Abstract
This year marks the 125th anniversary of the beginning of regeneration research in the ascidian Ciona intestinalis. A brief note was published in 1891 reporting the regeneration of the Ciona neural complex and siphons. This launched an active period of Ciona regeneration research culminating in the demonstration of partial body regeneration: the ability of proximal body parts to regenerate distal ones, but not vice versa. In a process resembling regeneration, wounds in the siphon tube were discovered to result in the formation of an ectopic siphon. Ciona regeneration research then lapsed into a period of relative inactivity following the purported demonstration of the inheritance of acquired characters using siphon regeneration as a model. Around the turn of the present century, Ciona regeneration research experienced a new blossoming. The current studies established the morphological and physiological integrity of the regeneration process and its resemblance to ontogeny. They also determined some of the cell types responsible for tissue and organ replacement and their sources in the body. Finally, they showed that regenerative capacity is reduced with age. Many other aspects of regeneration now can be studied at the mechanistic level because of the extensive molecular tools available in Ciona.
Keywords: Partial body regeneration, wounding, tissue repair, neural complex, siphons, oral siphon pigment organs, aging
INTRODUCTION
The ascidian tunicate Ciona intestinalis has contributed significantly to our understanding of the mechanisms of embryonic development (Satoh, 1994; 2014). Because of invariant cleavage and cell lineages and inability to regulate cell fate after specific blastomeres are removed or destroyed ascidians are characterized by determinate development, in which cell and regional fates are specified by intrinsic mechanisms (Jeffery, 2001; Nishida, 2002). Localized mRNAs were discovered in the early embryo that account for this phenomenon (Yamada et al., 2005). A crowning achievement was the identification of a muscle determinant, a maternal mRNA encoding the transcription factor Macho (Nishida and Sawada, 1998). Macho was initially discovered in the ascidian Halocynthia roretzi and later identified in Ciona intestinalis (Satou et al., 2002). The Macho mRNA is localized in the myoplasm, a cytoplasmic region of the egg that causes the blastomeres that contain it to adopt a muscle cell fate.
Like the majority of ascidian species, Ciona intestinalis embryos develop into tadpole-like larvae containing a dorsal central nervous system (CNS), a notochord, and a post-anal tail, which establishes the tunicates as members of the Phylum Chordata. Indeed, recent phylogenetic studies inferred that the tunicates might be the closest living relatives of vertebrates (Bourlat et al., 2006; Delsuc et al., 2006). The morphology of adult ascidians, whose body plan is essentially a sessile, filter-feeding sac, is distinct from the tadpole larva (Berrill, 1950). The larval features are absorbed or extensively modified and replaced by adult tissues and organs during metamorphosis. Metamorphosis may also be a cue that reverses the restriction of cell fates that is a hallmark of the embryonic period. Whereas removal of cells from an embryo causes permanent defects in larval development, injury to the post-metamorphic juvenile or adult can result in regeneration of the missing parts (Berrill, 1950; 1951). The extent of this regenerative power is variable, however, depending on whether an ascidian species has a solitary or colonial life history and exhibits only sexual reproduction or both sexual and asexual reproduction respectively.
Colonial (or compound) ascidians (e. g. Botryllus, Botrylloides, and Perophora species), which consist of zooids interconnected either by a common basal vasculature or a stolon, and that reproduce asexually through budding, have a complete capacity for regeneration, which has been termed whole body regeneration (Tiozzo et al., 2008). In Botryllids the entire colony can be replaced from the basal vasculature after zooids and buds are surgically removed (Rinkevich et al., 1995) or zooids are naturally destroyed at the conclusion of a weekly cycle of growth followed by apoptosis (Lauzon et al., 2002). In Perophora, a single type of blood cell can initiate the regeneration process (Freeman, 1964). In contrast, solitary ascidians, such as Ciona intestinalis and many other well-known forms that show only sexual reproduction, have lesser powers of regeneration. The latter animals have a type of regeneration termed here as partial body regeneration, in which only some parts of the body can regenerate the missing parts of an animal. Nevertheless, the development of adults with extensive regulatory powers (e. g. regeneration) from highly determinate embryos represents one of the most dramatic reversals of cell fate potential in any animal.
Ascidian budding and whole body regeneration has been the primary subject of several historical and recent reviews (Berrill, 1951; Tiozzo et al., 2008, Candia Carnevali and Burighel, 2010; Brown and Swalla, 2012). However, partial body regeneration, as exhibited in solitary ascidians, has received less attention, even though it may be the most primitive evolutionary state among ascidians (Zeng et al., 2006) and has a longer history of investigation. The purpose of this article is to review the past 125 years of research in regenerative biology in the solitary ascidian, Ciona intestinalis (hereafter called Ciona).
THE BEGINNING OF CIONA REGENERATIVE BIOLOGY
Ciona is commonly called “the sea vase” in the English speaking world and the “ghost ascidian” in Japan for good reason. It’s basket-like body is soft, almost transparent, and among the largest of all ascidians, making it an attractive subject for regenerative experiments. It also has excellent markers for regeneration along its to proximal (base) to distal axis (Millar, 1953), including the visceral organs (heart, gonad, stomach, and intestines), the pharynx containing a large branchial sac, the neural complex, and the oral and atrial siphons (Fig. 1A).
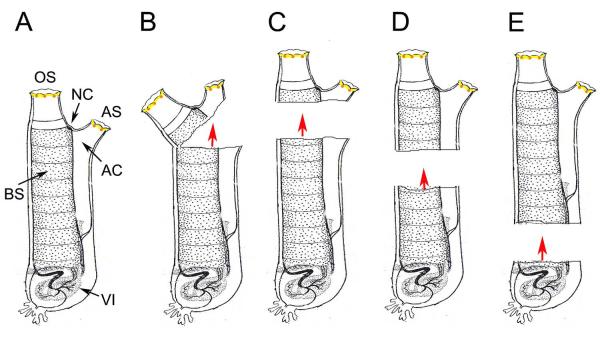
Fig. 1.
Schematic representation of the effects of separating the body into two parts on regeneration. A. Adult showing the oral siphon (OS), atrial siphon (AS), neural complex (NC), atrial cavity (AC), branchial sac (BS), and basal viscera (VI). B. Partial removal of distal portion of adults, including both siphons and the NC, as reported by Mingazzini (1891), resulted in regeneration of a complete distal part from the proximal part (directional red arrow) but no regeneration of the basal part by the distal flap. C-D. Examples of adults bisected into distal and proximal parts at several different levels, as reported by Hirschler (1914), resulted in regeneration of complete distal parts from the proximal parts, including the most basal portion of the body (D) (directional red arrows), but lack of regeneration of the proximal part by the distal part. All diagrams illustrate adult Ciona with distal shown at the top and proximal (or basal) at the bottom.
About 125 years ago Pio Mingazzini, working at the Stazione Zoologica in Naples (Fantini, 2000), published a note describing the first regeneration experiments in Ciona (Mingazzini, 1891). In this brief report, he described discoveries regarding the ability of distal body structures to be replaced after their removal. First, he reported that the oral and atrial siphons regenerated within about a month after amputation. However, it was also noted that the regenerated siphons were longer than the originals. Moreover, when two consecutive cycles of siphon amputation and regeneration were carried out the regenerated siphons became even longer. Second, he showed that a wound made within the siphon tube healed by producing an ectopic supernumerary siphon. Third, he discovered that the neural complex (NC), which contains the central ganglion (brain), the neural gland, and the ciliated duct and funnel (see Figs 4A-C, 6), could regenerate after removal of the distal part of the body. This was the first report of complete regeneration of the brain, which is considered to be a unique phenomenon among the chordates. Finally, he reported an experiment in which the distal part of the body, including the neural complex and both siphons, was incompletely severed, leaving it as a flap of tissue attached by a small isthmus to the base of the animal (Fig. 1B). Under the latter conditions, the original base part was found to be capable of regenerating new distal structures but the flap containing the distal organs degenerated without reproducing a new base. This was the first description of partial body regeneration.
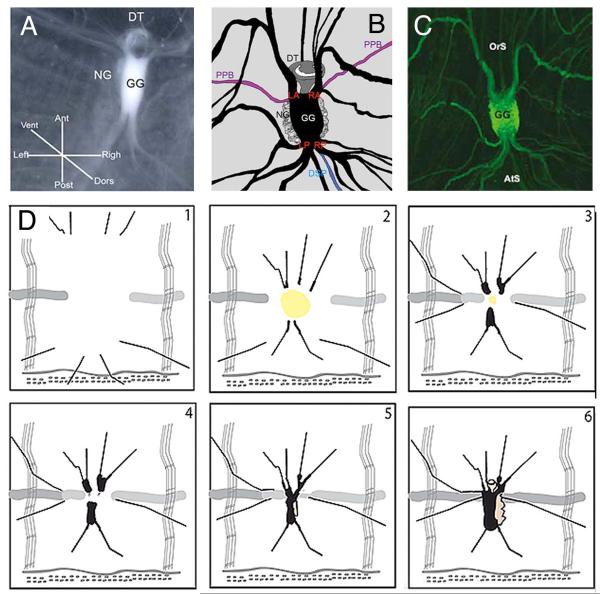
Fig. 4.
