Abstract
OBJECTIVE
The purpose of this study was to evaluate automated CT volumetry in the assessment of living-donor livers for transplant and to compare this technique with software-aided interactive volumetry and manual volumetry.
MATERIALS AND METHODS
Hepatic CT scans of 18 consecutively registered prospective liver donors were obtained under a liver transplant protocol. Automated liver volumetry was developed on the basis of 3D active-contour segmentation. To establish reference standard liver volumes, a radiologist manually traced the contour of the liver on each CT slice. We compared the results obtained with automated and interactive volumetry with those obtained with the reference standard for this study, manual volumetry.
RESULTS
The average interactive liver volume was 1553 ± 343 cm3, and the average automated liver volume was 1520 ± 378 cm3. The average manual volume was 1486 ± 343 cm3. Both interactive and automated volumetric results had excellent agreement with manual volumetric results (intraclass correlation coefficients, 0.96 and 0.94). The average user time for automated volumetry was 0.57 ± 0.06 min/case, whereas those for interactive and manual volumetry were 27.3 ± 4.6 and 39.4 ± 5.5 min/case, the difference being statistically significant (p < 0.05).
CONCLUSION
Both interactive and automated volumetry are accurate for measuring liver volume with CT, but automated volumetry is substantially more efficient.
Keywords: computer-assisted diagnosis, computer-assisted image analysis, CT, liver transplantation, organ volume
Seven thousand liver transplants are performed each year in the United States [1], and more than 16,000 people in the United States are currently waiting for liver grafts. Because the number of waiting patients is greater than the availability of livers from persons who have died, living-donor liver transplants are being increasingly performed. Assessment of donor livers for liver transplant includes evaluation of donor anatomy, liver parenchyma, and liver volume. Evaluation of total and segmental liver volumes is crucial because assuring appropriate graft size is one of the major predictors of a safe, successful outcome for both donor and recipient. A liver remnant measuring 30–40% of the original liver volume is required for the donor to survive. A minimum of 40% of the standard liver mass, which is calculated from body surface area, is needed by the recipient [2]. Therefore, accurate, noninvasive liver volume-try is necessary [3, 4] for planning a liver transplant. CT may be a good tool for this purpose because it is noninvasive and has high spatial and contrast resolution [5, 6].
Despite innovations and the availability of assist programs, manual tracing of the liver boundary on individual CT images is the standard technique for calculation of liver volume. Manual tracing yields accurate but subjective results, and the technique is time-consuming. It takes 25–40 minutes on average to assess a liver volume for one patient [7], and intraobserver and interobserver variation exists. Because of the long time required for manual volumetry, radiologists at some medical centers use commercially available interactive volumetry-assist software in routine practice. However, few serious published studies have been conducted to assess the accuracy of use of this software in comparison with manual volumetry.
With advanced technology, automated computerized liver volumetry may replace manual liver volumetry for accurate calculation of liver volume. Researchers have developed computerized liver extraction schemes for CT. Bae et al. [8] developed a semiautomated liver segmentation scheme based on thresholding and compared it with manual tracing in four cases. Gao et al. [9] developed an automated liver segmentation scheme based on thresholding and morphologic filtering. Nakayama et al. [7] developed an automated liver segmentation method based on thresholding, feature analysis, and region growing. In a comparison with manual tracing, the correlation coefficient was 0.883. Okada et al. [10] developed an automated scheme based on a probabilistic atlas and a statistical shape model and tested their scheme in eight cases. Kim et al. [11] developed an automated segmentation method for the right lobe. The advantage of their method was capability of estimating the weight of the lobe without blood. Karlo et al. [12] compared CT and MRI volumes of resected liver specimens with intraoperative volumes and weight measurements to derive conversion factors. Hermoye et al. [13] compared semiautomatic liver volumetry with manual volumetry in living liver donors.
Although the foregoing schemes were found potentially useful, there is room for improvement in the accuracy of liver extraction. Our purposes in this study were to develop and evaluate an automated liver extraction scheme for measuring liver volume at CT and to compare results of the technique with those of commercially available interactive volumetry-assist software and manual volumetry.
Materials and Methods
Our institutional review board approved this retrospective study. Informed consent for use of the cases in this study was waived by the institutional review board because patient data were removed from the records. This study complied with HIPAA and met all standards for good clinical research according to the U.S. National Institutes of Health and local institutional review board guidelines.
