Abstract
Pseudomonas putida strain DOT-T1E is highly tolerant to organic solvents, with a logPow (the logarithm of the partition coefficient of a solvent in a two-phase water-octanol system of ≥2.5. Solvent tolerant microorganisms can be exploited to develop double-phase (organic solvent and water) biotransformation systems in which toxic substrates or products are kept in the organic phase. We tested P. putida DOT-T1E tolerance to different aliphatic alcohols with a logPow value between 2 and 4, such as decanol, nonanol, and octanol, which are potentially useful in biotransformations in double-phase systems in which compounds with a logPow around 1.5 are produced. P. putida DOT-T1E responds to aliphatic alcohols as the second phase through cis-to-trans isomerization of unsaturated cis fatty acids and through efflux of these aliphatic alcohols via a series of pumps that also extrude aromatic hydrocarbons. These defense mechanisms allow P. putida DOT-T1E to survive well in the presence of high concentrations of the aliphatic alcohols, and growth with nonanol or decanol occurred at a high rate, whereas in the presence of an octanol double-phase growth was compromised. Our results support that the logPow of aliphatic alcohols correlates with their toxic effects, as octanol (logPow = 2.9) has more negative effects in P. putida cells than 1-nonanol (logPow = 3.4) or 1-decanol (logPow = 4). A P. putida DOT-T1E derivative bearing plasmid pWW0-xylE::Km transforms m-xylene (logPow = 3.2) into 3-methylcatechol (logPow = 1.8). The amount of 3-methylcatechol produced in an aliphatic alcohol/water bioreactor was 10- to 20-fold higher than in an aqueous medium, demonstrating the usefulness of double-phase systems for this particular biotransformation.
Biocatalysis using whole cells has emerged as an important tool in the industrial synthesis of bulk chemicals, pharmaceuticals, and agrochemical intermediates. However, the number of applications so far is rather restricted because some factors such as strain stability, reduced production rates or yields, and narrow substrate scope limit the number of applications (32). One important limitation of biocatalysis is that many interesting reactions involve organic components (substrates or products) that are poorly water soluble. Furthermore, in some cases the productivity of the biocatalyst was decreased because of end product inhibition (37). The use of a second organic phase in biotransformations has several advantages: if the product is continuously removed by a solvent phase, its toxic effects will decrease and the biocatalyst will remain active; if the concentration of the product increases in the organic phase, product recovery will be easier and less costly (6, 20).
The choice of organic solvents for a bioreactor depends on the purpose of the process. In an extractive biocatalysis process or in the production of a toxic compound, for instance, the product should dissolve preferentially in the organic solvent. The solvent must perform optimally both for the biocatalyst microorganism to be used in the process and for product recovery. The requirements of a “perfect” solvent include a favorable distribution coefficient for the substrate or product, high selectivity, low emulsion-forming tendency, low aqueous solubility, chemical and thermal stability, favorable properties for product recovery, nonbiodegradability, nonhazardous, inexpensiveness, and availability in bulk quantities (9, 18).
In addition, nontoxicity of the solvent for the biocatalyst is an absolute condition. Theoretical and computer-assisted solvent screening strategies to identify suitable solvents have been described elsewhere (5, 17), and these calculations can help to identify potential solvents. However, each biotransformation process requires a series of experiments to test the behavior of the biocatalyst in the system, which must be analyzed experimentally in vivo.
Organic solvents with a logPow (the logarithm of the partition coefficient of a solvent in a two-phase water-octanol system) between 1.5 and 4 are extremely toxic for most microorganisms (14, 29, 35). The isolation and identification of solvent-tolerant bacteria able to grow in the presence of organic solvents with a logPow of ≥2.5 (8, 13, 16, 25, 38) was one of the scientific breakthroughs that may help to overcome some limitations in industrial biotransformations and to expand the applications of biocatalysts. The use of solvent-tolerant bacteria as whole-cell biocatalysts in double-phase systems is an interesting new option for the production of toxic compounds with a low logPow value because these chemicals can be removed from the aqueous phase with the appropriate solvents.
Pseudomonas putida strain DOT-T1E is highly tolerant to organic solvents with a logPow of ≥2.5. In the case of aromatic hydrocarbons such as toluene, xylenes, ethylbenzene, and others, this tolerance is due to the extrusion of these compounds by constitutive and inducible efflux pumps and rigidification of the cell membranes via phospholipid alterations. The molecular mechanisms of toluene tolerance in this strain have been studied extensively (reviewed in references 24 and 34). Among these mechanisms, three efflux pumps have been proved to be necessary for maximal tolerance (21, 23, 30). Studies in our laboratory showed that sublethal concentrations of toluene increased the expression of the ttgDEF and ttgGHI efflux pumps (21, 30) whereas the constitutive level of expression of ttgABC was not altered in the presence of the aromatic hydrocarbon (10).