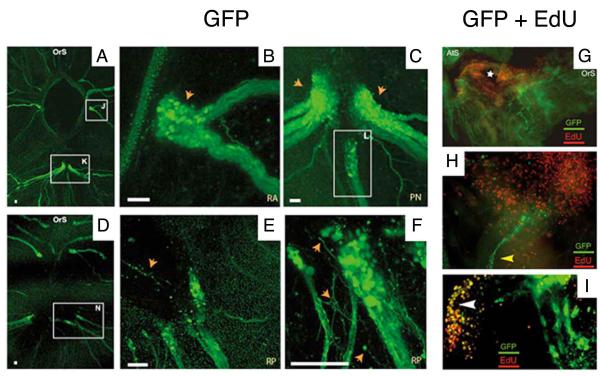
Neural complex regeneration. A-C. The components of the neural complex. A. Imaging of the neural complex in a living animal. B. Schematic diagram of a neural complex and surrounding nerves drawn according to C. C. GFP staining of the neural complex and surrounding nerves in an E15 transgenic animal. OrS: oral siphon. AtS: atrial siphon. GG: cerebral ganglion. NG: neural gland (note that the NC lies on the ventral side of the GG). DSP: dorsal strand plexus. DT: ciliated funnel. LA and RA: left and right anterior nerves. LP and AP: left and right posterior nerves. PPB: peripharyngeal band. DSP. Drawings 1-5 show progressive stages of NC regeneration as described in the text. From Dahlberg et al. (2009) with modifications.
Fig. 6.
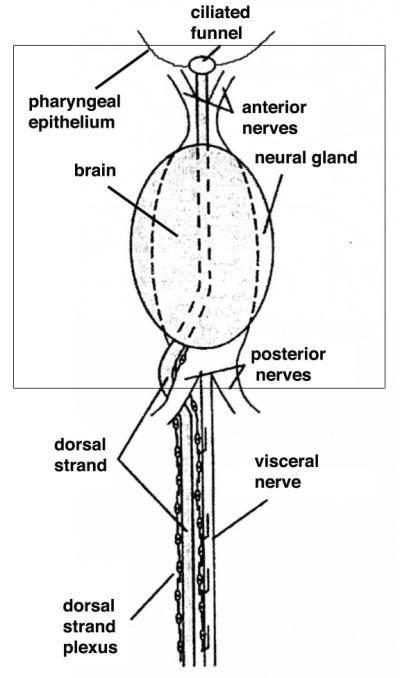
A schematic diagram showing the relationship of the dorsal strand and nerve plexus to the neural complex. The view is ventral. The dashed lines represent the position of the brain, which lies dorsal to the neural gland in the complex. The boxed area represents the approximate area of a typical neural complex ablation zone. From Bollner et al. (1997) with modifications.
EARLY YEARS IN CIONA REGENERATIVE BIOLOGY
Mingazzini’s report launched a flurry of research on Ciona regeneration around the turn of the 20th century, in which most of his discoveries were substantiated. L. S. Schultze excised the NC region and found that it was replaced in about a month (Schultze, 1899). All elements of the complex regenerated, and the brain was re-connected to the severed nerves of the CNS, although each regenerated organ was smaller than the originals. J. Loeb then confirmed NC regeneration and tested the effects of the missing brain on behavior (Loeb, 1905). He concluded the lack of a NC had only minor effects of on the general physiology of the animal.
J. Hirschler (1914) separated the body of young adults into distal and basal portions by bisecting them through several different planes along the body axis (Fig. 1C-E). In all cases, he observed that the basal part was able to regenerate a complete replica of the distal portion, including the NC and siphons, but the distal part, no matter how large, did not regenerate the basal portion, thus substantiating Mingazzini’s earlier demonstration of partial body regeneration. In the most extreme case, animals were separated into a large distal part containing almost the entire pharynx (branchial sac, endostyle, dorsal strand, etc), NC, and both siphons, and a smaller basal part, containing mostly the heart, intestine, stomach, and gonad (Fig. 1E). As previously, the distal large distal portion did not regenerate any of the proximal parts. The regeneration of distal parts from the small base was sometimes observed, but only when it contained a piece of the branchial sac or epicardium (the cavity containing blood cells lying immediately above the heart). To explain partial body regeneration, Hirschler (1914) proposed that cells migrated from proximal sites, probably the branchial sac and/or epicardium, into the wounded area. Consistent with this idea, he noted the mass migration of pigmented blood cells toward a wound in the body and the subsequent formation of pigmented organs along the wound site, which were structurally similar to those normally seen at the rims of both siphons. More recent studies suggest that this proposal is only partially true: under some circumstances (see below) progenitor cells involved in regeneration can also originate near the site of the injury (Auger et al., 2010)
During the following years, other investigators re-confirmed most of Mingazzini’s conclusions on NC and siphon regeneration (Fox, 1924; Sutton, 1953; Whittaker, 1975), and these organs became the major foci for modern regeneration research in Ciona.
REGENERATION OF LONGER SIPHONS: FACT OR ARTIFACT?
One of Mingazzini’s discoveries, the replacement of longer siphons in regenerating animals could not be universally confirmed. During the 1920s this uncertainty generated a great deal controversy, when siphon regeneration in Ciona was used as a model to test an unpopular non-Mendelian genetic hypothesis: the inheritance of acquired characters.
The Austrian biologist Paul Kammerer had previously published experiments purporting the inheritance of acquired characters in frogs and salamanders. The conclusions of these experiments were staunchly and vigorously defended by their perpetuator, but subsequently discredited and viewed as fraudulent by some contemporaries (see Koestler, 1971). After learning of Mingazzini’s work showing the regeneration of longer siphons in Ciona, Kammerer apparently decided to use siphon regeneration as a test case for the inheritance of acquired characters. He reported results similar to those of Mingazzini (1891): amputation of both siphons caused them to be replaced as longer structures, and repeated amputations resulted in even longer siphons (Kammerer, 1923). He went further by asserting that the longer siphons acquired during regeneration could be inherited in the next generation.
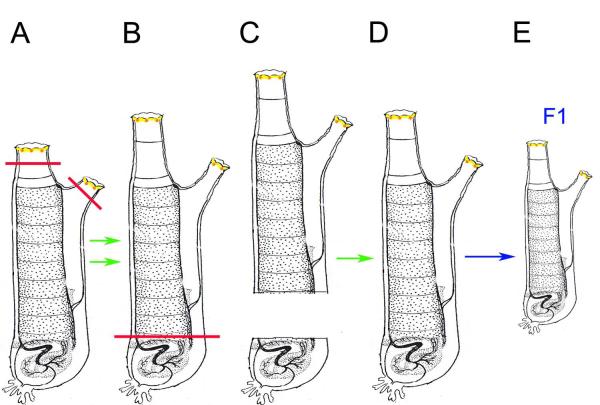
The experimental protocol described by Kammerer is illustrated in Fig. 2. After producing long siphoned animals by two cycles of amputation and regeneration, he reported removing the basal part of an animal, which contains the gonad and other viscera, and said that this caused the distal portion to regenerate a complete animal with a new gonad (Fig. 2). This was the first confounding result: the generation of a complete animal from a distal portion was not in line with the well-accepted idea of partial body regeneration (Mingazzini, 1891; Hirschler, 1914; Fig. 1). The second dubious result was purportedly obtained when gametes from the animals with long siphons and regenerated gonads were inter-crossed and reported to yield F1 progeny with the long siphons of their parents, even though they had not been subjected to a siphon amputation step during their lifetime.
Fig. 2.
Schematic representation of Kammerer’s experiments purporting the inheritance of acquired characters using Ciona siphon regeneration. A-B. Two cycles of amputation (solid black lines) and regeneration of both siphons were reported to result in animals with longer siphons. C-D. Bisection (solid red line) of long siphoned animals into two parts was reported to allow the distal half to regenerate a gonad, contrary to Hirschler’s (Hirschler, 1914) previous conclusions (Fig. 1 C-D). D-E. Animals with regenerated gonads were inter-crossed and reported to produce F1 progeny with long siphons. Green arrows: reported regenerations. Blue arrow: reported fertilization of gametes. Other details are the same as in Fig. 1. Diagram is redrawn after Whittaker (1973).
Neither of the two successive steps in Kammerer’s study has survived the test of time. H. M. Fox (Fox, 1924) and succeeding investigators (Wermel and Lopaschow, 1930; Whittaker, 1973) were unable to obtain longer siphons after one or more cycles of amputation and regrowth, casting doubt on Kammerer’s first experimental series (Fig. 2A-B). Furthermore, J. R. Whittaker (1973) was also unable to repeat the gonad regeneration results. Similar to Hirschler’s (1914) studies, in his hands, the distal parts of bisected animals did not regenerate any of the proximal parts, including the gonad; in fact, they were unable to survive the operation (Whittaker, 1973). There was no sound evidence for the inheritance of acquired characters based on siphon regeneration.