Hepatic CT Database
Our database consisted of dynamic contrast-enhanced hepatic CT scans of 18 prospective living liver donors (10 women, eight men; mean age, 33.1 ± 10.3 years; range, 20–52 years) consecutively registered from May 2006 to January 2008 at our institution. The mean age of the women was 33.2 ± 10.9 years, and that of the men was 33.0 ± 10.2 years. Under a liver transplant protocol, scans were obtained with a CT system with 16-, 40-, or 64-MDCT capability (Brilliance, Philips Healthcare). Nonionic contrast medium (120–150 mL iohexol; mean, 125 ± 8 mL; Omnipaque 350, GE Healthcare) was administered IV to the donors for acquisition of arterial and portal venous phase CT images. The CT parameters included collimation of 3 mm (11 donors), 4 mm (four donors), or 5 mm (three donors) and reconstruction intervals of 2.5 mm (two donors), 3.0 mm (13 donors), or 4.0 mm (three donors). Each reconstructed CT slice had a matrix size of 512 × 512 pixels with an in-plane pixel size of 0.53–0.85 mm (mean, 0.68 ± 0.08 mm).
Interactive Volumetry With Software Aid
A board-certified abdominal radiologist (10 years of experience) used commercial interactive volumetry-assist software (Volume Tracing in Advanced Vessel Analysis, Philips Healthcare) on a viewing workstation (Extended Brilliance Workspace v3.0.1.3200, Philips Healthcare) to determine the liver volume on CT images. The radiologist put a pointer on an axial CT image with a computer mouse and used the interactive software paint tool to select a homogeneous volume of a specified size at the location of the pointer. The radiologist could choose three volume sizes: low, 400 voxels; medium, 1500 voxels; and high, 4000 voxels). The radiologist dragged the pointer to paint the entire liver area on the axial CT image. The radiologist needed to paint every 5–20 slices depending on the chosen volume size and the homogeneity of the liver.
In the evaluating process, if the radiologist believed the appropriate area had been overestimated in the painting process, the exceeded volume could be removed with the eraser tool in a sphere of one of three diameter sizes: small, 5 voxels; medium, 10 voxels; large, 30 voxels. The radiologist could undo his actions as needed. The radiologist also could enlarge or shrink the entire liver volume by a certain number of voxels with an expansion tool and could fill in holes (unpainted spots often occurred because of a high noise level) in the painted liver volume by using a filling tool as needed.
The radiologist repeated the operations until visual assessment seemed appropriate. Therefore, the final painted liver volume determined with the in teractive software should have represented an accurate volume of the entire liver. However, the liver volume determined might not have been as accurate as that determined with manual tracing because of the precision limitation of the painting and eraser tools. The user time required for the radiolo-gist to complete the liver volumetry was recorded.
Reference Standard: Manual Volumetry
To establish the liver volumes that were the reference standard for this study, a board-certified abdominal radiologist specializing in liver imaging (15 years of experience) manually traced the contour of the liver on each CT slice on a liquid crystal display (1600 × 1200 pixel resolution, 20-inch [51 cm] display, 800:1 contrast ratio; LCD2070VX, NEC). The radiologist used a DICOM viewer with a tracing tool developed at our institution (Abras version 0.9.9). The number of slices in each case ranged from 44 to 77 (mean, 59.5 ± 10.1). The user time required to complete the manual tracing for each case was recorded. To calculate the entire liver volume in each case, we summed the volumes obtained by multiplying the areas of the manually traced regions in each slice by the reconstruction interval. Liver volumes obtained with our automated liver extraction scheme were compared with the interactive volume measurements obtained with the commercial software and the manual volume measurements that were the reference standard.
