FA_1mm) using non-linear transformations and visually inspected the normalized output for sufficient quality (Stage 5). We used the inverse transformation parameters from the previous step (FA to MNI normalization) to deproject each tract from template to native space (Stage 6). Each deprojected tract for each participant was visually inspected to ensure proper alignment with the white matter tract of interest. Finally, we visual inspected and adjusted the deprojected tracts as needed and extracted the diffusivity indices FA, AD, RD, and MD (Stage 7). Potential partial volume effects were minimized by setting the tract threshold at FA > .25 prior to data extraction and by demarcating the boundaries of the ROIs by at least two voxels within the visible termination of white matter to avoid partial intrusion of the gray matter or cerebrospinal fluid (CSF).
We preprocessed the DTI data with FSL 4.1.5 tools (FMRIB, Oxford University, UK, Smith et al. 2004). For each participant, the DWIs were concatenated into a 4D volume. Participants had between 1 and 5 DWI scans, which were all collected in a single session. We then applied five processing steps to the 4D volume, sequentially: a) brain extraction (Smith 2002); b) correction of eddy current-induced artifacts and head motion within and across scans using affine registration of the different scans to the first scan in the volume; c) rotation of the diffusion gradients to correct for shifts during the affine registration; d) averaging of the multiple scans into a single volume; and e) estimation of the DTI parameters FA, AD, RD, and MD. Following these processing steps, we visually inspected the alignment of the eigenvectors with the anatomical structures to ensure accuracy.
Statistical Analyses
Behavioral data
In the original studies, two behavioral measures were collected from all participants as part of the psychometric screening: Digit Symbol reaction time (RT) and Vocabulary. These two measures were used as our proxy for perceptual-motor speed and semantic memory, respectively. Initially, Vocabulary was the raw score on the subtest of the Wechsler Adult Intelligence Scale-Revised (WAIS, Wechsler 1981), and Digit Symbol was mean RT on a computer test of digit-symbol coding similar to the Digit Symbol Substitution subtest test of the WAIS. The final Vocabulary and Digit Symbol values were z-scores standardized using the mean and standard deviation from the younger group. This method allowed us to evaluate age-related differences in standard units relative to the younger cohort (Salthouse 1991b).
DTI data
To investigate the potential presence of global and tract-specific effects we conducted a single PCA across all participants for each of the four DTI measures, partialing out SFNR and the presence or absence of hypertension. This data reduction method computes orthogonal principal components (linear combinations of optimally weighted variables) from the covariance matrix of the data across the eight white matter tracts (Abdi and Williams 2010). PCA is well suited to discriminating between global and regional patterns because it reveals latent component(s) that characterize the maximal amount of unique variance in the manifest (observed) measures. Moreover, PCA, unlike ICA, provides an ordering of the extracted components (Hyvarinen and Oja 2000). For each of the DTI measures (FA, AD, RD, and MD), the PCA characterized the covariation across the eight white matter tracts (dependent variables). Moreover, for each DTI measure, PCA yields a component score for each participant which is a weighted estimate of how their individual data correlates to the overall component. In addition, a component loading is calculated for each tract, which reflects the degree to which variability in that tract contributes to the component. Thus, the pattern of results in terms of the number of components and the pattern across tracts (i.e., component loadings) can be used to infer global or regional patterns of variability, and can be analyzed with other variables of interest to investigate age-related differences. A PCA component was retained for further analysis when the eigenvalue was greater than 1.0 and confirmed by scree plot (Cattell 1966; Kaiser 1960). Component loadings greater than .70 were considered high, given our sample size and number of variables (Buja and Eyuboglu 1992).
Mediation analyses
To investigate the relations among white matter integrity, age, and behavior, we compared three different models of mediation (Salthouse 2011) with each PCA component. Mediation models were conducted using a multiple regression procedure (Baron and Kenny 1986; Judd and Kenny 1981). Model 1 proposes that the brain measure, one of the DTI components, is a mediator between the predictor variable, age, and the outcome variable, cognitive measure. In Model 2, the cognitive measure is the proposed mediator between age and the brain measure. In Model 3, age is the proposed mediator between the brain and cognitive measures. Following Baron and Kenny (1986), we considered mediation to be a warranted interpretation when, within a model, linear regression confirmed that all four of the following effects were significant: 1) between predictor and outcome variables; 2) between predictor and mediator variables; 3) between mediator and outcome variables, controlled statistically (partialed) for the predictor; and 4) a reduction or elimination of the relation between predictor and outcome variables, after controlling for the mediator. If mediation was observed, we then calculated the degree to which variance associated with the predictor was reduced (attenuated), when controlled for variance associated with the mediator (Salthouse 1991a). Given prior results in the literature, we hypothesized that the DTI components would mediate the relationship between age and perceptual-motor speed, but not the relationship between age and vocabulary (Madden et al. 2009b; Gold et al. 2010; Salami et al. 2012; Perry et al. 2009; Borghesani et al. 2013). However, to fully assess potential mediational relationships we tested the models formed by the alternative arrangement of the mediator and predictor variables (Salthouse 2011).
Results
Principal component analyses
The PCA components and associated loadings are presented in Table 2. The FA data yielded two components: the first with high loadings from the SLF and CST bilaterally, and the second with high loadings from the OR bilaterally. In contrast, analyses of AD, RD, and MD each yielded a single-component solution with high loadings from bilateral SLF and CST. In addition, the MD component also had high loadings from the genu, splenium, and bilateral OR; the AD component had additional high loadings from the genu and splenium; and the RD component had additional high loadings from bilateral OR.
Table 2.
Principal Component Analysis of DTI Variables
| Component Loadings | |||||
|---|---|---|---|---|---|
| FA 1 | FA 2 | AD 1 | RD 1 | MD 1 | |
| Genu | .38 | .38 | .76 | .57 | .73 |
| Splenium | .35 | .39 | .70 | .58 | .75 |
| SLF left | .85 | −.19 | .81 | .94 | .92 |
| SLF right | .82 | −.22 | .86 | .92 | .92 |
| CST left | .78 | −.42 | .88 | .85 | .93 |
| CST right | .84 | −.37 | .89 | .90 | .95 |
| OR left | .50 | .70 | .67 | .76 | .81 |
| OR right | .55 | .70 | .63 | .83 | .80 |
| Variance Explained (%) | |||||
| 44.28 | 20.90 | 60.76 | 64.69 | 73.23 | |
Note. Principal component analysis was conducted within the FA, AD, RD, and MD variables. FA 1 = first component for FA data; FA 2 = second component for FA data; AD 1 = first component for AD data; RD 1 = first component for RD data; MD 1 = first component for MD data. SLF = superior longitudinal fasciculus; CST = corticospinal tract; OR = optic radiations. Values are unrotated loadings and variance explained from the principal component analysis. Loadings > .70 (shown in bold font) are interpreted as high. The FA data yielded a two-component solution whereas the AD, RD, and MD data each yielded a single-component solution.