It is known that Ciona with unusually long siphons can be observed in nature or in the laboratory. Rather than attributed to regeneration, the long siphons seem to be an environmental effect related to abundance of algal food (Fuchs, 1914; Fox, 1924). Thus, Mingazzini’s and possibly also Kammerer’s results could have been obtained as a consequence of over-feeding in culture, although it is puzzling that this was not revealed in controls. Regardless, there is an important lesson to be learned from the long siphoned Ciona: growth processes, and even regenerative mechanisms, can be uncoupled between the siphons and the general body. This uncoupling was also demonstrated in recent siphon regeneration experiments (Auger et al. 2010).
CLOSING A WOUND WITH AN ECTOPIC SIPHON
In addition to the replacement of amputated body parts per se, early studies were also carried out to determine the effects of wounding. When the body is wounded, the injured area attracts migratory blood cells prior to re-sealing (Hirschler, 1914). Small wounds or punctures usually reseal in a few days. However, as described above, Mingazzini (1891) reported a different response to a large wound in one of the siphons: a new siphon tube was created in the wounded area. Subsequently, J. Loeb and T. H. Morgan confirmed Mingazzini’s results (Morgan, 1901; Loeb, 1905), and the formation of an ectopic siphon after wounding was also discovered in the solitary ascidian, Styela clava (George, 1937). The distal tissues of the ectopic siphon, namely the lobes, intervening pigmented organs, and rings of muscle fibers were formed in the correct proportions in the ectopic siphons. Indeed, Loeb even showed that multiple siphons could be formed in tandem from a row of individual wounds within the siphon tube of a single animal (Loeb, 1905).
Extensive studies of ectopic siphon formation after wounding were carried out by K. von Haffner (1933), who described the sequence of events leading to this condition after a transverse wound was made in the oral or atrial siphon (Fig. 3). First, the epidermis was repaired at the wound site, which entailed fusion between the severed inner pharyngeal and outer epidermal epithelia (Fig. 3A, E). The repair of the cut surface exhibited polarity: it always began on the proximal side of the wound and proceeded around the lesion to the distal side. Second, as the repair process moved from proximal to distal, pigmented organs appeared, first on the proximal side and then on the distal side of the wound (Fig. 3B, C, F, G). Third, lobes and circular muscle fibers appeared along the rim of the ectopic siphon (Fig. 3E, G). Finally, an extra siphon tube began to grow from the wound (Fig. 3D, G).
Fig. 3.
Wounding induces an ectopic siphon. A-G. The events of supernumerary siphon formation after a transverse wound is made in the oral (A-D) or atrial (E-G) siphon. After von Haffner (1933). H. Multiple pigmented organs are formed in vertical register along the oral siphon amputation site (arrow labeled 2) and both the distal and proximal (arrow labeled 1) sides of a transverse wound made in the tube of a regenerating oral siphon (Jeffery, unpublished).
The repair process involving the formation of an extra siphon was similar to the events of siphon regeneration after amputation, and the newly formed pigmented organs appeared around the wound in horizontal register with the pre-existing ones at the siphon rim. The latter point has been extended by an experiment in which a transverse wound was made in the siphon tube during oral siphon regeneration that resulted in multiple pigmented organs formed in vertical register between regeneration site along the siphon rim and the distal and proximal sides of the wound (Fig. 3H). These results suggest the existence of zones in the siphon dedicated to pigmented organ development or replacement, which were recently confirmed by the discovery of vertical niches of pigment organ precursors during siphon regeneration (Auger et al., 2010).
Since the mechanisms of wounding and tissue repair are now receiving renewed interest in regenerative biology, it would be worthwhile to apply modern methods to the study the Ciona system of ectopic siphon formation from wounds. One of the outstanding questions is whether the formation of siphons from wounds is restricted to the siphon tubes, which would suggest predetermined fields of developmental potential. According to von Haffner (1933), however, supernumerary siphons can also be formed from wounds in the region above the NC between the siphons, implying a broad zone of competence, but it is unclear whether or how far this zone spreads proximally along the body axis.
OTHER EARLY REGENERATION EXPERIMENTS
There is also evidence from early studies that individual Ciona organs are capable of regeneration. If a lesion is made in the tunic, this structure is replaced very quickly (Peres, 1948). In fact, when an entire siphon is amputated, the tunic is replaced in concert with the regenerating siphon (Sutton, 1953). Histological studies suggest that tunic regeneration occurs through production of a cellulose-rich extracellular matrix by the epidermis. Once the matrix is formed, mesenchymal cells cross the epidermis from the circulatory system to invade the reforming tunic. Whether or not these mesenchymal cells take part in laying down the matrix itself, or this is a sole function of the epidermis, remains to be explored.
It has also been reported that the gonad is capable of regeneration. Bourchard-Madrelle (1966) carefully removed the gonad through a small hole in the tunic and body wall, and a new gonad eventually reappeared. This experiment suggests that totipotent stem cells capable of producing gametes are present in the body outside the gonad. A similar conclusion can be made from more recent experiments in which the primordial germ cells of the endodermal strand were removed from larvae by tail amputation, yet the adult was still able to generate gonads (Takamura et al., 2002; Kawamura et al., 2011). If the organ removal procedures developed by Bourchard-Madrelle (1966) can be applied in general to Ciona, then it would be possible to study regeneration of other organs, including the heart and digestive system.
Also relevant to organ regeneration, the solitary ascidian Polycarpa has been reported to eviscerate its internal organs and completely regenerate them from the atrial epithelium (Selys-Longchamps, 1915). Whereas no such evisceration and replacement has been reported in Ciona, visceral regeneration in Polycarpa suggests that internal organs may have a capacity for regeneration in solitary ascidians that has not been demonstrated by experiments in which large pieces of the body are removed.
THE “DARK AGE” AND RENAISSANCE OF CIONA REGENERATIVE BIOLOGY
During the 1920s, Ciona regeneration research lapsed into a period of relative inactivity, a “dark age” possibly caused by fallout from the purported demonstration of acquired inheritance of siphon length and its discrediting. Beginning in the 1990s, however, new interest appeared in this type of research, fostering a mini-renaissance in Ciona regenerative biology. The renewed attention paralleled a general re-wakening of interest in tissue repair and regeneration, heralded by the emergence of stem cell biology. In the next part of this review, we will discuss recent studies focused on regeneration of two currently studied organs, the NC and the pigmented sensory organs of the siphons.
NEURAL COMPLEX AND BRAIN REGENERATION
The NC was the first organ to be revisited during the rebirth of Ciona regenerative biology. In a typical NC regeneration experiment, the entire complex of organs, including the cerebral ganglion, neural gland, and the ciliated duct and funnel, as well as the overlying epidermis are removed by ablation. Several questions have been the primary focus of recent research on brain and NC regeneration. First, is NC regeneration complete: are all structures and functions of the original NC consisting of several different organs replaced with fidelity? Second, what is the relationship between NC regeneration and ontogeny: does regeneration recapitulate the original sequence of post-metamorphic development? Third, what is the cellular basis for NC regeneration: are stem cells, trans-differentiating cells, or both involved in the process? Fourth, what is the source of cells for NC regeneration: is the source local, distant, or are there multiple sources? Below we discuss the current state of knowledge concerning these questions.
Earlier research relied on the interpretation of histological sections or electron micrographs to reconstruct the events of NC regeneration (Lender and Bouchard-Madrelle, 1964; Chambost, 1966). Recently, further knowledge of the NC regeneration process has been obtained using a combination of live imaging methods, labeling with the cell proliferation marker EdU, and tracking of nerve restoration in green fluorescent protein (GFP)-labeled transgenic animals (Dahlberg et al., 2009). This research used E15, a Ciona transgenic line that expresses GFP throughout the adult nervous system (Awazu et al., 2007; Sasakura, 2007). Accordingly, NC regeneration was divided into six stages, which are illustrated in Fig 4. From the earliest to the latest, these stages are: (1) healing of the wound, (2) further healing and merger of the severed nerve stumps at the anterior and posterior edges of the ablation site, (3) swelling, enlarging and fusing of the nerve stumps at their tips, (4) re-appearance of the brain and neural gland, (5) reconnection of the anterior and posterior nerves and enlargement of the NC, and (6) the restoration of a morphologically complete, albeit smaller, NC.