Automated Scheme for Measuring Liver Volume
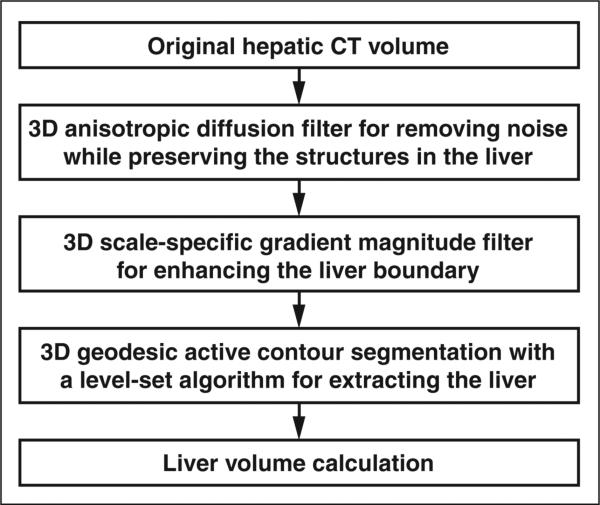
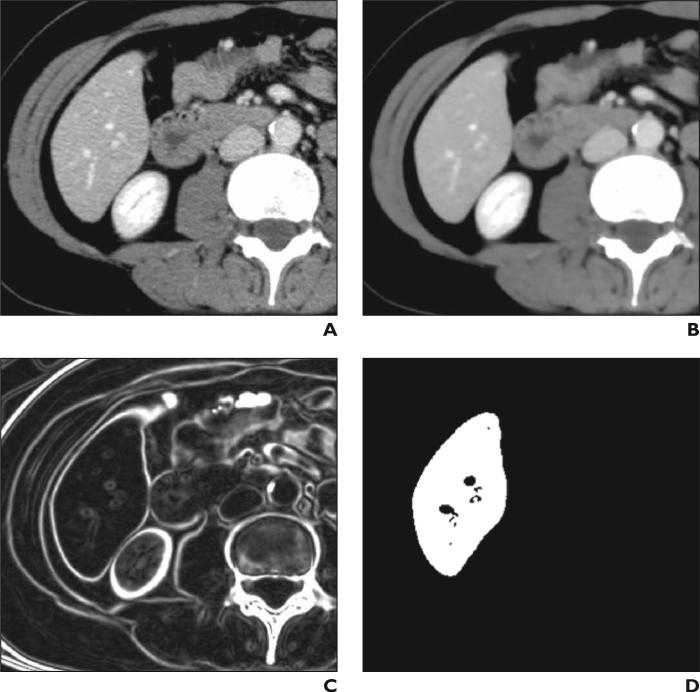
We developed an automated volumetric scheme [14] for liver CT based on four steps (Fig. 1) to yield liver volumes comparable in accuracy to the reference standard manual tracings. We applied the scheme to portal venous phase images to maximize the intensity difference between the liver parenchyma and nonliver tissue. First, a 3D anisotropic diffusion smoothing filter [15, 16] was applied to CT images (Fig. 2A) to remove noise while liver structures such as vessels and the liver border were preserved (Fig. 2B). A 3D scale-specific gradient-magnitude filter was applied to the noise-reduced CT images to enhance the liver boundary specifically (Fig. 2C). On the basis of the liver boundary– enhanced images, a 3D geodesic active-contour segmentation algorithm [17, 18] coupled with a level-set algorithm [19] was used for accurate extraction of the liver boundary (Fig. 2D). We calculated the liver volume based on the extracted liver volume by accounting for the reconstruction interval of each study. A user applied several initial points within the liver to establish the approximate location of the liver for the scheme. In this study, a radiologist provided four to six (average, 5.3 ± 0.6) initial points for each case. We have described the technical details previously [14].
Fig. 1.
Flowchart shows automated volumetry scheme for liver CT based on 3D anisotropic diffusion smoothing, 3D scale-specific edge enhancement, and 3D geodesic active contour liver extraction.
Fig. 2.
47-year-old woman. Examples of resulting image at each step in automated volumetry scheme.
A, Original axial CT image of liver.
B, Three-dimensional anisotropic diffusion noise reduction.
C, Three-dimensional scale-specific gradient magnitude calculation.
D, Three-dimensional geodesic active-contour segmentation.
Comparisons of Automated Volumetric With Interactive and Manual Volumetric Results
We compared the results obtained with automated liver volumetry with those obtained with interactive volumetry with the software aid and the reference standard manual volumetry in terms of mean and difference in liver volume. We defined a percentage volume error (E) for each automated and interactive volume (V) and the reference standard manual volume (VM) as E = (V – VM) / VM. Agreement between an automated volume or interactive volume and the manual volume was evaluated by intraclass correlation coefficient (ICC) [20, 21].
The two-way random-effect single-measure model, ICC(2,1), was used because we assumed that cases were chosen at random from the population and because we wanted to measure the reliability of each volumetric method as an individual method. ICC(2.1) is defined with the following equation:
where BMS is the between-subjects mean square, EMS is the error mean square, RMS is the between-raters mean square, k is the number of raters (i.e., volumetric methods), and n is the number of subjects tested. Analysis of variance was performed for obtaining the F statistic and statistical significance (from 0.0).