The aim of this study was to investigate the tolerance and growth of P. putida DOT-T1E in a two-phase system. Aliphatic alcohols such as 1-octanol (logPow 2.9), 1-nonanol (logPow 3.4), or 1-decanol (logPow 4) are suitable as a two-phase system for the extraction of compounds such as substituted catechols, with logPow around 1.5. In fact, at a 1 mM concentration of 3-methylcatechol, around 90, 94, and 98% of the substituted catechol partitions in a 1:1 (vol/vol) mixture of decanol-water, nonanol-water, and octanol-water (6, 12). We have subsequently studied the adaptation of P. putida DOT-T1E and its derivatives to medium-size chain aliphatic alcohols as the second phase and have constructed a P. putida DOT-T1E derivative strain able to produce alkylcatechols from aromatic hydrocarbons. This process is an example of the use of desirable agents for the bioproduction of fine chemicals in a two-phase bioreactor.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The strains used in this study were P. putida DOT-T1E (25) and its isogenic derivative P. putida DOT-T1EΔtodC1C2::kilAB (28). Strains were grown under routine conditions in Luria-Bertani (LB) liquid or solid medium or in a modified M9 minimal medium (1) supplemented with a C source as indicated in the text. The following antibiotics were added to the culture medium to the indicated final concentrations: rifampin (15 μg/ml), tetracycline (10 μg/ml), and kanamycin (25 μg/ml). Liquid cultures were incubated on orbital shakers operated at 200 strokes per min at 30°C.
To study the survival rate of P. putida DOT-T1E and P. putida DOT-T1EΔtodC1C2::kilAB against sudden shocks of 1-octanol, 1-nonanol, and 1-decanol, cells were grown on LB medium and the assays were performed as described before (23). In both media the cultures were preinduced with toluene (supplied through the vapor phase) or with octanol (1 mM) added to the culture medium.
Construction of a strain for the bioproduction of catechols.
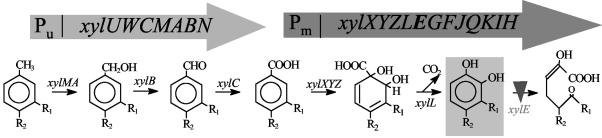
The pWW0 plasmid of P. putida encodes the catabolic pathways for the metabolism of toluene and related compounds (reviewed in references 11, 26, and 40) (Fig. 1). To produce catechols it is necessary to inactivate catechol-2,3-dioxygenase (the product of the xylE gene), the enzyme responsible for the conversion of catechol (or its derivatives) to the corresponding 2-hydroxymuconic acid semialdehyde (or its corresponding derivatives). Conjugation experiments were done by triparental mating, using P. putida KT2440(pWW0), Escherichia coli CC118λpir(pUT-Km), and E. coli HB101(pRK600), which was used as a helper because it provides the tra functions for mobilization of the pUT plasmid. Transconjugants were selected by their ability to grow on M9 minimal medium supplemented with citrate plus kanamycin and by their capacity to produce a brown color when grown on M9 minimal medium citrate plus 3-methylbenzoate. The appearance of the brown color identifies mutants that accumulate 3-methylbenzoate. One such mutant was found, and insertion of the mini-Tn5 into xylE was confirmed by Southern blot and PCR with appropriate primers (not shown).
FIG. 1.
Degradation of toluene and related hydrocarbons as specified by the TOL pWW0 plasmid and schematic gene organization. Toluene or xylenes are oxidized to benzoate or toluates through the upper pathway. The latter compounds are subsequently oxidized and decarboxylated to yield catechols, which upon ring cleavage are channeled to Krebs cycle intermediates by the enzymes encoded in the meta pathway. R1 = R2 = H; R1 = CH3, R2 = H; R1 = H, R2 = CH3; R1 = CH2-CH3, R2 = H. The inverted triangle indicates the inactivated step in pWW0:xylE::Km, which allows the transformation of m-xylene into 3-methylcatechol.
Plasmid pWW0::xylE::Km was then transferred to the P. putida DOT-T1E derivative strain in which todC1C2 (genes that code for the α and β subunits of the multicomponent toluene dioxygenase) had been knocked out by insertion of a tellurite-resistant cassette (28). The resultant strain is unable to grow on toluene as the only carbon source, is kanamycin resistant, and accumulates catechols when the corresponding precursors are added to the medium (see below).
Transformation of m-xylene in the aqueous phase.
P. putida DOT-T1EΔtodC1C2::kilAB bearing plasmid pWW0::xylE::Km was grown overnight (100 ml) in minimal medium M9 (0.5% [wt/vol] glucose) with kanamycin (25 μg/ml) and used as the inoculum for a Biostat B bioreactor (Braun Biotech International, Melsungen, Germany) containing 900 ml of M9 minimal medium with glucose as the carbon source. Temperature was controlled and set to 30°C (measured using a Pt −100 sensor); agitation was 600 rpm; pH was set to 7.1 and controlled by 40% H3PO4 and 32% NH4OH solutions (measured with a Crison pH electrode); aeration was set to 1 liter/min (dissolved pO2 was measured on line with an O2 sensor 12/220 from Ingold). Foam formation was controlled by adding polypropylene glycol P'2000 (Fluka, Madrid, Spain). After 1 h of growth, m-xylene was introduced into the fermentor via the air supply. Three-milliliter samples were taken at different times to measure turbidity and count viable cells; samples of supernatant were stored for further assays.
Transformation of m-xylene with an aliphatic alcohol as the second phase.