The regeneration of a smaller NC than the original NC brings up the question of whether the replaced set of organs is structurally and functionally complete. Because most studies have emphasized the brain and much less information is available about the other NC organs we will focus our discussion on cerebral ganglion regeneration. The Ciona brain has distinct anterior (oral siphon facing), central, and posterior (atrial siphon facing) parts, each consisting of a peripheral cortex containing neuronal cell bodies and an internal neuropile containing their axonal processes. The neuronal cells express Substance P (SP), cholecystokinin (CCK)-gastrin, neurokinin A (NKA), insulin, GABA, and gonadotropin-releasing hormone (GnRH), which have been used as markers for neurons with distinct localizations in the ganglion. In a series of studies, Bollner et al. (1992; 1993; 1995; 1997) compared the neuronal cells expressing these hormones and neurotransmitters, as well as a panel of monoclonal antibodies, in normal and regenerating brains. SP and CCK-gastrin producing cells are localized primarily in the anterior and posterior parts or the central portion of the ganglion respectively (Bollner et al., 1992). These cells reappear sequentially during regeneration, first only SP and CCK-gastrin positive neuroblasts were distributed randomly in the regenerating ganglion, but later cell processes indicative of differentiated neurons were formed with cell bodies in cortical positions resembling the original brain. GABA producing neurons are also found predominantly in the cortex of the normal brain, but are more abundant dorsally than ventrally (Fig. 5). They also reappear during regeneration, initially as neuroblasts and then as differentiated neurons, although there are more cell bodies clustered in the central and ventral portions of the regenerated ganglion than in the original brain (Bollner et al., 1993). GnRN expressing neurons are enriched in the posterior cortex of the original ganglion and reappear in a similar position during regeneration (Bollner et al., 1997). Lastly, cortical cells immunoreactive with monoclonal antibodies also reappear in their original positions in the regenerated brain (Bollner et al., 1995). In contrast to the above, however, two of the neuron markers originally present in the brain, NKA- and insulin-expressing cells, were not detected anywhere in the NC during the entire period of regeneration (Bollner et al., 1995). Although this result could have a trivial basis, such as a delay in the reappearance of these particular markers until after the period in which the regenerating brains were studied, it could also mean that regeneration is not complete at the cellular level.
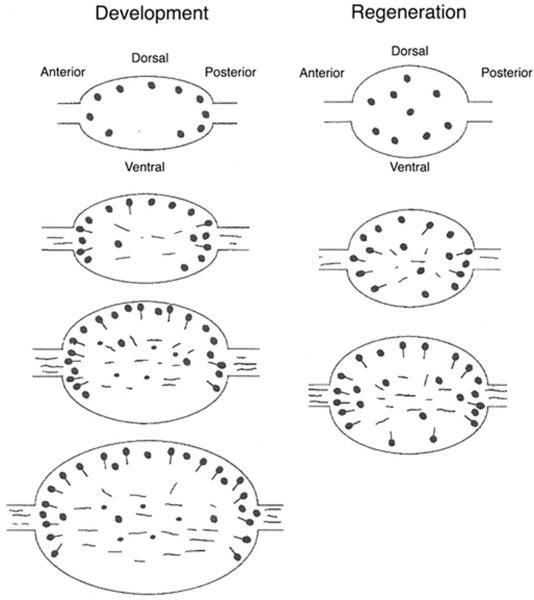
Fig. 5.
A schematic summary of GABA neuron replacement during post-metamorphic development (left) and regeneration (right) of the cerebral ganglion. Neuroblasts (filled circles) appear first followed by differentiated neurons with fibers (filled circles attached to lines or lines). In each column, development and regeneration proceed from the top to the bottom. From Bollner et al. (1993).
The fidelity of NC regeneration has also been addressed by functional assays. Loeb (1905) did not detect any specific physiological changes after NC ablation, but more recent studies have shown that the ascidian NC has important physiological roles in modulating the contractile behaviors of the muscular body wall and siphons, including the accurate performance of the so-called “cross-siphon” reflex (Mackie and Wyeth, 2000; Mackie et al., 2006). The NC-related behaviors are lost after NC ablation, and gradually reappear in sequence as connections are re-formed between nerve tracts of the CNS and the regenerating ganglion (Dahlberg et al., 2009). By the end of the process, the regenerated NC seems to function normally. Thus, even though some changes in the reappearance of neurons may occur during brain regeneration, this does not seem to affect the recovery of a cerebral ganglion that is able to guide normal behavioral activities.
The adult Ciona brain is formed from the anterior portion of the neural plate and larval CNS during post-metamorphic development (Manni et al., 2005; Horie et al., 2011) but its origin is less clear during regeneration, in which there is no pre-existing neural rudiment. Nevertheless, the sequence in which neuronal cells reappear during regeneration is similar if not identical to post-metamorphic development (Bollner et al., 1993; 1995; 1997). Thus, NC regeneration appears to mimic ontogeny with regard to neurogenesis.
Future studies on the completeness relationship of NC regeneration to post-metamorphic development would benefit from additional markers. It will be important to know whether the patterns of gene expression during regeneration are similar to those during post-metamorphic development. Furthermore, it will also be important to identify and include markers of the neural gland and ciliated duct/ funnel in future studies of NC regeneration. Recently, a proteome map has been obtained for the Ciona NC (Saxena et al., 2013) that could be used to explore the restoration of specific proteins during regeneration.
The identity and origin of the progenitor cells has been a priority since the beginning of research on NC regeneration. In this regard, a relevant observation may be that the cerebral ganglion can regenerate more rapidly when it is ablated while leaving the other NC components intact (Lender and Bouchard-Madrelle, 1964). This result could mean that the neural gland or the ciliated duct/funnel is a source of progenitor cells for brain regeneration. However, when the entire NC is removed, the non-ganglion parts must also be replaced, so the question is larger than the source of any single NC component.
Most previous and recent research suggests that NC replacement involves either pluripotent blood cells that rapidly invade the wound created by NC ablation (Lender and Bouchard-Madrelle, 1964; Bollner, 1990; Bollner et al. 1992) or undifferentiated neuroblasts migrating into the ablated region from the dorsal strand (Federle, 1938). The dorsal stand is a tube-like epithelial structure with an associated plexus of neurons extending posterior from the ciliated duct along the dorsal side of the branchial sac to the gonad (Fig. 6). Like the NC itself, the dorsal strand is thought to be a derivative of the larval CNS. The dorsal strand plexus also contains a population of GnRH expressing neurons and neuroblasts, which are situated in the part of this structure that lies close to the NC (Mackie, 1995; Bollner et al., 1997; Tsutsui et al., 1998) and has been proposed to be involved in NC regeneration (Federle, 1938). The evidence supporting NC progenitor cells from the dorsal strand is that GnRN positive neuroblasts are initially seen near the regenerating brain after NC ablation, where they subsequently develop axonal processes and eventually become incorporated into the posterior portion of the regenerating cerebral ganglion (Bollner et al., 1997). However, none of these GnRN positive neuronal cells were labeled with BrdU, a marker of DNA synthesis in proliferating cells, suggesting that they were born before the ablation (Bollner et al., 1997). In fact, even after long periods of BrdU labeling many cells of the regenerating NC remain unlabeled. Thus the progenitor cells could be a kind of neuroblast that does not require cell division to differentiate or could be produced by trans-differentiation of other cell types.
Recent experiments suggest that dividing cells also have a role in brain regeneration. In these studies, EdU was used as a marker of cell proliferation and neuronal cells were labeled by GFP fluorescence in the E15 transgenic line (Dahlberg et al. 2009). The results showed that neurons are formed near the tips of severed pre-existing nerve tracts on all sides of the regenerating ganglion, not only at the posterior end near the dorsal strand (Fig. 7A-F). The tips of the extending nerves were labeled with EdU, suggesting the presence of newly born cells in a blastema-a mass of cells capable of growth and regeneration-surrounding the cut stumps of pre existing nerves (Fig. 7G-I). Continuation of this activity by fusion of the growing nerves all sides of the ganglion would lead to its replacement and suggests that proliferating cells also make a make contribution to NC regeneration, at least with regard to the formation of a new cerebral ganglion.
Fig. 7.
Nerve regrowth and cell proliferation during neural complex regeneration. A-F. Neuronal cells stained with GFP in the E15 transgenic line. A. Nerve tract regeneration at 4 days post amputation (dpa) showing the bulbous tips of nerve tracts (boxed regions J and K) extending toward the ablation zone (dark hole in the center of photograph). B and C. Higher magnifications of boxed regions J and K respectively in A showing bulbous tips of regenerating nerves containing cell bodies (orange arrows and box L). Nerve tract regeneration at 9 dpa shows fine neurites extending from the tip of severed nerve tracts (boxed region N) into the ablation zone. E and F. Higher magnification images at 9 dpa showing neurites (orange arrows) extending from the tips of regenerating nerve tracts. All scale bars are 50 μm. G-I. Neuronal cells doubled labeled with GFP and EdU in the E15 transgenic line a 4 dpa. G. Low magnification showing a field of EdU labeled proliferating cells surrounding the NC ablation zone (star). H. A re-growing neurite (yellow arrowhead) surrounded by EdU labeled cells. I. A merged photograph showing a region of regenerating neuronal cells with overlapping EdU and GFP (yellow staining). OrS: oral siphon. AtS: atrial siphon. RP: right posterior nerve. RA: right anterior nerve. PN: posterior nerves. From Dahlberg et al. (2009) with modifications.