We performed post hoc power analysis by using the Walter- Eliasziw-Donner model for ICC-based reliability studies [22] to determine statistical power in this study. We assumed type 1 error (α) of 0.05 and type 2 error (β) of 0.20 in the analysis. Linear association was evaluated with Pearson product-moment correlation coefficient (r) as additional information. The Student t test for significance of correlation coefficient was performed. Bland-Altman analysis was performed to evaluate the level of agreement between two volumetric methods. The mean difference was used for measurement of the range of differences, expressed as 95% limits of agreement, between the two volume measurements likely to occur [23] and was calculated as follows: mean difference = (automated or interactive volume – manual volume) ± 1.96 × SD of the difference. The user times for manual volumetry, interactive volumetry, and automated volumetry were compared and evaluated by two-tailed multiple Student t test with Bonferroni correction. To assess the time of the user only, the time for loading DICOM images and calculating the liver volume from the determined liver areas were excluded from the user time.
Results
The average liver volume obtained with the automated scheme was 1520 cm3 (range, 956–2381 cm3). The average manual volume was 1486 cm3 (range, 984–2,439 cm3) with an average difference of 104 cm3 (E = 7.0%). The average liver volume obtained with the interactive software was 1553 cm3 (range, 1007–2435 cm3) with an average difference of 74 cm3 (E = 5.2%). Table 1 summarizes the comparison of liver volumes.
TABLE 1.
Comparison of Liver Volumes Measured With Three Methods
| Method | Volume (cm3) | Error (%) |
|---|---|---|
| Manual | 1486 ± 343 | Not applicable |
| Interactive | 1553 ± 343 | 5.2 ± 4.2 |
| Automated | 1520 ± 378 | 7.0 ± 4.7 |
Note—Values are average ± SD.
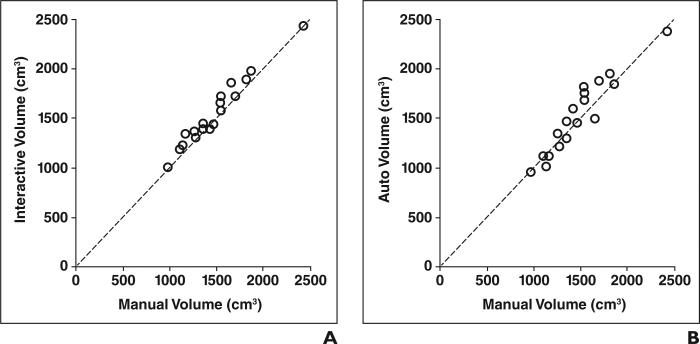
The relations between the interactive or automated volume and the manual volume are shown in Figure 3. Interactive (ICC, 0.96) and automated (ICC, 0.94) volumetry achieved excellent agreement with manual volumetry with (p = 0.0007) and without (p = 0.2672) statistical significance (Table 2). The analysis of variance table from the ICC analysis is presented in Table 3. The linear association in terms of Pearson correlation coefficient between interactive volumetry and manual volumetry was 0.98 at a level that was not statistically significant (p = 19.9). The association between automated volumetry and manual volumetry was 0.94, also at a level that was not statistically significant (p = 11.5).
Fig. 3.
Relations between interactive or automated volumetry and manual volumetry, which was reference standard for this study. Each volumetric method reached excellent agreement with reference standard (intraclass correlation coefficient, 0.96 and 0.94 for interactive and automated methods).
A, Graph shows volume measured with interactive method versus that obtained with manual method.
B, Graph shows volume measured with automated method versus that obtained with manual method.
TABLE 2.
Agreement Between Interactive or Automated Volumetry and Manual Volumetry and Results of Bland-Altman Analysis
| Comparison | Intraclass Correlation Coefficient | p | Bland-Altman Analysis |
|
|---|---|---|---|---|
| Bias (cm3) | 95% Limits of Agreement (cm3) | |||
| Interactive vs manual | 0.96 | 0.0007 | 66.4 | –67, 200 |
| Automated vs manual | 0.94 | 0.2672 | 33.7 | –211, 278 |
TABLE 3.