To determine if the components varied across age groups we submitted the component scores as dependent variables into a multivariate analysis of variance with Age Group and Component type as independent variables (it is important to note that SFNR and hypertension nuisance variables were already statistically controlled for in the initial PCA stage). There was a significant main effect of Age Group, F(1, 114) = 4.03, p < .05, and a significant Age Group x Component interaction, F(4, 456) = 37.81, p < .0001. The effect size associated with age group varied across the components, and univariate tests indicated that the age group difference was significant for each component individually (see Table 3), with F(1, 115) > 13.0, p < .001, in each case.
Table 3.
Principal Component Scores by Group
| Component Scores: Mean (SD) | |||||
|---|---|---|---|---|---|
| FA 1 | FA 2 | AD 1 | RD 1 | MD 1 | |
| Older | −.44 (.94) | −.38 (.96) | .30 (1.01) | .48 (.91) | .43 (.93) |
| Younger | .54 (.82)*** | .47 (.89)*** | −.37 (.91)** | −.60 (.84)*** | −.53 (.88)*** |
| Effect Size | .23 | .17 | . 10 | .27 | .21 |
Note. Principal component analysis was conducted within the FA, AD, RD, and MD variables. FA 1 = first component for FA data; FA 2 = second component for FA data; AD 1 = first component for AD data; RD 1 = first component for RD data; MD 1 = first component for MD data. Mean component scores and standard deviations (SD), across all tracts, are shown by group.
significant group difference at p < .0001,
significant group difference at p < .001.
Effect sizes are semi-partial omega-squared values calculated from univariate tests of Age Group.
Mediation analyses
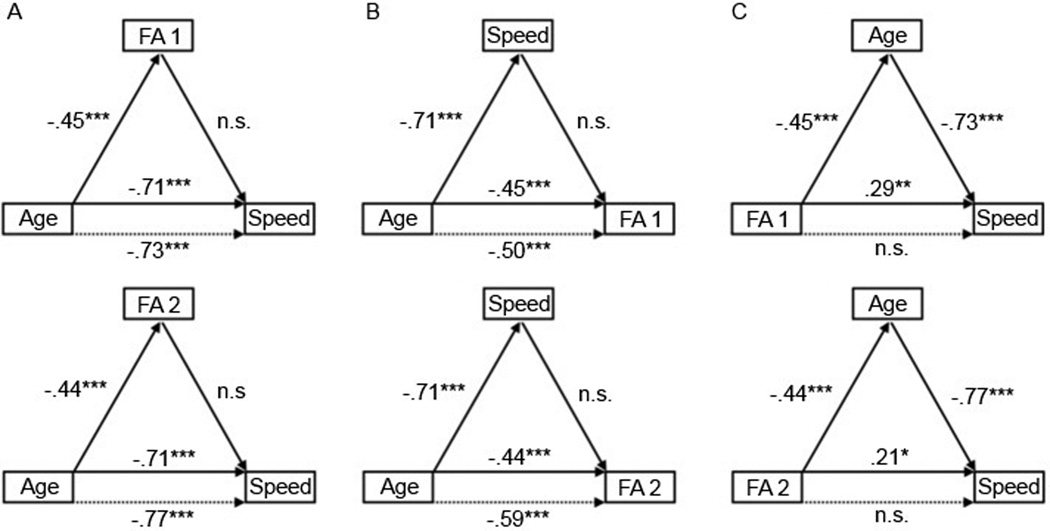
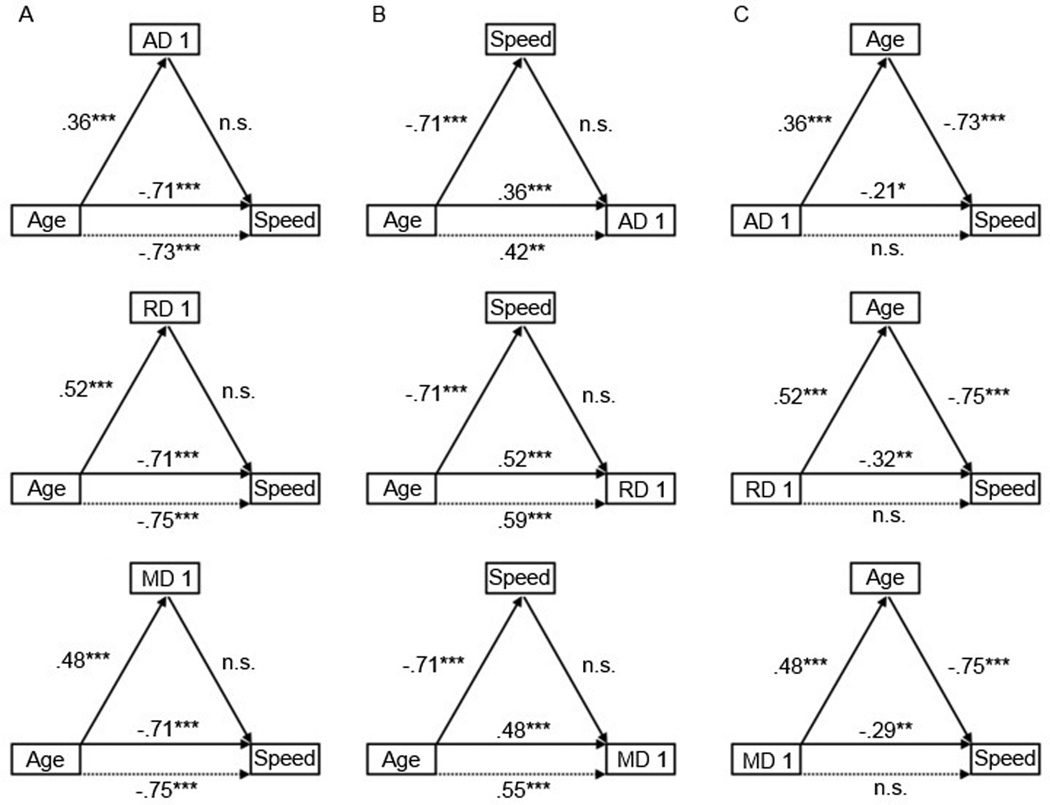
We used a four-step mediation procedure to test alternative models of the relations among age, the PCA-derived components of the DTI measures (which were corrected for SFNR and hypertension nuisance variables), and perceptual-motor speed (Baron and Kenny 1986; Salthouse 2011). For each of the DTI components, when we modeled the PCA component as a mediator between age and our cognitive measures (Model 1), the components were not statistically significant mediators of the age-related variance in speed (see Figures 3 and 4). Entering each of the components separately before age in a regression model predicting speed did not significantly reduce the original age-speed relation. Additionally, none of the components had an age-independent relation to perceptual-motor speed. This suggests that individual differences in the covariance of these properties of white matter, as defined by the PCA-derived components of the DTI measures, do not have a mediational role in the relation between age and perceptual-motor speed.