Thus, it seems likely that both cells born before and after ablation, and consisting of either pluripotent stem cells or more fate-restricted progenitor cells, give rise to the regenerating brain. Their source needs further investigation: they could be derived locally (around the site of the ablation itself), more distantly (from the dorsal stand or elsewhere in the body), or both.
SIPHON SENSORY ORGAN REGENERATION
The regeneration of the oral siphon (OS) and its ring of brightly pigmented oral siphon pigmented organs (OPO) have also received new attention during the latest period of Ciona regeneration research. The oral siphon (OS) is the external opening of the pharynx (Figs. 1A, 8A). It is formed after metamorphosis from a placode-like structure, which invaginates and fuses with the developing pharynx to form a continuous tube (Manni et al., 2005). The OS contains diffuse bands of circular muscle fibers and stripes of longitudinal muscle fibers that alternate with regions mostly free of muscle containing blood sinuses and nerves. The longitudinal stripes in the siphon grade into similar stripes extending into the body wall. The internal epidermal wall of the OS contains a ring of tentacles near its base and OPO near its rim. The OPO are sometimes termed ocelli, implying a function in photoreception, but this role has remained unproven (Dilly and Wolken, 1973). Another possibility is that the OPO are mechanoreceptors analogous to lateral line neuromasts in fishes and amphibians.
Fig. 8.
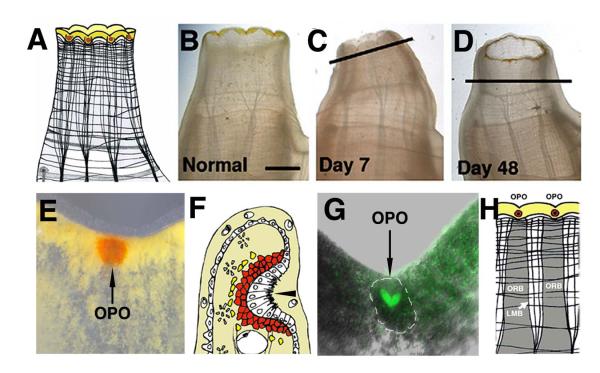
Oral siphon structure and regeneration. A. A diagram of an oral siphon showing the distal pigmented rim and muscle fiber organization. B. Oral siphon of a living animal prior to amputation. C-D. Oral siphon regeneration. The original amputation planes are indicated by diagonal or horizontal lines. Scale bar in B is 4 mm; magnification is the same in B-D. E. An oral siphon pigment organ (OPO) embedded in the yellow pigment band in a notch between the siphon lobes of a living animal. F. A diagram of a section through an oral siphon pigment organ showing the epithelial invagination (arrowhead) and underlying cup of pigment cells. G. GFP staining of the basal portion of an oral siphon pigment organ (outlined by dashed lines) in an E15 transgenic animal. H. A diagram showing the relationship between oral siphon pigment organs and the underlying oral siphon pigment organ regeneration band (ORB). LMB: longitundinal muscle band. From Auger et al. (2010).
Most Ciona individuals have eight OPO, although a small number of animals in natural populations show seven or nine OPO (Millar, 1953; Auger et al., 2010). The atrial siphon has only six OPO. Each OPO consists of a crypt of ciliated epithelial cells surrounded by a cup of orange pigment cells (Fig. 8E), which gives the OPO its brightly colored appearance in living animals (Fig. 9E-G). The most basal epithelial cells show intense GFP expression in E15 transgenic animals (Fig. 8G), suggesting a neuronal function and integration with the CNS (Auger et al., 2010). The OPO are spaced equidistantly in notches between siphon lobes, where they punctuate a band of yellow pigment cells that encircles the siphon rim. Each OPO is in register with a vertical stripe of tissue termed the oral siphon regeneration band (ORB) located below it (Fig. 9H), which probably has a role in replacing OPO after injury (Auger et al., 2010)
Fig. 9.
Diagrams depicting the five stages of oral siphon pigment organ regeneration (see text for details) along the oral siphon (thick black horizontal line) in young (A) and old (B) animals. B. The number and patterning of oral siphon pigment organs is affected by overproduction of pigment cells in old animals. ORB: oral siphon regeneration bands. LMB: longitundinal muscle bands. Open circle: undifferentiated cells. Red circles: red pigment cells, yellow circles: yellow pigment cells. Blue horizontal lines: longitundinal muscle fibers. From Jeffery (2012).
Early studies in Ciona established that both oral and atrial siphons are capable of regeneration, which is complete within about a month, and that OPO are replaced within several days after their removal (Hirschler 1914; Fox 1923; Sutton 1953; Whittaker 1973). The distal parts of the OS, the OPO and siphon lobes, are regenerated prior to the reformation of the proximal parts by intercalation, a process that also occurs during vertebrate limb regeneration (Echeverri and Tanaka, 2005).
Siphon regeneration was recently re-investigated focusing on the rapid restoration of the OPOs (Auger et al., 2010). The same four questions introduced above for NC regeneration-degree of completeness, developmental origin, replacement cell type, and source of precursor cells-were asked about OPO regeneration. Auger et al. (2010) demonstrated that when the distal part of an OS containing the OPOs was amputated the OS stump responded by replacing the OPOs with precise fidelity in both structure and number. The steps involved in this replacement are illustrated in Fig. 9A. First, red pigment cells differentiate in the ORB; second, the red pigment cells line up in single file along the rim of the severed siphon; third, the pigment cells condense into spots that presage the subsequent appearance of OPO; fourth, the epidermal epithelium invaginates to form a crypt and the pigment cells are organized as a cup surrounding this pit; and fifth, GFP positive neuronal cells appear at the base of the pit. The OPO regeneration process resembles OPO ontogeny during post-metamorphic development (Chiba et al., 2004; Auger et al., 2010).
Each OPO appears to regenerate as an independent unit: single OPOs can be removed or destroyed and are then replaced with no marked affects on other OPO in the ring (von Haffner, 1933; Auger et al., 2010). These studies indicate a that there is a degree of independence between regenerative events in the oral siphon and the body, which is expected considering the ability of siphon and body growth to be uncoupled under other circumstances.
Precise fidelity of OPO replacement was observed after four repetitive cycles of OS amputation at its distal end, indicating that the patterning mechanisms underlying the OPO regeneration process are extremely robust (Auger et al, 2010). However, amputation of the OS at its base (e.g. complete removal of the OS) resulted in the disruption of normal patterning, with regenerates sometimes showing OPO duplication (up to 16 OPO) along the rim of a normally sized OS. Thus, OPO regeneration has strong fidelity, although the patterning mechanism is robust only in the siphon.
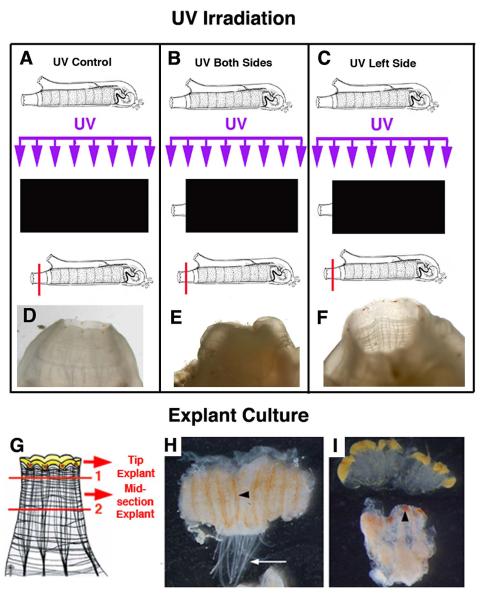
Hirschler (1914) originally suggested that the siphons and their ring of OPO were replaced by migration of cells from a source outside the regenerating area. As described above for the early steps in NC regeneration, the results of histological studies also indicate an extensive migration of blood cells into the wounded areas following siphon amputation (Sutton, 1953). Recently, the source of cells involved in OPO replacement has been studied in two different ways (Auger et al., 2010). First, the OS was irradiated with ultraviolet (UV) light prior to amputation (Fig. 10A-F). This would affect the ability of cells already present in the OS, particularity those that require a proliferation step, to participate in OPO regeneration. In contrast, if OPO replacement involves cells originating from outside the siphon, the effects of UV irradiation would be less pronounced. When the OS was irradiated OPO regeneration was retarded, suggesting a local source of cells for OPO replacement. Second, after OS amputation at the distal tip, the remaining siphon stump was cultured as an explant (Fig. 10G-I), which would exclude the contribution of migratory cells from the body in OPO regeneration. However, new OPO appeared in the distal region of the explants, indicating that OPO replacement is accomplished at least in part by the differentiation of local cells. Indeed, orange pigment cells appear to differentiate in the ORB of siphon explants prior to the appearance of regenerated OPO (Fig. 10H). Accordingly, the ORB has been proposed as a localized niche for OPO progenitor cells (Auger et al., 2010).
Fig. 10.