Analysis of Variance Table From Intraclass Correlation Coefficient Analysis
| Comparison | Interactive vs Manual | Automated vs Manual | ||||||
|---|---|---|---|---|---|---|---|---|
| df | Sum of Squares | Mean Square | F | df | Sum of Squares | Mean Square | F | |
| Between raters | 1 | 39,736 | 39,736 | 17.2 | 1 | 10,240 | 10,240 | 1.3 |
| Between cases | 17 | 3,952,360 | 232,492 | 100.7 | 17 | 4,291,912 | 252,465 | 32.4 |
| Within case | 18 | 78,968 | 4,387 | 18 | 142,520 | 7,918 | ||
| Residual | 17 | 39,232 | 2,308 | 17 | 132,280 | 7,781 | ||
| Total | 35 | 4,031,328 | 35 | 4,434,432 | ||||
We performed Walter-Eliasziw-Donner post hoc power analysis [22] to determine statistical power in this study. Because the number of cases was 18 and the actual ICC between interactive and manual volumetry was 0.96 and that between automated and manual volumetry was 0.94, the lowest ICCs between interactive and manual volumes and between automated and manual volumes that we should have been able to detect were 0.88 and 0.81. Thus this study with 18 cases had the power to detect a bias-to-error ratio of 0.13 in ICC.
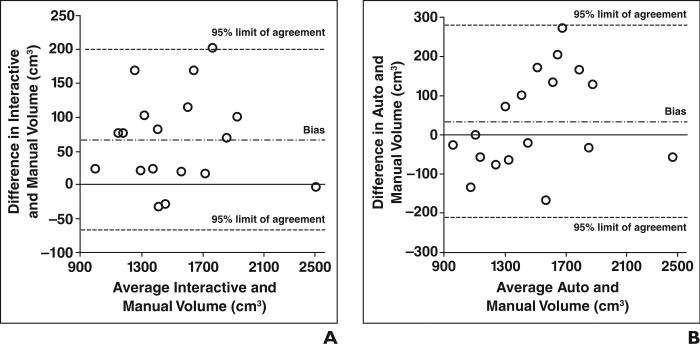
Bland-Altman plots for assessing agreement between interactive or automated volumetry and manual volumetry are shown in Figure 4. No notable trend was seen in the plot for interactive volumetry other than a larger positive bias (Fig. 5A), whereas variation tended to be greater around medium-sized livers (Fig. 5B). As shown in Table 3, interactive volumetry had greater bias than automated volumetry, whereas automated volumetry had larger 95% limits of agreement than interactive volumetry. The greater positive bias for interactive volumetry was probably caused by overestimation of the liver boundary due to the limited precision of the tool, as exemplified in Figure 3C.
Fig. 4.
Bland-Altman plots for agreement between manual volumetry and interactive or automated volumetry.
A, Plot for interactive and manual volumetry.
B, Plot for automated and manual volumetry.
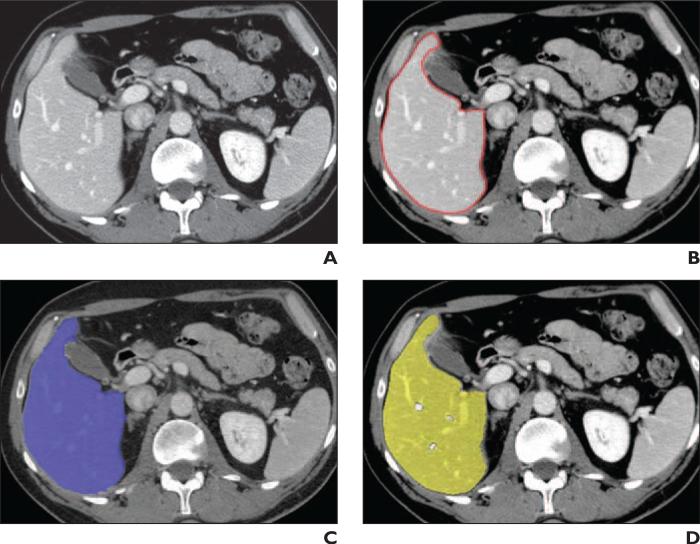
Fig. 5.
49-year-old man. CT images show liver area determined with three volumetric methods.
A, Original axial image of liver.
B, Reference standard manually-traced liver contour (red).
C, Liver area determined with interactive volumetry entailing use of software aid (purple).
D, Liver area determined with automated method (yellow).
Figure 5 shows an axial CT slice of the liver superimposed on extracted images of the liver obtained with the three volumetric methods. Although the liver boundaries determined with interactive volumetry and automated volumetry agreed with the manually determined liver contour (Fig. 5B), interactive volumetry (Fig. 5C) was associated with slight overextraction and underextraction due to the precision limits of the tool, whereas automated volumetry (Fig. 5D) was associated with slight underextraction. The portal venous branches were excluded from the liver area determined with automated volumetry (see Discussion).