The fermentor conditions were as described above except that the 100-ml inoculum was grown in M9 minimal medium with glucose as a carbon source with 1 mM octanol to preadapt the cells when octanol was used as the second phase. We allowed cells to grow for 1 h, and then we added the second phase with the substrate: 0.5 liter of the aliphatic alcohol containing 120 mmol of m-xylene or the aromatic hydrocarbons to be biotransformed. Aeration was increased to 2 liters/min and agitation was set at 1,200 rpm. We controlled in the reactor the same parameters as in single-phase cultures. Aqueous and organic phases were separated by centrifugation, and the organic phase was kept for further analysis. An aliquot of the aqueous phase was withdrawn with a syringe to avoid contamination by the aliphatic alcohol and was also kept for analysis. Cell viability was monitored as CFU per milliliter.
Analytical methods.
Glucose concentration in the supernatant was measured with an Accutrend Sensor (Roche Laboratories, Madrid, Spain). The concentration of 3-methylcatechol in the aqueous phase was measured by the Arnow assay (2) or by high-performance liquid chromatography with a C18 reverse column (159 by 3.9 mm; 60 Å, 4 μm; Nova-Pack, Waters) according to the method of Bühler et al. (7). 3-Methylcatechol was detected spectrophotometrically at 210 nm. Product concentration in the octanol phase was measured by high-performance liquid chromatography with normal-phase chromatography using a Nucleosil 100CN (5-μm) column (250 by 3 mm; Scharlab, Barcelona, Spain). The mobile phase was hexane-isopropanol (95:5), and the flow rate was 1.5 ml/min. The effect of aliphatic alcohols on the profile of fatty acids was assayed in P. putida DOT-T1E cultures growing in M9 minimal medium with glucose (0.5% [wt/vol]) at an optical density at 660 nm of 0.8 after 30 min of incubation with 1% (vol/vol) 1-octanol, 1-nonanol, and 1-decanol. Phospholipids were extracted as described by Bligh and Dyer (4). To quantify fatty acids, phospholipids were saponified as described by Bannon and coworkers (3). Methyl esters were analyzed by gas chromatography-mass spectrometry (model HP 6890/6890, with a capillary column HP5 [30 m by 250 μm; 0.25 μm]).
RESULTS
P. putida DOT-T1E is highly tolerant towards several medium-size chain aliphatic alcohols.
P. putida DOT-T1E has been identified as a highly solvent-tolerant strain because of its capacity to thrive in the presence of different aromatic hydrocarbons such as toluene, xylenes, ethylbenzene, propylbenzene, and others (30). However, its tolerance toward aliphatic alcohols has not been tested. We studied the survival rates of P. putida DOT-T1E cultures exposed to sudden shocks of each of the three aliphatic alcohols at a concentration of 0.3% (vol/vol). As shown in Table 1, 100% of the P. putida DOT-T1E cells survived the sudden addition of 0.3% (vol/vol) 1-nonanol or 1-decanol, whereas only about 10% of the cells tolerated the same shock with 1-octanol.
TABLE 1.
Survival of P. putida DOT-T1E cells after organic-solvent shocka
| Solvent | Rate of survival
|
|
|---|---|---|
| Noninduced | Induced | |
| None | 1 | 1 |
| Toluene (logPow = 2.4) | 10−4 | 0.8 |
| Octanol (logPow = 2.9) | 10−1 | 1 |
| 1-Nonanol (logPow = 3.4) | 1 | 1 |
| 1-Decanol (logPow = 4) | 1 | 1 |
P. putida DOT-T1E cells were grown on LB medium in the absence or in the presence of toluene supplied via the gas phase as described previously (23). When cells reached the mid-log growth phase (optical density at 660 nm = 0.8-1), cultures were split into aliquots and the indicated solvent was added to each of them to a final concentration of 0.3% (vol/vol). The values given are the survival ratios with respect to the control, to which no solvent was added. Values are the average rates of survival in six independent killing assays.
It was previously shown that toluene tolerance in P. putida DOT-T1E is an inducible process; only about 1 out of 104 cells survived sudden toluene shocks of 0.3% (vol/vol), although when cells had been preexposed to sublethal concentrations of toluene, between 80 and 100% of the cells tolerated a 0.3% (vol/vol) toluene shock (23; see also Table 1). The survival rate of the cultures shocked with 0.3% (vol/vol) 1-octanol preinduced with toluene increased so that almost 100% of the cells survived the octanol shock (Table 1). As expected, 100% of the induced cells survived a sudden shock of 1-nonanol or 1-decanol (Table 1).
We also tested whether sublethal concentrations of octanol itself could induce tolerance to high concentrations of this aliphatic alcohol. To this end P. putida DOT-T1E cells were cultured on LB medium with 1 mM 1-octanol, and when cells reached a turbidity of about 0.8 at 660 nm, the culture was split in two aliquots and octanol was added to one of them to 0.3% (vol/vol). It was found that almost 100% of the cells survived this sudden shock. We also tested whether octanol-induced cells were tolerant to a sudden 0.3% (vol/vol) toluene shock. We found that almost 100% of the cells survived this shock. Hence, P. putida DOT-T1E cells seemed to share the same defense mechanisms against toluene and octanol.
Together these results indicated that P. putida DOT-T1E was highly tolerant to all three aliphatic alcohols tested, and that in biotransformation processes mediated by P. putida DOT-T1E in a two-phase bioreactor these organic solvents could be used as a second phase. However, when octanol is used preinduction of the cells would be a prerequisite to avoid cell killing.
Defense mechanisms involved in tolerance towards aliphatic alcohols.