Figure 3.
Results from the mediation analyses of the relations among years of age (Age), perceptual-motor speed (Speed), and both the first (FA 1) and the second FA component (FA 2). A. Model 1, which posited each FA component as the mediator of the Age-Speed relation, failed because each FA component did not significantly predict Speed after controlling for Age and because controlling for each FA component did not reduce the Age-Speed relation (dashed line). B. Model 2, which posited Speed as the mediator between Age and each FA component, similarly failed. C. Model 3, which posited Age as the mediator between each FA component and Speed, passed the criteria for mediation because controlling for Age (dashed line) statistically eliminated the original relation between each FA component and Speed. Standardized regression coefficients are shown for each link with corresponding significance: n.s. = p > .05, * = p < .05, ** = p < .001, and *** = p < .0001.
Figure 4.
Results from the mediation analyses of the relations among years of age (Age), perceptual-motor speed (Speed), and the diffusivity components AD 1, RD 1, and MD 1. A. Model 1, which posited each component as the mediator of the Age-Speed relation, failed because each component did not significantly predict Speed after controlling for Age and because controlling for each component did not reduce the Age-Speed relation (dashed line). B. Model 2, which posited Speed as the mediator between Age and each component, similarly failed. C. Model 3, which posited Age as the mediator between each component and Speed, passed the criteria for mediation because controlling for Age (dashed line) statistically eliminated the original relation between each component and Speed. Standardized regression coefficients are shown for each link with corresponding significance: n.s. = p > .05, * = p < .05, ** = p < .001, and *** = p < .0001.
We tested an alternative model (Model 2) in which the cognitive measure is the proposed mediator between age and the brain measure (see Figures 3 and 4). However, as with Model 1, perceptual-motor speed was not a statistically significant mediator of the relation between age and DTI measures, primarily because speed was not related to any of the components after the effect of age was controlled.
A third possibility (Model 3) is that age was related to both the DTI components and perceptual-motor speed, and that this relationship accounted for some or all of the variance between each component and speed. The results indicated that this model was significant for each DTI component (see Figures 3 and 4). Age was a significant predictor of each component and a significant predictor of speed independently of each component. Initially, each component significantly predicted speed. However, after the inclusion of age in the regression, each component no longer predicted speed. These results are consistent with age-related variance completely mediating the relation between the DTI components and perceptual-motor speed.
Follow-up stepwise regressions were conducted to estimate the amount of attenuation in each component-speed relation after controlling for age. The original variance in speed explained by the first FA component (R2 = .0834), the second FA component (R2 = .0482), the AD component (R2 = .0474), the RD component (R2 = .1009), and the MD component (R2 = .0845) were nearly eliminated (FA 1: R2 = .0010; FA 2: R2 = .0169; AD: R2 = .0070; RD: R2 = .0055; MD: R2 = .0071) after controlling for age.
To directly measure the size of these mediation effects, effect sizes were calculated by multiplying the standardized regression coefficients of the intermediary paths (Preacher and Kelley 2011). To directly test the significance of these mediation effects, Sobel tests (Sobel 1982) and bootstrapping tests (Preacher and Hayes 2004) were conducted. The effect size (ab) was substantial for the first FA component (.33), the second FA component (.34), the AD component (−.26), the RD component (−.39), and the MD component (−.36). The Sobel test was highly significant for all components: first FA component (Sobel Z = 4.75; p < .0001), second FA component (Sobel Z = 4.66; p < .0001), AD component (Sobel Z = −3.82; p < .0001), RD component (Sobel Z = −5.40; p < .0001), and MD component (Sobel Z = −5.02; p < .0001). Bootstrapping tests, which provide the advantage of not assuming normality in the indirect effect (Hayes 2009), showed that the confidence intervals of the indirect effect did not include 0, thus further confirming the significance of age as mediator of the component-speed relation.
We also repeated the exact same mediational steps outlined above with vocabulary z-scores as the cognitive measure; however, none of the models passed the tests for mediation. This was due to non-significant relations either between vocabulary and age or between vocabulary and the DTI components.
Discussion
Principal component analyses
Recent research with healthy older adults has demonstrated that individual DTI variables are correlated across tracts, making a strong case for the usefulness of dimension-reduction techniques such as PCA to more clearly characterize age-related differences by characterizing inter-tract covariation. However, it is not yet clear whether individual differences in white matter tract integrity, as defined by DTI variables, represent global, brain-general influences (Penke et al. 2010) or regional, tract-specific influences (Lövdén et al. 2012). In the present study, we extended investigations into this issue with PCAs conducted for the first time on DTI data in both older and younger adults. Moreover, we assessed whether these structural components mediated cognitive performance in perceptual-motor speed and vocabulary.
Considering first the FA data, our PCA results revealed two components (Table 2), each with variable loadings across tracts, suggesting that a single, global effect did not capture the FA data sufficiently. The first FA component had high loadings within superior longitudinal fasciculi (SLF) and corticospinal tracts (CST), whereas the second component had high loadings within the optic radiations (OR). Both FA component scores were higher for younger adults than for older adults (Table 3), indicating that these measures of inter-tract covariation were also sensitive to the well-known age-related differences in FA (Bennett and Madden 2013).