The average processing time for the automated scheme was 3.6 ± 1.5 min/case (range, 1.7–7.0 min/case) on the computer used (2.66-GHz Xeon, Intel). Because the time the radiologist spent in automated volumetry was the time only for providing several initial points within the liver, we considered it user time. The average user time for automated volumetry was 0.57 ± 0.06 min/case (range, 0.4–0.7), whereas that for the interactive method was 27.3 ± 4.6 min/case (range, 20–39), and that for the manual method was 39.4 ± 5.5 min/case (range, 24–48). All differences between average user times were statistically significant (p < 0.025).
Discussion
Although automated volume measurements had a strong correlation with measurements obtained with the reference standard manual liver volumetry (r = 0.94), the correlation did not achieve the previously reported [24] minimal variation between expert radiologists, that is, 0.997 interobserver correlation between two radiologists’ manual volumes. In automated liver extraction, occasional false-positive and false-negative extractions require manual correction, which can be accomplished rapidly with routine manipulations. The substantial amount of time saved by use of the computerized volumetric method may justify the small volume error rate (E = 7.0%) compared with the manual process, which averages approximately 39 min/case. We did not perform any manual modifications on the automated liver volumes in this study because one of the aims was to compare the accuracy of the automated liver volumetry alone with that of the study reference standard, manual volumetry.
Our automated volumetric technique excluded the major hepatic vessels from the liver volume. In the evaluation, this exclusion was counted as false-negative extraction because manual volumetry included hepatic vessels. However, studies [5, 7] have shown that liver volume measured with CT volumetry is overestimated compared with the actual liver volume measured after resection. One possible explanation for this phenomenon is that actual liver volumes are measured with less blood in the hepatic vessels. Therefore, exclusion of major hepatic vessels may be desirable. Kim et al. [11] developed automated volumetry without blood for the right lobe.
Although liver weight and volume at surgery are the reference standard in liver transplantation, we considered manual CT volumetry the reference standard for this study because it is the reference standard technique for liver volume calculation for preoperative measurements in radiology. We therefore aimed at reducing the difference between automated volumetric results and the manual volumetric results used as the reference standard in this study. Reducing the difference between the manually measured volume on images and liver volume (or weight) measured at surgery is a next step in future research. One possible way to do this is to exclude blood from the liver volume, as we partially did in our automated method and as Kim et al. [11] have proposed.
Because it entailed a powerful nonlinear noise reduction technique for preprocessing, our automated scheme should have been robust against changes in noise characteristics on CT images across examinations and patients. In addition, variable liver density across examinations and patients may result in inaccurate liver volumetry because density depends on acquisition timing and on the manner of the contrast injection. Our automated scheme entailed primarily edge information, which is relatively robust against such variations.
Automated liver CT volumetry is potentially useful for preoperative assessment of residual hepatic volume. Accurate assessment of resection volume and vascular anatomy is mandatory in preoperative planning for safe curative hepatectomy, which is most often performed for treatment of patients with liver tumors. Because hepatectomy reduces liver volume, it must be ensured that the residual volume is sufficient to maintain liver function. If treatment is complicated by chronic liver disease, a larger residual volume is required [25]. Researchers have tried to predict segmental liver volume accurately on CT images by using virtual hepatectomy [26].
We considered the time a radiologist spent for the automated volumetry the user time. When we include the processing time in the automated scheme, the average total time becomes 4.1 ± 1.5 min/case (range, 2.3–7.7 min/case). This time is still substantially shorter than the 39.4 min/case needed for manual volumetry and the 27.3 min/case needed for interactive volumetry.
Although the 95% limits of agreement between our automated and manual CT volumes of –211 and 278 cm3 are not small, they are comparable to those between actually measured liver volumes and manual CT volumes of –256 and 261 cm3 in a study by Nakayama et al [7]. In addition, the range of our 95% limits of agreement (ratios of automated to manual volume of 0.86 and 1.19; for comparison, the physical unit was converted into the ratio) is smaller than that between actually measured graft volumes and manual CT volumes of 0.65 and 1.96 in another study [13]. Our 95% limits of agreement also are smaller than those between their automated volumes and manual volumes of –230 and 328 cm3 in the study by Nakayama et al. Although cases with large differences between automated and manual volumes may require manual correction, the substantial time reduction with automated volumetry would justify the differences.