A summary of oral siphon UV irradiation and explant experiments demonstrating a local source of progenitor cells for oral siphon pigment organ regeneration. Top. UV irradiation. A, D. UV irradiation of a shielded control animal resulted in normal oral pigment organ regeneration. B, E. UV irradiation of the both sides of an oral siphon prior to distal amputation resulted in no oral pigment organ regeneration. C, F. UV irradiation of the left side of an oral siphon prior to distal amputation resulted in no oral pigment organ regeneration on the left side. Black rectangles indicate the areas shaded during UV irradiation (purple arrows). Horizontal red lines indicate the amputation planes. Bottom. Explant cultures. G. Diagram of the two-step procedure used to obtain siphon distal and mid-section explants. H. A siphon midsection explant after 6 days in culture showing pigment cell accumulation in the oral siphon pigment organ regeneration bands (arrowhead). Arrow shows the siphon tentacles. I. Siphon distal (top) and mid-section (bottom) explants after 9 days in culture showing the regeneration of oral siphon pigment organs (arrowhead) in the latter. From Auger et al. (2010).
Experiments using EdU and phospho-histone H3 as markers of cell division show the existence of a blastema of proliferating cells at the OS amputation site, although the regenerated OPO themselves appear to be unlabeled (Auger et al., 2010). Thus, like the situation for NC regeneration, OS replacement may involve the participation of cells that have recently divided as well as those that have not divided. Pluripotent stem cells, undifferentiated precursor cells, trans-differentiated cells, or multiple cell types could be responsible for OPO regeneration.
The position of the amputation site might also be relevant to the type of cells involved in OPO regeneration. When the entire OS siphon, rather than a part of it, is amputated, replacement cells are derived from the body rather than within the siphon, and are potentially different from those stored in the ORB niches. Furthermore, different mechanisms may be involved in OPO replacement, which occurs rapidly, than in the regeneration of other parts of the OS, which proceeds more slowly. Clearly, there is much more to be learned about the mechanisms of siphon and OPO regeneration.
As in the case of the NC, one of the next phases in Ciona OS and OPO regeneration research should be analysis of the molecular mechanisms underlying the cellular and tissue related phenomena that are reviewed above. The Ciona system is well suited for this purpose due to the extensive molecular tool kit that has been developed, offering the possibility of detailed analysis at the genome (Dehal et al., 2002), transcriptome/EST (Azumi et al., 2007; Matsuoka et al., 2013), and proteome (Serafini et al. 2005; Saxena et al., 2013) levels.
THE NERVOUS SYSTEM AND SIPHON REGENERATION
The requirement of nerves for vertebrate limb regeneration (Kumar et al., 2007) brings up the question of whether the NC and/or the siphon nerves are involved in OPO and siphon regeneration. Nerve tracts emanate from cerebral ganglion into the OS where they radiate into a network leading to the ring of OPOs (Markman, 1958; Auger et al., 2010). The possible role of the central nervous system in OPO regeneration was examined by amputating the OS in animals with an ablated NC (Sutton, 1953; Auger et al., 2010). When only the distal portion of the OS with its ring of OPO were removed the OPOs were replaced rapidly (Auger et al., 2010). In contrast, when the entire OS was removed OPO siphon regeneration proceeded much more slowly (Sutton, 1953). These results do not support a role for the NC itself in OS and OPO regeneration. However, they point to the importance of nerve tracts remaining in the OS after amputation, which would have been missing after complete OS removal.
It was once believed that all cell bodies of the ascidian central nervous system are present in the cerebral ganglion, however, the recent imaging studies of GFP expression in transgenic Ciona demonstrate the existence of small cell bodies outside the ganglion, including along the nerve tracts leading to the OS (Dahlberg et al., 2009). Thus, the ability of OPOs to be replaced after NC removal could be related to the persistence and regeneration of siphon nerves in the portion of the oral siphon remaining after amputation. This interpretation is supported by regeneration studies in the E15 transgenic line, in which OS regeneration is accompanied by regrowth of nerves from the OS stumps into the blastema (Fig. 11A). The growing nerves separate into fine process near the re-forming OPO and could be involved in the replacement of its neural component (Fig. 11B). Further studies in which siphon nerves are manipulated independently of the NC will be necessary to determine their roles in OS and OPO regeneration.
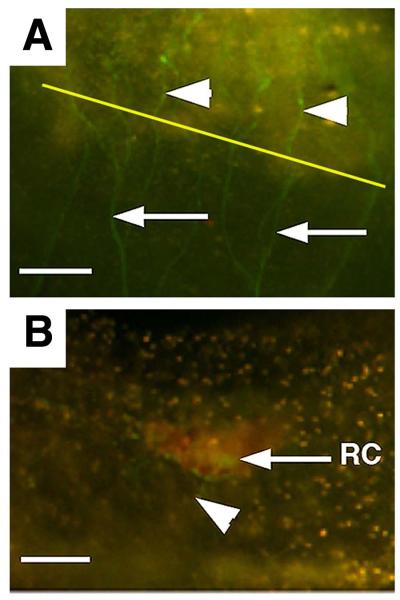
Fig. 11.
Nerve regeneration following oral siphon amputation in a GFP labeled E15 transgenic animal. A. Nerve fibers (arrowheads) develop in the blastema 5 days after amputation that emanate from the original siphon nerve tracts (arrows). Diagonal yellow line indicates the amputation plane. B. A fine nerve fiber (arrowhead) connecting to the basal neural receptor (RC) of an oral siphon pigment organ at 7 days post amputation. Scale bars are 50 μm in A and 20 μm in B. From Auger et al. (2010).
RELATIONSHIP BETWEEN REGENERATION AND AGING
Ciona is currently being developed to study the relationship between regeneration and aging (Jeffery, 2014). Recent studies on NC regeneration have established an inverse relationship between the rate of regeneration and size (Dahlberg et al., 2009). Auger et al., (2010) also found a strong negative correlation between the size and OPO regeneration. In a natural population, the smallest animals regenerated OPO in only 2-3 days, whereas the largest animals required about 8-10 days to completely replace their OPOs. It was subsequently shown that the largest animals in a wild population were unable to regenerate their OS after amputation, probably because they lacked the ability to form a blastema of proliferating cells in the OS stump (Jeffery, 2012). The largest animals in the population also showed defects in the process of OPO regeneration hallmarked by the formation of supernumerary and malformed OPO (Fig. 9B), similar to the effects on OPO replacement encountered after the entire OS was removed in younger animals (Auger et al., 2010). The inability to produce normal OPO after multiple amputation cycles suggested a breakdown in the robustness of patterning during regeneration (Jeffery, 2012). Indeed, explant cultures of OS stumps from large animals did not form OPO, possibly as consequence of this defective patterning, but instead differentiated an excessive number of red pigment cells in their ORBs, perhaps explaining overproduction of OPO in vivo (Jeffery, 2012). Since size can be used as a proxy for age in wild caught Ciona (Berrill, 1947; Millar, 1952; Dybern, 1965; Peterson et al., 1995), these results suggest that OPO and OS regeneration may be compromised during aging. The relationship between aging regeneration needs further study in laboratory-reared Ciona that offer the opportunity of studying individuals of a precise age.
SPECULATION ON MODES OF REGENERATION AND EVOLUTION
The two types of ascidian regeneration-partial body regeneration in solitary ascidians (exemplified by Ciona) and whole body regeneration in colonial ascidians-seem strikingly different when considering the variety of different fates they produce. However, the differences in regeneration potential could be superficial and subject to interpretation according to the experimental operations and manipulations that evoked them. On one hand, it is likely that colonial ascidians are also capable of partial body regeneration, although the author is unaware of experiments that have been specifically carried out to test this possibility. On the other hand, solitary ascidians might also to be capable of regeneration beyond the expectations of partial body regeneration. Ciona is apparently able to regenerate the gonad (Bourchard-Madrelle, 1966), hinting of a totipotent stem cell cache somewhere in the body, and another solitary ascidian species has been reported to replace all internal body organs after evisceration (Selys-Longchamps, 1915). These reports need substantiation but it is interesting to speculate that experiments done under circumstances in which survival of regenerates is maximized could reveal a whole body-like of regeneration mode in solitary ascidians. For instance, because regeneration capacity declines with age (Dahlberg et al., 2009; Auger et al., 2010; Jeffery, 2012) there could be a stage in post-metamorphic development with extensive regeneration that disappears, as juveniles become adults.
The origin of whole body regeneration probably occurred in concert with the evolution of brooding, asexual reproduction by budding, and the colonial life style (Tiozzo et al., 2008; Brown and Swalla, 2012). Different ascidian families contain exclusively colonial species or some solitary and some colonial species, suggesting a polyphyletic origin of the colonial life style. It is difficult to infer the direction of evolution, whether the colonial life history is basal or derived, however, because of poor resolution in phylogenetic analysis for most families (Yokobori et al., 2006; Brown and Swalla, 2012). However, in one family, the stolidobranchs (which include the Botryllid species used as a model for whole body regeneration), the solitary life style has been inferred to be basal and colonial organization to be derived (Zeng et al., 2006). At least in this case, whole body regeneration is likely to have evolved from a sexually developing solitary lineage originally lacking this capability. It is also thought that strong regenerative capacity was probably the ancestral condition in most animal phyla (Bely, 2010; Bely and Nyberg, 2010). Therefore, the evolution of ascidians with more comprehensive regeneration capacities from those with restricted regeneration may underscore the evolutionary flexibility of regenerative mechanisms.