The regional covariation of tract FA which we observed is consistent with similar studies showing that FA covaries across bilateral tract sets (Lövdén et al. 2012) or along groups of tracts or tract segments (Li et al. 2012). It is also consistent with studies showing differential rates of age-related changes across different white matter tracts (Raz and Rodrigue 2006; Westlye et al. 2010), with studies reporting the presence of an anterior-posterior gradient (Brickman et al. 2012; Davis et al. 2009; Gunning-Dixon et al. 2009; Salat et al. 2005; Sullivan et al. 2006) and a superior-inferior gradient (Sullivan et al. 2010), and others suggesting that association tracts are more affected by aging than projection tracts (Stadlbauer et al. 2008). The deterioration of myelin sheaths is one hypothesized mechanism of aging-related differences in white matter (Meier-Ruge et al. 1992) and this process has been shown to be regionally heterogeneous (Bartzokis et al. 2004), consistent with the retrogenesis hypothesis that proposes that tracts which develop myelin later are among the first to demyelinate (Brickman et al. 2012). Other potential mechanisms of age-related white matter decline are axonal loss and degeneration (Charlton et al. 2006; Meier-Ruge et al. 1992), which may be further influenced by cerebrovascular disease resulting in lesions, gliosis, and infarcts (Braffman et al. 1988b, a; de Groot et al. 2001; DeCarli et al. 2005; Scheltens et al. 1995). Local manifestations of these microstructural alterations may translate into regional variability within macrostructural measures of tracts such as FA.
Although the regional variation in our FA components is consistent with previous reports, the anatomical basis for the different tract loadings on the two FA components is not entirely clear. The grouping of the tracts, for example, did not dissociate along a functional dimension. The highest loadings for the first FA component were from tracts associated with sensory-motor regions (CST) and frontoparietal language regions (SLF). The OR tracts, responsible for the transmission of visual sensory information from the lateral geniculate nucleus of the thalamus to the visual cortex, loaded on a separate component from the CST and SLF. Additionally, the two FA components were not defined by the direction of the tract. The first component contained tracts with a superior-inferior orientation (CST) and those with an anterior-posterior orientation (SLF). The two-component solution may be driven by the qualitatively different functions associated with the CST and SLF (motor and cognitive) versus the OR (vision).
In contrast to our dual-component solution for FA, we observed a single-component solution for the PCA on each of the diffusivity measures, AD, RD, and MD (Table 2). Similar to the first FA component, the diffusivity components also demonstrated strong covariation between the bilateral SLF and CST systems, along with the genu and splenium and/or bilateral OR. A single component upon which most or all tracts load highly indicated that the majority of variance in the diffusivity data from these tracts was shared (roughly 60–75% variance explained in our data, see Table 2), which is consistent with a global model as proposed by Penke et al. (2010). However, it is interesting to note that even within this predominantly global pattern there persisted some regional variation in the loadings. There has been support for global variation in diffusion-based measures (Nusbaum et al. 2001; Rovaris et al. 2003; Stadlbauer et al. 2012), although Lövdén et al. (2012) found that a regional model captured the most variance. Indeed, it is well documented that, throughout most of the brain, older adults generally show increases in MD (Kochunov et al. 2012; Lebel et al. 2012; Voineskos et al. 2012; Westlye et al. 2010), and age-related differences in AD and RD also occur with the most pronounced effects for RD (Bennett and Madden 2013), perhaps reflecting greater age-related demyelination (Bennett et al. 2010; Burzynska et al. 2010).
These findings suggest that global and regional effects may depend on the specific aspect of white matter being examined, a conclusion also offered by Lövdén et al. (2012). Simultaneous global and regional effects might occur due to differential rates or magnitudes of change across different tracts. In other words, although aging affects cerebral white matter in general (allowing global trends to be interpreted from the data), some fiber systems (e.g., association and frontal pathways) experience age-related changes more strongly (allowing regional trends to emerge).
There are some important differences between the present study and previous research that might account for the different findings. First, the Penke et al. (2010) and Lövdén et al. (2012) included only older adults, whereas the present data estimated the PCA structure for younger and older adults combined. To address this issue, we conducted an additional PCA on both age groups separately which was similar to the combined-groups analysis (see Supplementary Table 1 and Supplementary Table 2). Thus, inclusion of younger adults does not seem to be driving the differences. Additionally, the PCA structure of the older group remained nearly identical even after partialing out years of age (see Supplementary Table 3), demonstrating the age-independent nature of these brain-general and regional trends within the older group.
Second, studies differed in the tracts that were investigated which may contribute to the relative prominence of global versus tract-specific effects. Similar to our study, Lövdén et al. (2012) included bilateral SLF and CST, but they did not include optic radiations, genu or splenium. While Penke et al. (2010) included the genu and splenium of the corpus callosum as we did, they also included tracts that we did not (i.e., bilateral arcuate fasciculi, cingulum bundles, and uncinate fascicule). Moreover, the Penke et al. results show some variability in tract loadings suggesting additional variability beyond the global effect. One potentially useful method to address the issue of tract selection is to adopt a voxel-wise whole brain approach (Li et al. 2012). But one limitation of this skeleton-based approach is that only voxels at the center of tracts are analyzed, thus losing some sensitivity.
We also observed significant differences between age-groups on all of the PCA components, which is consistent with age-related decline in FA and increased diffusivity (Bennett and Madden 2013; Bennett et al. 2010; Burzynska et al. 2010; Carmichael and Lockhart 2012; Davis et al. 2009; Madden et al. 2012; Madden et al. 2009a; Sullivan and Pfefferbaum 2006, 2011). While some have suggested that age-related effects in cerebral white matter are the results of differences in myelin integrity and/or macrostructural organization (leading primarily to changes in FA and RD), rather than axonal loss per se (leading primarily to changes in AD), it is important to note that differences associated with individual DTI variables are the result of many influences and cannot as yet be attributed to specific physiological mechanisms (Jones et al. 2012; Wheeler-Kingshott and Cercignani 2009).
Mediation
Consistent with previous behavioral investigations of cognitive aging (Park et al. 2002; Salthouse 1991b), our results confirmed strong age-related decline in perceptual-motor speed but not vocabulary (Table 1). However, in contrast to our initial hypothesis, none of the DTI components mediated the age-related variance in speed. Thus, although the components exhibited some sensitivity to age-related differences, they did not mediate the age-related differences in speed as might be expected from previous findings (Gold et al. 2010; Madden et al. 2009b; Perry et al. 2009; Salami et al. 2012; Borghesani et al. 2013). These previous studies, however, used more cognitively demanding tasks, as compared to our relatively simple test of perceptual-motor speed. Additionally, we tested for relations between the DTI components and speed within each age group, however, none of these regressions were significant.
We did observe mediation in an alternative model that hypothesized age as the mediator of the component-speed relation. In this case, age accounted for a substantial portion of the variance shared by speed and each component. Although each component was initially associated with speed, this was due to the fact that these components shared variance with age. Other variables associated in common with age and the tracts measured, such as efficiency of visual sensory (for OR) or motor functioning (for CST) may be influencing perceptual-motor speed. Our results highlight the importance of testing alternative models of the relations among DTI measures, aging, and cognitive measures (Salthouse 2011).