A limitation of this study was the small number of cases. We used 18 cases to evaluate the automated scheme and the interactive volumetry, whereas other investigators used four cases [8], 35 cases [7], 10 cases [9], and nine cases [10]. None of these previous studies compared automated with interactive volumetry. In general, a small number of cases limits variation among cases. Case variation in our study, however, would be relatively small compared with those in studies involving abnormal cases, as in computer-aided diagnosis [27], because the livers in prospective donor cases are normal.
A limit on the evaluation in this study was that the manual volumes were determined by a single expert radiologist. Reference standard manual volumes are ideally determined by several expert radiologists experienced in liver diagnosis. This ideal evaluation would not be feasible at all institutions because not many institutions have a number of such radiologists who are sufficiently experienced in liver diagnosis. Although researchers have evaluated their automated liver extraction schemes with reference standard manual volumes [7–10, 28], none of the studies was conducted with several radiologists. We used manual volumes determined very carefully by a single experienced radiologist (more than 15 years of experience in liver diagnosis) as a reference standard because we thought that volumes determined manually by a mixture of inexperienced and experienced radiologists or by several inexperienced radiologists might be less reliable. More important, because the interob-server variation of CT volumetry is considered small (the interobserver correlation between two radiologists’ manual volumes was 0.997 in one study [24]), volumes determined by radiologists and those by a single radiolo-gist would not differ greatly.
In summary, we developed an automated liver extraction scheme for measuring liver volumes at CT and compared the automated volumetric assessment based on the scheme with the findings at interactive volumetry performed with commercially available assist software, which is becoming a standard procedure in hospitals, and with manual volumetry, which was the reference standard. The values obtained with automated and interactive CT liver volumetry agreed with the values obtained with manual volumetry (ICC, 0.94 and 096). Automated volumetry required substantially less user time (less than 1 min/case) than manual volume-try (≈40 min/case) and interactive volumetry (≈30 min/case). Therefore, automated volumetry is an efficient, accurate, useful way of measuring liver volume at CT.
Acknowledgment
The authors thank E. F. Lanzl for improving the manuscript.
Supported in part by NIH grants S10 RR021039 and P30 CA14599.
References
- 1.2008 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1998–2007. U.S. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; Rockville, MD: 2008. [Google Scholar]
- 2.Lo CM, Fan ST, Liu CL, et al. Adult-to-adult living donor liver transplantation using extended right lobe grafts. Ann Surg. 1997;226:261–269. doi: 10.1097/00000658-199709000-00005. discussion 269–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamel IR, Kruskal JB, Warmbrand G, Goldberg SN, Pomfret EA, Raptopoulos V. Accuracy of volu-metric measurements after virtual right hepatectomy in potential donors undergoing living adult liver transplantation. AJR. 2001;176:483–487. doi: 10.2214/ajr.176.2.1760483. [DOI] [PubMed] [Google Scholar]
- 4.Radtke A, Sotiropoulos GC, Nadalin S, et al. Pre-operative volume prediction in adult living donor liver transplantation: how much can we rely on it? Am J Transplant. 2007;7:672–679. doi: 10.1111/j.1600-6143.2006.01656.x. [DOI] [PubMed] [Google Scholar]
- 5.Lemke AJ, Brinkmann MJ, Schott T, et al. Living donor right liver lobes: preoperative CT volumetric measurement for calculation of intraoperative weight and volume. Radiology. 2006;240:736–742. doi: 10.1148/radiol.2403042062. [DOI] [PubMed] [Google Scholar]
- 6.Emiroglu R, Coskun M, Yilmaz U, Sevmis S, Ozcay F, Haberal M. Safety of multidetector computed tomography in calculating liver volume for living-donor liver transplantation. Transplant Proc. 2006;38:3576–3578. doi: 10.1016/j.transproceed.2006.10.101. [DOI] [PubMed] [Google Scholar]
- 7.Nakayama Y, Li Q, Katsuragawa S, et al. Auto mated hepatic volumetry for living related liver transplantation at multisection CT. Radiology. 2006;240:743–748. doi: 10.1148/radiol.2403050850. [DOI] [PubMed] [Google Scholar]