Given the cross-tolerance of octanol- and toluene-induced cells, we decided to examine the implication of strain P. putida DOT-T1E well-established defense mechanisms against aromatic hydrocarbons in tolerance to aliphatic alcohols. Efflux pumps of the resistance-nodulation-cell division family exhibit a wide substrate specificity and are able to remove many different chemicals. The TtgABC and TtgGHI efflux pumps are able to remove a number of antibiotic compounds with different structures in addition to aromatic hydrocarbons, i.e., ampicillin, tetracycline, chloramphenicol, and others (10, 30, 36). We decided to test whether the Ttg efflux pumps could remove aliphatic alcohols. To test this hypothesis we used a series of knockout mutants in the Ttg efflux pumps (30): the three single mutants in each pump, three mutants lacking two pumps and a triple mutant lacking all three efflux pumps. The assays were first performed with noninduced cells, i.e., cells growing exponentially in LB culture medium. As shown in Table 2, 100% of the wild-type and single mutant cells survived a sudden shock of nonanol and decanol. Single mutants lacking the TtgABC, TtgDEF, or TtgGHI efflux pump were as tolerant as the wild-type strain to the sudden addition of 0.3% (vol/vol) octanol. Although almost 100% of the cells in the double-mutant P. putida DOT-T1E-82 (with ttgABC and ttgDEF knockouts) and in P. putida DOT-T1E-PS30 (lacking TtgDEF and TtgGHI) tolerated a shock of nonanol or decanol, only around 1 out of 10 to 102 cells survived the shock with 0.3% (vol/vol) 1-octanol. In the PS32 mutant increased susceptibility was found in response to 1-nonanol and 1-decanol (survival rate in the range of 1 out of 102 to 103), and in particular to 1-octanol, survival was only around 1 out of 104 to 105 cells. In the triple mutant in which none of the three efflux pumps were functional, the decrease in survival rate was exacerbated; i.e., only 1 out of 106 to 107 cells survived the octanol shock, and a significant decrease in survival rate was observed when 1-nonanol or 1-decanol was added.
TABLE 2.
Survival of P. putida DOT-T1E of the sudden addition of organic solventsa
| P. putida strain | Relevant characteristic | Rate of survival
|
|||||
|---|---|---|---|---|---|---|---|
| Noninduced
|
Induced
|
||||||
| 1-Octanol | 1-Nonanol | 1-Decanol | 1-Octanol | 1-Nonanol | 1-Decanol | ||
| DOT-T1E | Wild type | 10−1 | 1 | 1 | 1 | 1 | 1 |
| DOT-T1E-18 | ttgB::phoA-Km | 10−1 | 1 | 1 | 1 | 1 | 1 |
| DOT-T1E-1 | ttgD::kilAB | 10−1 | 1 | 1 | 1 | 1 | 1 |
| DOT-T1E-28 | ttgH::ΩSm | 10−1 | 1 | 1 | 10−1 | 1 | 1 |
| DOT-T1E-82 | ttgB::phoA-Km ttgD::kilAB | 10−1 | 1 | 1 | 1 | 1 | 1 |
| DOT-T1E-PS30 | ttgD::kilAB ttgH::ΩSm | 10−2 | 1 | 1 | 10−1 | 1 | 1 |
| DOT-T1E-PS32 | ttgB::′phoA-Km ttgH::ΩSm | 10−4-10−5 | 10−2-10−3 | 10−2 | 10−3 | 10−2 | 10−1 |
| DOT-T1E-PS34 | ttgB::′phoA-Km ttgD::kilAB ttgH::ΩSm | 10−6-10−7 | 10−4 | 10−2-10−3 | NDb | ND | ND |
1-Octanol, 1-nonanol, and 1-decanol were added at concentrations of 0.3% (vol/vol). Conditions were the same as those described in the footnote for Table 1, except that the strains used and their relevant characteristics are as indicated and that induced cells were grown on LB with toluene supplied in the gas phase.
ND, not determined.
This series of assays was also performed with cells cultured in the presence of toluene supplied through the gas phase prior to the solvent shock with octanol and decanol. As expected, survival of the wild type, all single mutants, and all double mutants, but not that of the ttgH mutant, increased by at least 1 order of magnitude. The triple mutant, however, failed to grow in the presence of toluene in the gas phase, and it was therefore not possible to test the survival of induced cells in response to these solvents. The same results were obtained when the cultures were preinduced with 1 mM octanol in the medium. The results suggest that all three efflux pumps are involved in the extrusion of aliphatic alcohols.
It should be noted that in the double mutant in which the TtgDEF and the TtgABC efflux pumps were not functional (P. putida DOT-T1E-PS82), the survival rate in response to the addition of octanol was similar to that of the wild type, suggesting that from a quantitative point of view the TtgGHI efflux pump may play the most important role in tolerance to aliphatic alcohols. However, the fact that double mutants in TtgGHI and one of the other pumps are more sensitive to these aliphatic alcohols than the single TtgGHI mutant, and the fact that the triple mutant is even more sensitive than any of the double mutants in TtgGHI, suggest that all three efflux pumps work additively to achieve maximal tolerance to aliphatic alcohols. This finding is in agreement with previous observations published by Lee et al. (19), who suggested that efflux pumps of the same family and with overlapping substrate specificity work additively.