Limitations
Our data were acquired from two different scanners (see Supplementary Figure 1 for example gradient and FA images) with differences in field strength and number of diffusion-weighted gradients, factors which are known to affect signal to noise ratio (SNR), measurement accuracy, and variability (Jones 2004; Polders et al. 2011; Reischauer et al. 2009; Vollmar et al. 2010; Zhang et al. 2009; Zhu et al. 2011). We addressed these potential effects by partialing out participant-specific SFNR values (derived from a phantom) in the PCA analyses, thereby removing the associated inter-scanner variance (Friedman and Glover 2006; Zhu et al. 2011). To further address this limitation of inter-scanner differences, we conducted an additional PCA on data from only one scanner which revealed a very similar PCA structure (see Supplementary Table 4).
Another potential limitation of the present study is the selection of white matter tracts. Based on previous research, we selected tracts that support inter-hemispheric communication (genu, splenium), as well as tracts that connect cortical and subcortical regions associated with visual perception (OR), attention and language (SLF), and motor control (CST). Although comparable to the number of tracts investigated by other studies, these represent only a subset of all white matter tracts. Moreover, our use of regions of interest as opposed to a tractography-based approach presents a higher chance that the results may have been more influenced by partial-voluming effects or from crossing fibers.
Another potential limitation is that our indices of perceptual-motor speed and semantic memory were limited to single measures (e.g., the Digit Symbol task for speed and WAIS vocabulary for semantic memory). A more robust method would be to use composite scores across a variety of speed-based tasks, such as the PCA-based processing speed factor used by Penke et al. (2010), because such methods more clearly disentangle the manifest and latent variables associated with the behavioral performance measures. We also acknowledge that mediation analysis based on cross-sectional data may not be as accurate as mediation analysis based on longitudinal data (Lindenberger et al. 2011; Maxwell and Cole 2007). Future studies investigating the potential mediational role of PCA-derived white matter components in the relation between age and perceptual-motor speed could benefit from longitudinal data where available.
We note that although we chose to combine all participants from both groups into one PCA, an alternative method could have been to first conduct PCA in an independent dataset (either younger or older only, or combined) and then use those component loadings to generate new component scores (or weight the diffusion variables) in a different data set. This method could produce a more robust estimate. We partly addressed this issue by demonstrating that separate PCAs on the younger (Supplementary Table 1) and older groups (Supplementary Table 2 and 3) yielded highly similar patterns to the PCA structure of both groups combined.
One final point is that while PCA, unlike ICA, provides the advantage of extracting uncorrelated components that are ordered by the amount of unique variance explained, it does not determine that those components are statistically independent, as ICA does. We chose to use PCA because of its advantage of ordering the components by unique variance explained in the data. Interestingly, the only study that we know of to have used ICA to characterize aging of white matter integrity (Li et al. 2012) reported a regional pattern of covariation within the FA data, similar to the dual-component solution for FA which we report here.
Conclusion
We used PCA to characterize the relative contribution of global and regional influences on individual differences in DTI variables. The PCA analysis of FA yielded two components, which is consistent with a pattern of regional variation. In contrast, analyses of the diffusivity variables yielded in each case a single component with high loadings from most or all tracts, consistent with a pattern of global or brain-general variation. We hypothesized that PCA-derived components of white matter integrity would mediate the age-related differences associated with perceptual-motor speed. However, in contrast to our predictions we found that each component was related to speed only by virtue of its relation to age. These data highlight the potential for using PCA to investigate age-related trends in white matter properties, and the importance of testing alternative models of age-related effects in the context of mediation.
Supplementary Material
Acknowledgments
This research was supported by grants R01 AG034138 (MTD) and R01 AG039684 (DJM) from the National Institute on Aging. We thank Guy Potter, Ying-hui Chou, Emily L. Parks, David A. Hoagey, Sally Ann B. Cocjin, and Maxwell Horowitz for assistance. We also thank the staff and scientists at the Brain Imaging and Analysis Center, especially the center director, Allen W. Song, for their support of this project.
Abbreviations
- AC-PC
anterior commissure – posterior commissure
- AD
axial diffusivity
- CST
corticospinal tract
- DTI
diffusion tensor imaging
- DWI
diffusion-weighted image
- fSPGR
fast spoiled gradient echo imaging
- FA
fractional anisotropy
- FMRIB
functional MRI of the brain
- FOV
field of view
- ICA
independent component analysis
- MD
mean diffusivity
- MNI
Montreal Neurological Institute
- MR
magnetic resonance
- MRI
magnetic resonance imaging
- OR
optic radiations
- PCA
principal component analysis
- RD
radial diffusivity
- RF
radio frequency
- ROI
region of interest
- RT
reaction time
- SFNR
signal fluctuation to noise ratio
- SLF
superior longitudinal fasciculus
- SNR
signal to noise ratio
- TE
echo time
- TR
repetition time
- WAIS
Wechsler adult intelligence scale
References