ACKNOWLEDGEMENTS
The author is grateful to colleagues who generously agreed to allow original figures from their publications to be reproduced in this article. The preparation of this article was assisted by the support of NIH grant R01AG037918.
LITERATURE CITED
- Auger H, Sasakura Y, Joly J-S, Jeffery WR. Regeneration of oral siphon pigment organs in the ascidian Ciona intestinalis. Dev Biol. 2010;339:374–389. doi: 10.1016/j.ydbio.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awazu S, Matsuoka T, Inaba K, Satoh N, Sasakura Y. High-throughput enhancer trap by remobilization of transposon Minos in Ciona intestinalis. Genesis. 2007;45:307–317. doi: 10.1002/dvg.20290. [DOI] [PubMed] [Google Scholar]
- Azumi K, Sabau SV, Fugie M, Usami T, Koyanagi R, Kawashima T, Fujiwara S, Ogasawara M, Satake M, Nonaka M, Wang HG, Satou Y, Satoh N. Gene expression profile during the life cycle of the urochordate Ciona intestinalis. Dev Biol. 2007;308:572–582. doi: 10.1016/j.ydbio.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Bely AE. Evolutionary loss of animal regeneration: pattern and process. Integr Comp Biol. 2010;50:515–527. doi: 10.1093/icb/icq118. [DOI] [PubMed] [Google Scholar]
- Bely AE, Nyberg KG. Evolution of animal regeneration: re-emergence of a field. Trends Ecol Evol. 2010;25:161–170. doi: 10.1016/j.tree.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Berrill NJ. The development and growth of Ciona. J Mar Biol Assoc UK. 1947;26:616–625. doi: 10.1017/s0025315400013825. [DOI] [PubMed] [Google Scholar]
- Berrill NJ. The Tunicata with an Account of the British Species. Ray Society; London: 1950. pp. 1–354. [Google Scholar]
- Berrill NJ. Regeneration and budding in tunicates. Biol Rev. 1951;26:456–475. [Google Scholar]
- Bourchard-Madrelle C. Régéneration de l’ovaire chez une Ascidie simple Ciona intestinalis L. (Prochordé) Bull Soc Zool Fr. 1966;91:107–114. [Google Scholar]
- Bollner T. Regeneration of the central nervous system of the ascidian Ciona intestinalis. In: Kiortsis V, Koussoulakos S, editors. Recent Trends in Regeneration Research. Plenum; New York: 1989. pp. 281–287. [Google Scholar]
- Bollner T, Beesley PW, Thorndike M. Pattern of substance P- and cholecytokinin-like immunoreactivity during regeneration of the neural complex in the ascidian Ciona intestinalis. J Comp Neurol. 1992;325:572–580. doi: 10.1002/cne.903250409. [DOI] [PubMed] [Google Scholar]
- Bollner T, Beesley PW, Thorndike MC. Distribution of GABA-like immunoreactivity during post-metamorphic development and regeneration of the nervous system in the ascidian Ciona intestinalis. Cell Tiss Res. 1993;272:553–561. [Google Scholar]
- Bollner T, Howalt S, Thorndyke MC, Beesley PW. Regeneration and post-metamorphic development of the central nervous system in the protochordate Ciona intestinalis: a study with monoclonal antibodies. Cell Tiss Res. 1995;279:421–432. doi: 10.1007/BF00318500. [DOI] [PubMed] [Google Scholar]
- Bollner T, Beesley PW, Thorndike MC. Investigation of the contribution from peripheral GnRH-like immunoreactive ‘neuroblasts’ to the regenerating central nervous system in the protochordate Ciona intestinalis. Proc R Soc Lond B. 1997;264:1117–1123. [Google Scholar]
- Bourlat SJ, Juliusdottir T, Lowe CJ, Freeman R, Aronowicz J, Kirschner M, Lander ES, Thorndyke M, Nakano M, et al. Deuterostome phylogeny reveals monophyletic chordates and the new phylum Xenoturbellida. Nature. 2006;444:85–88. doi: 10.1038/nature05241. [DOI] [PubMed] [Google Scholar]
- Brown FD, Swalla BJ. Evolution and development of budding by stem cells: Ascidian coloniality as a case study. Dev Biol. 2012;369:151–162. doi: 10.1016/j.ydbio.2012.05.038. [DOI] [PubMed] [Google Scholar]
- Candia Carnevali MD, Burighel P. Regeneration in echinoderms and ascidians. In: Encyclopedia of Life Sciences. John Wiley & Sons, Ltd; Chicester: 2010. pp. 1–15. [Google Scholar]
- Chambost D. Le complexe neural de Ciona intestinalis. Etude comparative du ganglion nerveux et de la glande asymétrique aux microscope optiques et électroniques. C R Acad Sc Paris Série D. 1966:969–971. [Google Scholar]
- Chiba S, Sasaki A, Nakayama A, Takamura K, Satoh N. Development of Ciona intestinalis juveniles (Through 2nd Ascidian Stage) Zool Sci. 2004;21:285–298. doi: 10.2108/zsj.21.285. [DOI] [PubMed] [Google Scholar]
- Dahlberg C, Auger H, Dupont S, Sasakura Y, Thorndyke M, Joly J-S. Refining the model system of central nervous system regeneration in Ciona intestinalis. PLoS One. 2009;4:e4458. doi: 10.1371/journal.pone.0004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehal P, Satou Y, Campbell RK, Chapman J, Degnan B, De Tomaso A, Davidson B, Di Gregorio A, Gelpke M, Goodstein M, et al. The draft genome of Ciona intestinalis: insights into chordate and vertebrate origins. Science. 2002;298:2157–2167. doi: 10.1126/science.1080049. [DOI] [PubMed] [Google Scholar]
- Delsuc F, Brinkmann H, Chourrout D, Phillipe H. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature. 2006;439:965–968. doi: 10.1038/nature04336. [DOI] [PubMed] [Google Scholar]
- Dilly PN, Wolken JJ. Studies on the receptors of Ciona intestinalis: IV. The ocellus in the adult. Micron. 1973;4:11–29. [Google Scholar]
- Dybern BI. The life cycle of Ciona intestinalis (L) f. typical; in relation to environmental temperature. Oikos. 1965;16:109–131. [Google Scholar]
- Echeverri K, Tanaka E. Proximodistal patterning during limb regeneration. Dev Biol. 2005;279:391–401. doi: 10.1016/j.ydbio.2004.12.029. [DOI] [PubMed] [Google Scholar]
- Fantini B. The “Stazione Zoologica Anton Dohrn” and the history of embryology. Int J Dev Biol. 2000;44:523–535. [PubMed] [Google Scholar]
- Fedele M. Il sistema nervoso delgli ‘Ascidiacea’ nel piano di organizzazione dell’ Cordati. Atti del’ Academ Naìionale Lincei Rendiconti, Series. 1938;6:370–376. [Google Scholar]
- Fox HM. Note on Kammerer’s experiments with Ciona concerning the inheritance of an acquired character. J Genet. 1924;14:89–91. [Google Scholar]
- Freeman G. The role of blood cells in the process of asexual reproduction in the tunicate Perophora. J Exp Zool. 1964;156:157–184. doi: 10.1002/jez.1401560204. [DOI] [PubMed] [Google Scholar]
- Fuchs HM. The effect of abundant food on the growth of young Ciona intestinalis. Biol Zentralbl. 1914;34:439–444. [Google Scholar]
- George WC. The formation of new siphon openings in the tunicate, Styela plicata. J. Elisha Mitchell Sci Soc. 1937;53:87–91. [Google Scholar]
- von Haffner K. Die Überzaligen Siphon and Ocellen von Ciona intestinalis L. (Experimentell-morpholosiche Untersunchungen) Z Wiss Zool. 1933;143:16–52. [Google Scholar]
- Hirschler J. Über die Restitutions-und Involutionsvoränge bei operierten Exemplaren von Ciona intestinalis Flem. (Teil I) nebst Bemurkungen über den Wert des Negativen für das Potenzproblem. Arch mikr Anat. 1914;85:205–227. [Google Scholar]
- Horie T, Shinki R, Ogura Y, Kusakabe TG, Satoh N, Sasakura Y. Ependymal cells of chordate larvae are stem-like cells that form the adult nervous system. Nature. 2011;469:525–528. doi: 10.1038/nature09631. [DOI] [PubMed] [Google Scholar]
- Jeffery WR. Determinants of cell and positional fate in ascidian embryos. Int Rev Cytol. 2001;203:3–62. doi: 10.1016/s0074-7696(01)03003-0. [DOI] [PubMed] [Google Scholar]