The fact that survival of a 0.3% (vol/vol) octanol shock by the double mutants was much higher in induced that in noninduced cultures led us to test whether the efflux pumps were induced in response to aliphatic alcohols. We used the ttgABC, ttgDEF, and ttgGHI promoters fused to a promoterless β-galactosidase gene in the low-copy-number promoter probe plasmid pMP220 (10, 31, 36). β-Galactosidase assays showed that expression from all three efflux pump promoters was two- to threefold higher in the presence of 1 mM 1-octanol, 1-nonanol, or 1-decanol than in the control without the addition of these alcohols (Table 3). These induction levels were similar to those observed for the ttgDEF and ttgGHI operon in the presence of toluene (10, 21, 30). Duque et al. showed that the ttgABC operon was not induced by toluene (10). We confirmed this observation but found that this efflux pump can be induced by all three aliphatic alcohols tested. These compounds should then be added to tetracycline and chloramphenicol, antibiotics also expelled by the TtgABC pump, as effectors for the expression of the ttgABC operon (36). The results reinforce our earlier findings for the contribution of the TtgABC efflux pump to survival when cells are exposed to octanol.
TABLE 3.
Expression of efflux pumps in response to different aliphatic alcoholslegend
| Efflux pump | Activity (mean ± SD) after growth on:
|
|||
|---|---|---|---|---|
| Control | Octanol | Nonanol | Decanol | |
| PttgA::lacZ | 51 ± 14 | 108 ± 13 | 114 ± 35 | 85 ± 16 |
| PttgD::lacZ | 6 ± 2 | 13 ± 6 | 18 ± 7 | 15 ± 7 |
| PttgG::lacZ | 408 ± 89 | 1,474 ± 361 | 1,585 ± 254 | 919 ± 204 |
P. putida DOT-T1E bearing the pED13 (PttgA::lacZ), pMTT (PttgD::lacZ), or pANA96 (PttgG::lacZ) plasmid was grown on LB medium without or with a 1 mM concentration of the indicated aliphatic alcohol. Four hours later β-galactosidase activity (in Miller units) was determined. The results are the averages of five independent assays done in duplicate.
We also tested whether aliphatic alcohols induced changes in the phospholipid composition, as happened with toluene. We found that the cis/trans ratio of unsaturated fatty acids was 8.5 ± 0.5 for noninduced cells versus 1.7 ± 0.3 for cells induced with toluene; 0.92 ± 0.1 when 1% (vol/vol) octanol was added to the medium, 1.86 ± 0.2 with nonanol and 1.13 ± 0.3 with 1% (vol/vol) decanol. Hence, all three aliphatic alcohols increased the packing of fatty acids in the membrane and induced the solvent efflux pumps.
Growth of P. putida DOT-T1E in the presence of octanol, nonanol, and decanol.
The above results suggested that sublethal concentrations of aliphatic alcohols would enhance the survival of P. putida DOT-T1E in response to the addition of large amounts of these compounds. To test this hypothesis P. putida DOT-T1E cells were grown overnight in minimal medium M9 with glucose and with 1 mM octanol. The next day the culture was diluted 1/100 in the same medium but with 0, 0.1, 1, 10, and 100% (vol/vol) 1-octanol, 1-nonanol, or 1-decanol. In these series of assays growth was determined by counting viable cells on LB plates. With 1-nonanol or 1-decanol no inhibition of growth was found. The growth rate on minimal medium with glucose as the carbon source in the presence of these alcohols was similar to that seen in the absence of the aliphatic compounds (doubling time = 65 ± 2 min). The addition of octanol at a concentration of ≥1% inhibited cell growth although the number of viable cells in the culture remained constant during the first 24 h of incubation. Thereafter, we observed a continuous decrease in cell viability in the culture with the highest concentration of 1-octanol (100% [vol/vol]), so that after 42 h the number of viable cells was around 102 CFU/ml.
Our results therefore support the argument that 1-nonanol and 1-decanol are not toxic for P. putida DOT-T1E and can be used as a second phase without previous preinduction of the cells. The findings also indicate that although P. putida DOT-T1E cannot grow in the presence of supersaturating concentrations of 1-octanol, cell viability is maintained for long periods if the culture has been preinduced with sublethal amounts of octanol (1 mM).
Production of 3-methylcatechol by strain P. putida DOT-T1EΔtodC1C2::kilAB(pWW0::xylE::Km) in an aqueous system and in double-phase systems with different aliphatic alcohols.
P. putida DOT-T1EΔtodC1C2::kilAB is a tellurite-resistant mutant of P. putida DOT-T1E that cannot metabolize toluene because the toluene dioxygenase pathway is blocked. This strain was generated previously in our laboratory for use in the biotransformation of substituted toluenes (28). In the present study it was chosen as the host for a TOL plasmid with an xylE inactivated by a kanamycin cassette (see Materials and Methods), which would be useful to transform m- and p-xylene and m- and p-chlorotoluene (and other substituted toluenes) into the corresponding catechols. We subsequently tested the survival of this mutant strain in the presence of aliphatic alcohols as described above for the parental strain. The results obtained were very similar to those with the parental strain P. putida DOT-T1E (not shown), and we concluded that neither inactivation of toluene dioxygenase nor the extra genetic load imposed by the TOL plasmid influenced tolerance to aliphatic compounds.