- Abdi H, Williams LJ. Principal component analysis. WIREs: Computational Statistics. 2010;2(4):433–459. [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bartolomeo P, Thiebaut de Schotten M, Doricchi F. Left unilateral neglect as a disconnection syndrome. Cereb Cortex. 2007;17(11):2479–2490. doi: 10.1093/cercor/bhl181. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Sultzer D, Lu PH, Nuechterlein KH, Mintz J, Cummings JL. Heterogeneous age-related breakdown of white matter structural integrity: implications for cortical “disconnection” in aging and Alzheimer’s disease. Neurobiol Aging. 2004;25(7):843–851. doi: 10.1016/j.neurobiolaging.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8(7–8):333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Madden DJ. Disconnected aging: Cerebral white matter integrity and age-related differences in cognition. Neuroscience. 2013 doi: 10.1016/j.neuroscience.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett IJ, Madden DJ, Vaidya CJ, Howard DV, Howard JH., Jr Age-related differences in multiple measures of white matter integrity: A diffusion tensor imaging study of healthy aging. Hum Brain Mapp. 2010;31(3):378–390. doi: 10.1002/hbm.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett IJ, Madden DJ, Vaidya CJ, Howard JH, Jr, Howard DV. White matter integrity correlates of implicit sequence learning in healthy aging. Neurobiol Aging. 2011;322317(12):e2311–e2312. doi: 10.1016/j.neurobiolaging.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett IJ, Motes MA, Rao NK, Rypma B. White matter tract integrity predicts visual search performance in young and older adults. Neurobiol Aging. 2012;33433(2):e421–e431. doi: 10.1016/j.neurobiolaging.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghesani PR, Madhyastha TM, Aylward EH, Reiter MA, Swarny BR, Warner Schaie K, Willis SL. The association between higher order abilities, processing speed, and age are variably mediated by white matter integrity during typical aging. Neuropsychologia. 2013;51(8):1435–1444. doi: 10.1016/j.neuropsychologia.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braffman BH, Zimmerman RA, Trojanowski JQ, Gonatas NK, Hickey WF, Schlaepfer WW. Brain MR: pathologic correlation with gross and histopathology. 1. Lacunar infarction and Virchow-Robin spaces. AJR Am J Roentgenol. 1988a;151(3):551–558. doi: 10.2214/ajr.151.3.551. [DOI] [PubMed] [Google Scholar]
- Braffman BH, Zimmerman RA, Trojanowski JQ, Gonatas NK, Hickey WF, Schlaepfer WW. Brain MR: pathologic correlation with gross and histopathology. 2. Hyperintense white-matter foci in the elderly. AJR Am J Roentgenol. 1988b;151(3):559–566. doi: 10.2214/ajr.151.3.559. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Meier IB, Korgaonkar MS, Provenzano FA, Grieve SM, Siedlecki KL, Wasserman BT, Williams LM, Zimmerman ME. Testing the white matter retrogenesis hypothesis of cognitive aging. Neurobiol Aging. 2012;33(8):1699–1715. doi: 10.1016/j.neurobiolaging.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucur B, Madden DJ, Spaniol J, Provenzale JM, Cabeza R, White LE, Huettel SA. Age-related slowing of memory retrieval: contributions of perceptual speed and cerebral white matter integrity. Neurobiol Aging. 2008;29(7):1070–1079. doi: 10.1016/j.neurobiolaging.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde MD, Kim JH, Liang HF, Schmidt RE, Russell JH, Cross AH, Song SK. Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magn Reson Med. 2007;57(4):688–695. doi: 10.1002/mrm.21200. [DOI] [PubMed] [Google Scholar]
- Buja A, Eyuboglu N. Remarks on Parallel Analysis Multivariate Behavioral Research. 1992;27:509–540. doi: 10.1207/s15327906mbr2704_2. [DOI] [PubMed] [Google Scholar]
- Burke DM, Mackay DG. Memory, language, and ageing. Philosophical Transactions of the Royal Society of London. 1997;352(1363):1845–1856. doi: 10.1098/rstb.1997.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzynska AZ, Preuschhof C, Backman L, Nyberg L, Li SC, Lindenberger U, Heekeren HR. Age-related differences in white matter microstructure: region-specific patterns of diffusivity. Neuroimage. 2010;49(3):2104–2112. doi: 10.1016/j.neuroimage.2009.09.041. [DOI] [PubMed] [Google Scholar]
- Carmichael O, Lockhart S. The role of diffusion tensor imaging in the study of cognitive aging. Current Topics in Behavioral Neuroscience. 2012;11:289–320. doi: 10.1007/7854_2011_176. [DOI] [PubMed] [Google Scholar]
- Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17(1):77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Cattell RB. The scree test for the number of factors. Multivariate Behavioral Research. 1966;1(2):245–276. doi: 10.1207/s15327906mbr0102_10. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Barrick TR, McIntyre DJ, Shen Y, O’Sullivan M, Howe FA, Clark CA, Morris RG, Markus HS. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology. 2006;66(2):217–222. doi: 10.1212/01.wnl.0000194256.15247.83. [DOI] [PubMed] [Google Scholar]
- Christiansen MH, MacDonald MC. A usage-based approach to recursion in sentence processing. Language Learning. 2009;59:126–161. [Google Scholar]
- Davis SW, Dennis NA, Buchler NG, White LE, Madden DJ, Cabeza R. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage. 2009;46(2):530–541. doi: 10.1016/j.neuroimage.2009.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot JC, de Leeuw FE, Oudkerk M, Hofman A, Jolles J, Breteler MM. Cerebral white matter lesions and subjective cognitive dysfunction: the Rotterdam Scan Study. Neurology. 2001;56(11):1539–1545. doi: 10.1212/wnl.56.11.1539. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, Beiser A, D’Agostino R, Wolf PA. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiol Aging. 2005;26(4):491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Filley CM. White matter and behavioral neurology. Ann N Y Acad Sci. 2005;1064:162–183. doi: 10.1196/annals.1340.028. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friedman L, Glover GH. Reducing interscanner variability of activation in a multicenter fMRI study: controlling for signal-to-fluctuation-noise-ratio (SFNR) differences. Neuroimage. 2006;33(2):471–481. doi: 10.1016/j.neuroimage.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Gold BT, Powell DK, Xuan L, Jicha GA, Smith CD. Age-related slowing of task switching is associated with decreased integrity of frontoparietal white matter. Neurobiol Aging. 2010;31(3):512–522. doi: 10.1016/j.neurobiolaging.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Brickman AM, Cheng JC, Alexopoulos GS. Aging of cerebral white matter: a review of MRI findings. Int J Geriatr Psychiatry. 2009;24(2):109–117. doi: 10.1002/gps.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. Beyond Baron and Kenny: Statistical Mediation Analysis in the New Millenium. Communication Monographs. 2009;76(4):408–420. [Google Scholar]
- Hyvarinen A, Oja E. Independent component analysis: algorithms and applications. Neural Netw. 2000;13(4–5):411–430. doi: 10.1016/s0893-6080(00)00026-5. [DOI] [PubMed] [Google Scholar]
- Jones DK. The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: a Monte Carlo study. Magn Reson Med. 2004;51(4):807–815. doi: 10.1002/mrm.20033. [DOI] [PubMed] [Google Scholar]
- Jones DK. Studying connections in the living human brain with diffusion MRI. Cortex. 2008;44(8):936–952. doi: 10.1016/j.cortex.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Jones DK, Knosche TR, Turner R. White matter integrity, fiber count, and other fallacies: The do’s and don’ts of diffusion MRI. Neuroimage. 2012 doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Judd CM, Kenny DA. Process analysis: estimating mediation in treatment evaluations. Eval Rev. 1981;5(5):602–619. [Google Scholar]