- Jeffery WR. Siphon regeneration capacity is compromised during aging in the ascidian Ciona intestinalis. Mech Ageing Dev. 2012;133:629–636. doi: 10.1016/j.mad.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery WR. The tunicate Ciona: a model system for studying the relationship between regeneration and aging. Invert Reprod Dev. 2014 doi: 10.1080/07924259.2014.925515. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammerer P. Breeding experiments on the inheritance of acquired characters. Nature. 1923;111:637–640. [Google Scholar]
- Kawamura K, Tiozzo S, Manni L, Sunanaga T, Burighel P, De Tomaso AW. Germline formation and gonad regeneration in solitary and colonial ascidians. Dev Dynam. 2011;240:299–308. doi: 10.1002/dvdy.22542. [DOI] [PubMed] [Google Scholar]
- Koestler A. The Case of the Midwife Toad. Random House; New York: 1971. pp. 1–187. [Google Scholar]
- Kumar A, Godwin JW, Gates PB, Garza-Garcia AA, Brockles JP. Molecular basis for the nerve dependence of limb regeneration in an adult vertebrate. Science. 2007;318:772–777. doi: 10.1126/science.1147710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauzon RJ, Ishizuka KJ, Weissman IL. Cyclical generation and degeneration of organs in a colonial urochordate involves crosstalk between old and new: A model for development and regeneration. Dev Biol. 2002;249:333–348. doi: 10.1006/dbio.2002.0772. [DOI] [PubMed] [Google Scholar]
- Lender T, Bouchard-Madrelle C. Études expérimental de la régéneration du complex neural de Ciona Intestinalis (Prochodé) Bull Soc Zool. 1964;89:546–554. [Google Scholar]
- Loeb J. Studies in General Physiology. University of Chicago Press; Chicago: 1905. pp. 1–423. [Google Scholar]
- Mackie GO. On the “visceral nervous system” of Ciona. J Mar Biol Assoc UK. 1995;75:141–151. [Google Scholar]
- Mackie GO, Wyeth RC. Conduction and coordination in deganglionated ascidians. Can J Zool. 2000;78:1626–1639. [Google Scholar]
- Mackie GO, Burighel P, Caicci F, Manni L. Innervation of ascidian siphons and their responses to stimulation. Can J Zool. 2006;84:1146–1162. [Google Scholar]
- Manni L, Agnoletto A, Zaniolo G, Burighel P. Stomodeal and neurohypophysial placodes in Ciona intestinalis: insights into the origin of the pituitary gland. J Exp Zool B Mol Dev Evol. 2005;304:324–339. doi: 10.1002/jez.b.21039. [DOI] [PubMed] [Google Scholar]
- Markman B. On the peripheral nervous system of ascidians. Acta Zool. 1958;34:13–18. [Google Scholar]
- Matsuoka T, Ikead T, Fujimaki K, Satou Y. Transcriptome dynamics in early embryos of the ascidian, Ciona intestinalis. Dev Biol. 2013;384:375–385. doi: 10.1016/j.ydbio.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Millar RH. The annual growth and reproductive cycle in four ascidians. J Mar Biol Assoc UK. 1952;31:41–61. [Google Scholar]
- Millar RH. Ciona. In: Colman JS, editor. LMBC Memoirs on Typical British Marine Plants and Animals. Liverpool University; Liverpool: 1953. pp. 1–123. [Google Scholar]
- Mingazzini P. Sulla rigenerazione nei Tunicati. Boll Dell Soc Natur Napoli. 1891;5:76–79. [Google Scholar]
- Morgan TH. Regeneration. The Macmillan Company; New York: 1901. pp. 1–311. [Google Scholar]
- Nishida H. Specification of developmental fates in ascidian embryos: Molecular approach to maternal determinants and signaling molecules. Int Rev Cytol. 2002;217:227–276. doi: 10.1016/s0074-7696(02)17016-1. [DOI] [PubMed] [Google Scholar]
- Nishida H, Sawada K. macho-1 encodes a localized mRNA in ascidian eggs that specifies muscle fate during embryogenesis. Nature. 2001;409:724–728. doi: 10.1038/35055568. [DOI] [PubMed] [Google Scholar]
- Peres JM. Researche sur la genese et la régéneration de la tunique chez Ciona intestinalis L. Bull Institut Oceanograph Monaco. 1948;145:1–12. [Google Scholar]
- Peterson JK, Chou O, Thor P. Growth and energetics in the ascidian Ciona intestinalis. Mar Ecol Prog Ser. 1995;120:175–184. [Google Scholar]
- Rinkevich B, Shlemberg Z, Fishelson L. Whole body protochordate regeneration from totipotent blood cells. Proc Natl Acad Sci USA. 1995;92:7695–7699. doi: 10.1073/pnas.92.17.7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakura Y. Germline transgenesis and insertional mutagenesis in the ascidian Ciona intestinalis. Dev Dyn. 2007;236:1758–1767. doi: 10.1002/dvdy.21111. [DOI] [PubMed] [Google Scholar]
- Satoh N. Developmental Biology of Ascidians. University Press; Cambridge: 1994. pp. 1–234. [Google Scholar]
- Satoh N. Developmental Genomics of Ascidians. Wiley; New York: 2014. pp. 1–201. [Google Scholar]
- Satou Y, Yagi K, Imai KS, Yamada L, Nishida H, Satoh N. macho-1 related genes in Ciona embryos. Dev Genes Evol. 2002;212:87092. doi: 10.1007/s00427-002-0218-3. [DOI] [PubMed] [Google Scholar]
- Saxena S, Dupont S, Meghah V, Meena-Lakshimi MG, Singh SK, Brahmendra Swamy CV, Idris MM. Proteome map of the neural complex of the tunicate Ciona intestinalis, the closet living relative to vertebrates. Proteomics. 2013;13:860–865. doi: 10.1002/pmic.201200148. [DOI] [PubMed] [Google Scholar]
- Schultze LS. Die Regeneration des Ganglions von Ciona intestinalis L und über das Verhaltneiss der Regeneration und Knospung zur Keimenblatterlehre. Jena Z f Naturwiss. 1899;33:263–344. [Google Scholar]
- Selys-Longchamps M. de. Autonomie et régéneration des visceres chex Polycarpa tenera Lacaze et Delage. C R Acad Sci Paris. 1915;160:566–569. [Google Scholar]
- Serafini L, Hann JB, Kutz D, Tomenek L. The proteomic response of sea squirts (genus Ciona) to acute heat stress: A global perspective on the thermostability of proteins. Comp Biochem Physiol D: Genom Proteom. 2005;6(3):322–334. doi: 10.1016/j.cbd.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Sutton MF. The regeneration of the siphons of Ciona intestinalis L. J Mar Biol Assoc UK. 1953;32:249–286. [Google Scholar]
- Takamura K, Fugimura M, Yamaguchi Y. Primordial germ cells originate from the endodermal strand cells the ascidian Ciona intestinalis. Dev Genes Evol. 2002;212:11–18. doi: 10.1007/s00427-001-0204-1. [DOI] [PubMed] [Google Scholar]
- Tiozzo S, Brown FD, De Tomaso AW. Chapter 6. Regeneration and stem cells in ascidians. In: Bosch TCG, editor. Stem Cells: From Hydra to Man. Springer; New York: 2008. pp. 95–112. [Google Scholar]
- Tsutsui H, Yamamoto N, Ito H, Oka Y. GnRH-immunoreactive neuronal system in the presumptive ancestral chordate Ciona intestinalis (Ascidian) Gen Comp Endcrinol. 1998;112:426–432. doi: 10.1006/gcen.1998.7160. [DOI] [PubMed] [Google Scholar]
- Wermel J, Lopaschow GW. Über den Einfluss der Regeneration und Überernährung auf die Siphonenlänge bei Ciona intestinalis L. Ein Beitrag zu Kammerer’s Experientmenten. Arch. Entwicklungsmech. Org. 1930;122:22–47. doi: 10.1007/BF00576964. [DOI] [PubMed] [Google Scholar]
- Whittaker JR. Siphon regeneration in Ciona. Nature. 1975;255:224–225. doi: 10.1038/255224a0. [DOI] [PubMed] [Google Scholar]
- Yamada L, Kobayashi Y, Satou Y, Satoh N. Microarray analysis of localization of maternal transcripts in eggs and early embryos of the ascidian, Ciona intestinalis. Dev Biol. 1995;284:536–550. doi: 10.1016/j.ydbio.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Yokobori S, Kurabayashi A, Neilan BA, Maruyama T, Hirose E. Multiple origins of the ascidian-Prochloron symbiosis: molecular phylogeny of photosymbiotic and non-symbiotic colonial ascidians inferred from 18S rDNA sequences. Mol Phylogenet Evol. 2006;40:8–1. doi: 10.1016/j.ympev.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Zeng L, Jacobs MW, Swalla BJ. Coloniality and sociality has evolved once in stolidobranch ascidians. Integr Comp Biol. 2006;46:255–268. doi: 10.1093/icb/icj035. [DOI] [PubMed] [Google Scholar]