Cultures of strain P. putida DOT-T1EΔtodC1C2::kilAB(pWW0::xylE::Km) were grown in 250-ml conical flasks with 100 ml of M9 minimal medium with 0.5% (wt/vol) glucose, 10 mM citrate, or 10 mM glycerol as the carbon source and with m-xylene supplied in the vapor phase, to determine the capacity of the strain to convert m-xylene into 3-methylcatechol. A maximum concentration of 3 mM of 3-methylcatechol was reached with glucose in the medium after 10 h of incubation; similar concentrations were reached with the other two carbon sources but took about 24 h to reach this concentration. We used glucose as the source of C for growth of the strain in subsequent biotransformation assays.
In the above assay glucose remained available after the maximum amount of 3-methylcatechol was accumulated. To test whether this maximum concentration of 3-methylcatechol reflected the intrinsic toxicity of the product, we added increasing concentrations of 3-methylcatechol to cells growing on M9-minimal medium with glucose and checked the effect on growth. We found that 3 mM 3-methylcatechol fully inhibited growth of P. putida DOT-T1EΔtodC1C2::kilAB(pWW0::xylE::Km). Taken together, the results suggested that the most probable cause of low production was toxicity of 3-methylcatechol for biocatalyst P. putida DOT-T1E.
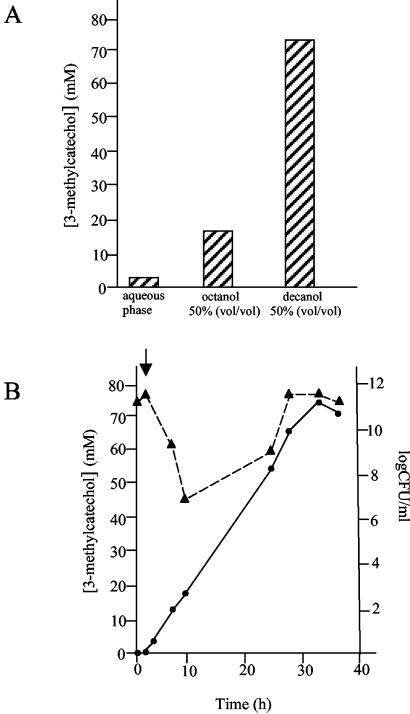
3-Methylcatechol production from m-xylene was then assayed in a bioreactor, as described in Materials and Methods. First, it was done in the aqueous phase where a maximum concentration of 3 mM was reached with m-xylene introduced through the air supply. Thereafter, production was tested with a second phase of 50% (vol/vol) 1-decanol or 1-octanol to remove the catechol. After the addition of 50% of either aliphatic alcohol we observed a decrease in cell viability, which was five orders of magnitude higher with octanol than with decanol as the second phase. Concentrations as high as 17 mM (2.6 g/liter) 3-methylcatechol were found with octanol after 90 h of incubation (Fig. 2A). Production of the alkylcatechol in this octanol double-phase system showed a 30-h lag phase, after which the production rate was linear (0.12 g liter−1 h−1) (not shown). Production, however, reached 70 mM alkylcatechol with decanol as a second phase (Fig. 2A). After a 2-h lag (which correlates with a decrease in cell viability), the production was linear and alkylcatechols accumulated at a rate of (0.60 g 1iter−1 h−1) (Fig. 2B).
FIG. 2.
Time course production of 3-methylcatechol using P. putida ΔtodC1C2::kilAB(pWW0:xylE::Km) as a biocatalyst. (A) Maximal production. The assays were performed in the aqueous phase or in a double-phase fermentor system using octanol or decanol as the second phase. Conditions are given in Materials and Methods. The concentration of catechol was determined in the aqueous and organic phases. Concentrations given are the addition of concentrations in the aqueous phase plus the organic phase. On average, when octanol was the second phase, almost 96% 3-methylcatechol was in the organic phase and the alkylcatechol in the aqueous phase was always at concentrations below 0.7 mM. With decanol almost 90% of 3-methylcatechol was in the organic phase and at the end of the production period 3-methylcatechol in water was close to 3 mM. (B) Time course production of 3-methylcatechol in a fermentor with decanol as the second phase. Conditions are as above except that 3-methylcatechol (circles) was determined at the given times. The number of viable cells was also determined (triangles).
DISCUSSION
P. putida DOT-T1E is known for its high tolerance to different aromatic hydrocarbons and because it can be easily manipulated, constituting a host of interest for biotransformations (22, 27, 28). The solvent tolerance properties of P. putida DOT-T1E make it a suitable candidate for two-phase bioreactor systems. The use of a second phase in which the substrate and product partition preferentially in an organic phase adds value to the process in the sense that it solves the problem of solubility. Moreover, in the case of toxic chemicals such partitioning can improve the performance of the system. However, not all organic solvents are biocompatible with the strain that bears the machinery for the desired reactions, and the choice of the organic phase to be used in a biotransformation process is not trivial. The organic solvent must meet several criteria and in particular it must be biocompatible with the chosen microorganism. Although P. putida strain DOT-T1E was characterized as highly resistant toward different aromatic hydrocarbons, these compounds are in general harmful for the environment and for persons who work with them and are therefore not appropriate for industrial processes. Because of their lipophilic character, nontoxicity, price, and other characteristics, aliphatic alcohols are good candidates for use as a second phase in biotransformation processes of interest (39). Our results showed that P. putida DOT-T1E is also highly tolerant toward these chemicals, although maximal tolerance to octanol requires preexposure to sublethal concentrations of this compound and very high amounts of octanol prevent cell growth. Tolerance of P. putida DOT-T1E to these chemicals involves rigidification of the cell membrane through cis→trans isomerization (15, 38) and a number of efflux pumps that prevent accumulation of medium size-chain aliphatic alcohols in the cells.