- Kaiser HF. The application of electronic computers to factor analysis. Educational and Psychological Measurement. 1960;20:141–151. [Google Scholar]
- Kennedy KM, Raz N. Aging white matter and cognition: differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia. 2009;47(3):916–927. doi: 10.1016/j.neuropsychologia.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klawiter EC, Schmidt RE, Trinkaus K, Liang HF, Budde MD, Naismith RT, Song SK, Cross AH, Benzinger TL. Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. Neuroimage. 2011;55(4):1454–1460. doi: 10.1016/j.neuroimage.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Williamson DE, Lancaster J, Fox P, Cornell J, Blangero J, Glahn DC. Fractional anisotropy of water diffusion in cerebral white matter across the lifespan. Neurobiol Aging. 2012;33(1):9–20. doi: 10.1016/j.neurobiolaging.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer AF, Madden DJ. Attention. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. Third edn. New York: Psychology Press; 2008. pp. 189–249. [Google Scholar]
- Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nature Reviews Neuroscience. 2003;4(6):469–480. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 2012;60(1):340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Li YO, Yang FG, Nguyen CT, Cooper SR, LaHue SC, Venugopal S, Mukherjee P. Independent component analysis of DTI reveals multivariate microstructural correlations of white matter in the human brain. Hum Brain Mapp. 2012;33(6):1431–1451. doi: 10.1002/hbm.21292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberger U, von Oertzen T, Ghisletta P, Hertzog C. Cross-sectional age variance extraction: what’s change got to do with it? Psychol Aging. 2011;26(1):34–47. doi: 10.1037/a0020525. [DOI] [PubMed] [Google Scholar]
- Lövdén M, Laukka EJ, Rieckmann A, Kalpouzos G, Li TQ, Jonsson T, Wahlund LO, Fratiglioni L, Backman L. The dimensionality of between-person differences in white matter microstructure in old age. Hum Brain Mapp. 2012 doi: 10.1002/hbm.21518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu PH, Lee GJ, Tishler TA, Meghpara M, Thompson PM, Bartzokis G. Myelin breakdown mediates age-related slowing in cognitive processing speed in healthy elderly men. Brain Cogn. 2013;81(1):131–138. doi: 10.1016/j.bandc.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Burzynska A, Potter GG, Chen NK, Song AW. Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochim Biophys Acta. 2012;1822(3):386–400. doi: 10.1016/j.bbadis.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Song AW. Cerebral white matter integrity and cognitive aging: contributions from diffusion tensor imaging. Neuropsychol Rev. 2009a;19(4):415–435. doi: 10.1007/s11065-009-9113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Costello MC, Bucur B, White LE, Cabeza R, Davis SW, Dennis NA, Provenzale JM, Huettel SA. Cerebral white matter integrity mediates adult age differences in cognitive performance. J Cogn Neurosci. 2009b;21(2):289–302. doi: 10.1162/jocn.2009.21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell SE, Cole DA. Bias in cross-sectional analyses of longitudinal mediation. Psychol Methods. 2007;12(1):23–44. doi: 10.1037/1082-989X.12.1.23. [DOI] [PubMed] [Google Scholar]
- McIntosh AR. Towards a network theory of cognition. Neural Netw. 2000;13(8–9):861–870. doi: 10.1016/s0893-6080(00)00059-9. [DOI] [PubMed] [Google Scholar]
- Meier-Ruge W, Ulrich J, Bruhlmann M, Meier E. Age-related white matter atrophy in the human brain. Ann N Y Acad Sci. 1992;673:260–269. doi: 10.1111/j.1749-6632.1992.tb27462.x. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol. 1990;28(5):597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Miller KL, Stagg CJ, Douaud G, Jbabdi S, Smith SM, Behrens TE, Jenkinson M, Chance SA, Esiri MM, Voets NL, Jenkinson N, Aziz TZ, Turner M, Johansen-Berg H, McNab JA. Diffusion imaging of whole, post-mortem human brains on a clinical MRI scanner. Neuroimage. 2011;57(1):167–181. doi: 10.1016/j.neuroimage.2011.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S. Introduction to diffusion tensor imaging. Amsterdam: Elsevier; 2007. [Google Scholar]
- Nusbaum AO, Tang CY, Buchsbaum MS, Wei TC, Atlas SW. Regional and global changes in cerebral diffusion with normal aging. AJNR Am J Neuroradiol. 2001;22(1):136–142. [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Faria AV, Van Zijl PCM, Mori S. MRI Atlas of Human White Matter. Second edn. Academic Press; 2011. [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychol Aging. 2002;17(2):299–320. [PubMed] [Google Scholar]
- Penke L, Munoz Maniega S, Murray C, Gow AJ, Hernandez MC, Clayden JD, Starr JM, Wardlaw JM, Bastin ME, Deary IJ. A general factor of brain white matter integrity predicts information processing speed in healthy older people. J Neurosci. 2010;30(22):7569–7574. doi: 10.1523/JNEUROSCI.1553-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry ME, McDonald CR, Hagler DJ, Jr, Gharapetian L, Kuperman JM, Koyama AK, Dale AM, McEvoy LK. White matter tracts associated with set-shifting in healthy aging. Neuropsychologia. 2009;47(13):2835–2842. doi: 10.1016/j.neuropsychologia.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polders DL, Leemans A, Hendrikse J, Donahue MJ, Luijten PR, Hoogduin JM. Signal to noise ratio and uncertainty in diffusion tensor imaging at 1.5, 3.0, and 7.0 Tesla. J Magn Reson Imaging. 2011;33(6):1456–1463. doi: 10.1002/jmri.22554. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36(4):717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Kelley K. Effect size measures for mediation models: quantitative strategies for communicating indirect effects. Psychol Methods. 2011;16(2):93–115. doi: 10.1037/a0022658. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30(6):730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reischauer C, Staempfli P, Jaermann T, Boesiger P. Construction of a temperature-controlled diffusion phantom for quality control of diffusion measurements. J Magn Reson Imaging. 2009;29(3):692–698. doi: 10.1002/jmri.21665. [DOI] [PubMed] [Google Scholar]
- Rovaris M, Iannucci G, Cercignani M, Sormani MP, De Stefano N, Gerevini S, Comi G, Filippi M. Age-related changes in conventional, magnetization transfer, and diffusion-tensor MR imaging findings: study with whole-brain tissue histogram analysis. Radiology. 2003;227(3):731–738. doi: 10.1148/radiol.2273020721. [DOI] [PubMed] [Google Scholar]