- 8.Bae KT, Giger ML, Chen CT, Kahn CE., Jr Automatic segmentation of liver structure in CT images. Med Phys. 1993;20:71–78. doi: 10.1118/1.597064. [DOI] [PubMed] [Google Scholar]
- 9.Gao L, Heath DG, Kuszyk BS, Fishman EK. Automatic liver segmentation technique for three-dimensional visualization of CT data. Radiology. 1996;201:359–364. doi: 10.1148/radiology.201.2.8888223. [DOI] [PubMed] [Google Scholar]
- 10.Okada T, Shimada R, Hori M, et al. Automated segmentation of the liver from 3D CT images using probabilistic atlas and multilevel statistical shape model. Acad Radiol. 2008;15:1390–1403. doi: 10.1016/j.acra.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Kim KW, Lee J, Lee H, et al. Right lobe estimated blood-free weight for living donor liver transplantation: accuracy of automated blood-free CT volumetry—preliminary results. Radiology. 2010;256:433–440. doi: 10.1148/radiol.10091897. [DOI] [PubMed] [Google Scholar]
- 12.Karlo C, Reiner CS, Stolzmann P, et al. CT- and MRI-based volumetry of resected liver specimen: comparison to intraoperative volume and weight measurements and calculation of conversion factors. Eur J Radiol. 2010;75:e107–e111. doi: 10.1016/j.ejrad.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Hermoye L, Laamari-Azjal I, Cao Z, et al. Liver segmentation in living liver transplant donors: comparison of semiautomatic and manual methods. Radiology. 2005;234:171–178. doi: 10.1148/radiol.2341031801. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki K, Kohlbrenner R, Epstein ML, Obajuluwa AM, Xu J, Hori M. Computer-aided measurement of liver volumes in CT by means of geodesic active contour segmentation coupled with level-set algorithms. Med Phys. 2010;37:2159–2166. doi: 10.1118/1.3395579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perona P, Malik J. Scale-space and edge detection using anisotropic diffusion. IEEE Trans Pattern Anal Mach Intell. 1990;12:629–639. [Google Scholar]
- 16.Whitaker RT, Xue X. Variable-conductance, level-set curvature for image denoising.. Proceedings of the IEEE International Conference on Image Processing; Los Alamitos, CA. 2001; IEEE; pp. 142–145. [Google Scholar]
- 17.Caselles V, Kimmel R, Sapiro G. Geodesic active contours. Int J Comput Vis. 1997;22:61–79. [Google Scholar]
- 18.Sethian JA. A fast marching level set method for monotonically advancing fronts. Proc Natl Acad Sci USA. 1996;93:1591–1595. doi: 10.1073/pnas.93.4.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sethian JA. Level set methods and fast marching methods. 2nd ed. Cambridge University Press; New York, NT: 1999. [Google Scholar]
- 20.Portney LG, Watkins MP. Foundations of clinical research applications and practice. Appleton & Lange; Norwalk, CT: 1993. pp. 509–516. [Google Scholar]
- 21.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 22.Walter SD, Eliasziw M, Donner A. Sample size and optimal designs for reliability studies. Stat Med. 1998;17:101–110. doi: 10.1002/(sici)1097-0258(19980115)17:1<101::aid-sim727>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 23.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327:307–310. [PubMed] [Google Scholar]
- 24.Sandrasegaran K, Kwo PW, DiGirolamo D, Stockberger SM, Jr, Cummings OW, Kopecky KK. Measurement of liver volume using spiral CT and the curved line and cubic spline algorithms: reproducibility and interobserver variation. Abdom Imaging. 1999;24:61–65. doi: 10.1007/s002619900441. [DOI] [PubMed] [Google Scholar]
- 25.Okamoto E, Kyo A, Yamanaka N, Tanaka N, Kuwata K. Prediction of the safe limits of hepatectomy by combined volumetric and functional measurements in patients with impaired hepatic function. Surgery. 1984;95:586–592. [PubMed] [Google Scholar]
- 26.Yamanaka J, Saito S, Fujimoto J. Impact of preoperative planning using virtual segmental volume-try on liver resection for hepatocellular carcinoma. World J Surg. 2007;31:1249–1255. doi: 10.1007/s00268-007-9020-8. [DOI] [PubMed] [Google Scholar]
- 27.Giger ML, Suzuki K. Computer-aided diagnosis (CAD). In: Feng DD, editor. Biomedical Information Technology. Academic Press; Boston, MA: 2007. pp. 359–374. [Google Scholar]
- 28.Selver MA, Kocaoglu A, Demir GK, Dogan H, Dicle O, Guzelis C. Patient oriented and robust automatic liver segmentation for pre-evaluation of liver transplantation. Comput Biol Med. 2008;38:765–784. doi: 10.1016/j.compbiomed.2008.04.006. [DOI] [PubMed] [Google Scholar]