Our results support the argument that at least three broad-substrate efflux pumps were induced at a moderate level by 1-octanol, 1-nonanol, and 1-decanol. Furthermore, we have demonstrated the importance of the so-called TtgGHI efflux pump in aliphatic alcohol tolerance: mutants lacking this pump were more sensitive to octanol than those without TtgABC or TtgDEF. This is in agreement with our observation that the TtgGHI efflux pump seemed to be also the most important efflux pump in toluene tolerance (30).
The maximum production of 3-methylcatechol using Pseudomonas sp. strain S12 or DOT-T1E in an aqueous system is around 3 mM. This limitation seems to be due to the intrinsic toxicity of the product, which severely impedes the growth of P. putida DOT-T1E at that concentration. A similar observation had been reported by Wery et al. (39) showing that in rich LB medium a solvent-resistant derivative of P. putida S12 bearing the todC1C2BAD genes of P. putida F1 under a salicylate-inducible promoter grew up to concentrations of about 5 mM in the culture medium. When a second phase of n-octanol was used, more than 96% of the 3-methylcatechol partitioned into the organic phase, which allowed the product to accumulate in the organic phase in larger amounts. In our assays, the addition of a second phase, made up of an aliphatic alcohol, to the bioreactor improved 3-methylcathecol production in quantitative terms by 10- to 20-fold in comparison to production in the aqueous medium. Although all three aliphatic alcohols tested (1-octanol, 1-nonanol, and 1-decanol) were biocompatible with P. putida DOT-T1E cells if preinduced, catechol production was highest with decanol, probably because octanol is more toxic than decanol as shown by growth inhibition by octanol and hence cells need less energy to avoid the intrinsic toxicity of the second phase system. The toxic effect of octanol was also shown in the biotransformation assay, where the decrease in cell viability after the addition of the second phase to the reactor was five orders of magnitude higher than with decanol (not shown). We reached concentrations of 3-methylcatechol in the range of 70 mM with decanol, which is close to the maximum expected concentrations based on the partition coefficient of 3-methylcatechol in the water-decanol system (under these conditions 3 mM 3-methylcatechol was measured in the aqueous phase). The production levels of 3-methylcatechol achieved in this study were obtained in M9 minimal medium, which allowed us to recover a highly pure product. The system described by Husken et al. (12) can achieve similar levels of 3-methylcatechol, although, probably because the strain is not as robust as DOT-T1E (33), these high concentrations of 3-methylcatechol were only achieved in rich LB medium. This can compromise the purity of the product in the recovery process and may require a longer incubation time. It should be mentioned that the production system based on the TOL plasmid allowed the production at similarly high concentrations of catechol and a number of substituted catechols, i.e., 4-methylcatechol and 3- and 4-chlorocatechol when toluene, p-xylene, and m- or p-chlorotoluene were used as substrates. This contrasts with the limited range of catechols that can be produced by the toluene dioxygenase system used by Husken et al. (12) that was limited to the production of 3-methylcatechol from toluene. Substrate specificity of the toluene dioxygenase enzymes are generally narrower than those of TOL enzymes (1, 22).
In short, it is feasible to provide P. putida DOT-T1E with suitable catabolic segments to carry out useful biotransformations. The production yields can be enhanced if nontoxic organic solvents are used as a second phase to remove toxic products.
Acknowledgments
This study was supported by grants from the European Commission (QLK3-CT-2001-00435) and a grant from the Spanish Ministry for Science and Technology (CICYT-2003).
We thank Carmen Lorente and Karen Shashok for checking the English in the manuscript. We thank G. Ionidis and D. Meyer for advice in the fermentation assays and P. Godoy for fatty acid analysis performance.