- Salami A, Eriksson J, Nilsson LG, Nyberg L. Age-related white matter microstructural differences partly mediate age-related decline in processing speed but not cognition. Biochim Biophys Acta. 2012;1822(3):408–415. doi: 10.1016/j.bbadis.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, Rosen BR, Fischl B, Corkin S, Rosas HD, Dale AM. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging. 2005;26(8):1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Mediation of adult age differences in cognition by reductions in working memory and speed of processing. Psychol Sci. 1991a;2(3):179–183. [Google Scholar]
- Salthouse TA. Theoretical perspectives on cognitive aging. Hillsdale, NJ: Erlbaum; 1991b. [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103(3):403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Aging and measures of processing speed. Biol Psychol. 2000;54(1–3):35–54. doi: 10.1016/s0301-0511(00)00052-1. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Neuroanatomical substrates of age-related cognitive decline. Psychol Bull. 2011;137(5):753–784. doi: 10.1037/a0023262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Madden DJ. Information processing speed and aging. In: Deluca J, Kalmar J, editors. Information processing speed in clinical populations. New York: Psychology Press; 2007. pp. 221–241. [Google Scholar]
- Saur D, Kreher BW, Schnell S, Kummerer D, Kellmeyer P, Vry MS, Umarova R, Musso M, Glauche V, Abel S, Huber W, Rijntjes M, Hennig J, Weiller C. Ventral and dorsal pathways for language. Proceedings of the National Academy of Sciences. 2008;105(46):18035–18040. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheltens P, Barkhof F, Leys D, Wolters EC, Ravid R, Kamphorst W. Histopathologic correlates of white matter changes on MRI in Alzheimer’s disease and normal aging. Neurology. 1995;45(5):883–888. doi: 10.1212/wnl.45.5.883. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Smith EE, Eichler FS, Filley CM. Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann N Y Acad Sci. 2008;1142:266–309. doi: 10.1196/annals.1444.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sobel ME. Asymptotic intervals for indirect effects in structural equations models. Sociological methodology. 1982:290–312. [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20(3):1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Stadlbauer A, Ganslandt O, Salomonowitz E, Buchfelder M, Hammen T, Bachmair J, Eberhardt K. Magnetic resonance fiber density mapping of age-related white matter changes. Eur J Radiol. 2012;81(12):4005–4012. doi: 10.1016/j.ejrad.2012.05.029. [DOI] [PubMed] [Google Scholar]
- Stadlbauer A, Salomonowitz E, Strunk G, Hammen T, Ganslandt O. Quantitative diffusion tensor fiber tracking of age-related changes in the limbic system. Eur Radiol. 2008;18(1):130–137. doi: 10.1007/s00330-007-0733-8. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Pfefferbaum A. Selective age-related degradation of anterior callosal fiber bundles quantified in vivo with fiber tracking. Cereb Cortex. 2006;16(7):1030–1039. doi: 10.1093/cercor/bhj045. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neurosci Biobehav Rev. 2006;30(6):749–761. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. In: Diffusion tensor imaging in aging and age-related neurodegenerative disorders. Jones DK, editor. New York: Diffusion MRI: Theory, methods, and applications Oxford University Press; 2011. pp. 624–643. [Google Scholar]
- Sullivan EV, Zahr NM, Rohlfing T, Pfefferbaum A. Fiber tracking functionally distinct components of the internal capsule. Neuropsychologia. 2010;48(14):4155–4163. doi: 10.1016/j.neuropsychologia.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Gandevia SC. Noninvasive stimulation of the human corticospinal tract. J Appl Physiol. 2004;96(4):1496–1503. doi: 10.1152/japplphysiol.01116.2003. [DOI] [PubMed] [Google Scholar]
- Toosy AT, Ciccarelli O, Parker GJ, Wheeler-Kingshott CA, Miller DH, Thompson AJ. Characterizing function-structure relationships in the human visual system with functional MRI and diffusion tensor imaging. Neuroimage. 2004;21(4):1452–1463. doi: 10.1016/j.neuroimage.2003.11.022. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Cerella J. Aging, executive control, and attention: A review of meta-analyses. Neurosci Biobehav Rev. 2002;26(7):849–857. doi: 10.1016/s0149-7634(02)00071-4. [DOI] [PubMed] [Google Scholar]
- Voineskos AN, Rajji TK, Lobaugh NJ, Miranda D, Shenton ME, Kennedy JL, Pollock BG, Mulsant BH. Age-related decline in white matter tract integrity and cognitive performance: a DTI tractography and structural equation modeling study. Neurobiol Aging. 2012;33(1):21–34. doi: 10.1016/j.neurobiolaging.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmar C, O’Muircheartaigh J, Barker GJ, Symms MR, Thompson P, Kumari V, Duncan JS, Richardson MP, Koepp MJ. Identical, but not the same: intra-site and inter-site reproducibility of fractional anisotropy measures on two 3.0T scanners. Neuroimage. 2010;51(4):1384–1394. doi: 10.1016/j.neuroimage.2010.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl M, Li YO, Ng J, Lahue SC, Cooper SR, Sherr EH, Mukherjee P. Microstructural correlations of white matter tracts in the human brain. Neuroimage. 2010;51(2):531–541. doi: 10.1016/j.neuroimage.2010.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Psychological. New York: Corporation; 1981. Wechsler adult intelligence scale-revised. [Google Scholar]
- Westlye LT, Walhovd KB, Dale AM, Bjornerud A, Due-Tonnessen P, Engvig A, Grydeland H, Tamnes CK, Ostby Y, Fjell AM. Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cereb Cortex. 2010;20(9):2055–2068. doi: 10.1093/cercor/bhp280. [DOI] [PubMed] [Google Scholar]
- Wheeler-Kingshott CA, Cercignani M. About “axial” and “radial” diffusivities. Magn Reson Med. 2009;61(5):1255–1260. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- Zacks RT, Hasher L, Li KZH. Human memory. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 2nd edn. Mahwah, NJ: Erlbaum; 2000. pp. 293–357. [Google Scholar]
- Zahr NM, Rohlfing T, Pfefferbaum A, Sullivan EV. Problem solving, working memory, and motor correlates of association and commissural fiber bundles in normal aging: a quantitative fiber tracking study. Neuroimage. 2009;44(3):1050–1062. doi: 10.1016/j.neuroimage.2008.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Deng Z-S, Wang F, Wang X-Y. The effect of different number of diffusion gradients on SNR of diffusion tensor-derived measurement maps. Journal of Biomedical Science and Engineering. 2009;2:96–101. [Google Scholar]
- Zhu T, Hu R, Qiu X, Taylor M, Tso Y, Yiannoutsos C, Navia B, Mori S, Ekholm S, Schifitto G, Zhong J. Quantification of accuracy and precision of multi-center DTI measurements: a diffusion phantom and human brain study. Neuroimage. 2011;56(3):1398–1411. doi: 10.1016/j.neuroimage.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.