REFERENCES
- 1.Abril, M. A., C. Michán, K. N. Timmis, and J. L. Ramos. 1989. Regulator and enzyme specificities of the TOL plasmid-encoded upper pathway for degradation of aromatic hydrocarbons and expansion of the substrate range of the pathway. J. Bacteriol. 171:6782-6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnow, L. E. 1937. Colorimetric determination of the components of 3,4-dihydroxhyphenylalanine-tyrosine mixtures. J. Biol. Chem. 118:531-537. [Google Scholar]
- 3.Bannon, C. D., G. J. Breen, J. D. Craske, N. T. Hai, N. L. Harper, and K. L. O'Rourke. 1982. Analysis of fatty acid methyl esters with high accuracy and readability. III. Literature review of an investigation into the development of a rapid procedure for methoxide catalysed methanolysis of fats and oils. J. Chromatogr. 247:71. [Google Scholar]
- 4.Bligh, E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911-917. [DOI] [PubMed] [Google Scholar]
- 5.Brink, L. E. S., and J. Tramper. 1985. Optimization of organic solvent in multiphase biocatalysis. Biotechnol. Bioeng. 27:1258-1269. [DOI] [PubMed] [Google Scholar]
- 6.Bruce, L. J., and A. J. Daugulis. 1991. Solvent selection strategies for extractive biocatalysis. Biotechnol. Prog. 7:116-124. [DOI] [PubMed] [Google Scholar]
- 7.Bühler, B., A Schmid, B. Hauer, and B. Witholt. 2000. Xylene monooxygenase catalyzes the multistep oxygenation of toluene and pseudocumene to corresponding alcohols, aldehydes, and acids in Escherichia coli JM101. J. Biol. Chem. 275:10085-10092. [DOI] [PubMed] [Google Scholar]
- 8.Cruden, D. L., J. H. Worfram, R. D. Rogers, and D. T. Gibson. 1992. Physiological properties of a Pseudomonas strain which grows with p-xylene in a two phase (organic-aqueous) medium. Appl. Environ. Microbiol. 58:2723-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daugulis, A. J. 1988. Integrated reaction and product recovery in bioreactor systems. Biotechnol. Prog. 4:113-122. [Google Scholar]
- 10.Duque, E., A. Segura, G. Mosqueda, and J. L. Ramos. 2001. Global and cognate regulators control the expression of the organic solvent efflux pumps TtgABC and TtgDEF of Pseudomonas putida. Mol. Microbiol. 39:1100-1106. [DOI] [PubMed] [Google Scholar]
- 11.Harayama, S., and K. N. Timmis. 1988. Catabolism of aromatic hydrocarbons by Pseudomonas, p. 151-174. In D. A. Hopwood and K. F. Chater (ed.), Genetics of bacterial diversity Academic Press, New York, N.Y.
- 12.Husken, L. E., M. C. F. Dalm, J. Tramper, J. Wery, J. A. M. de Bont, and R. Beeftink. 2001. Integrated bioproduction and extraction of 3-methylcatechol. J. Biotechnol. 88:11-19. [DOI] [PubMed] [Google Scholar]
- 13.Inoue, A., and K. Horikoshi. 1989. A Pseudomonas thrives in high concentrations of toluene. Nature 338:264-265. [Google Scholar]
- 14.Isken, S., and J. A. M. de Bont. 1998. Bacteria tolerant to organic solvents. Extremophiles 2:229-238. [DOI] [PubMed] [Google Scholar]
- 15.Junker, F., and J. L. Ramos. 1999. Involvement of the cis/trans isomerase CtiT1 in solvent resistance in Pseudomonas putida DOT-T1E. J. Bacteriol. 181:5693-5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, K., S. Lee, K. Lee, and D. Lim. 1998. Isolation and characterization of toluene-sensitive mutants from the toluene-resistant bacterium Pseudomonas putida GM73. J. Bacteriol. 180:3692-3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kollerup, F., and A. J. Daugulis. 1985. Screening and identification of extractive fermentation solvents using a database. Can. J. Chem. Eng. 63:919-927. [Google Scholar]
- 18.Laane, C., S. Boeren, K. Vos, and C. Veeger. 1987. Rules for optimization of biocatalysis in organic solvents. Biotechnol. Bioeng. 30:81-87. [DOI] [PubMed] [Google Scholar]
- 19.Lee, A., W. Mao, M. S. Warren, A. Mistry, K. Hoshino, R. Okumura, H. Ishida, and O. Lomovskaya. 2000. Interplay between efflux pumps may provide either additive or multiplicative effect on drug resistance. J. Bacteriol. 182:3142-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leon, R., P. Fernandes, H. M. Pinheiro, and J. M. S Cabral. 1998. Whole-cell biocatalysis in organic media. Enzyme Microb. Technol. 23:483-500. [Google Scholar]
- 21.Mosqueda, G., and J. L. Ramos. 2000. A set of genes encoding a second toluene efflux system in Pseudomonas putida DOT-T1E is linked to the tod genes for toluene metabolism. J. Bacteriol. 182:937-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosqueda, G., M. I. Ramos-González, and J. L. Ramos. 1999. Toluene metabolism by the solvent-tolerant Pseudomonas putida DOT-T1 strain, and its role in solvent impermeabilization. Gene 232:69-76. [DOI] [PubMed] [Google Scholar]
- 23.Ramos, J. L., E. Duque, P. Godoy, and A. Segura. 1998. Efflux pumps involved in toluene tolerance in Pseudomonas putida DOT-T1E. J. Bacteriol. 180:3323-3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramos, J. L., E. Duque, M. T. Gallegos, P. Godoy, M. I. Ramos-González, A. Rojas, W. Terán, and A. Segura. 2002. Mechanisms of solvent tolerance in gram-negative bacteria. Annu. Rev. Microbiol. 56:743-768. [DOI] [PubMed] [Google Scholar]
- 25.Ramos, J. L., E. Duque, M. J. Huertas, and A. Haïdour. 1995. Isolation and expansion of the catabolic potential of a Pseudomonas putida strain able to grow in the presence of high concentrations of aromatic hydrocarbons. J. Bacteriol. 177:3911-3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramos, J. L., S. Marqués, and K. N. Timmis. 1997. Transcriptional control of the Pseudomonas TOL plasmid catabolic operons is achieved through an interplay of host factors and plasmid encoded regulators. Annu. Rev. Microbiol. 51:341-373. [DOI] [PubMed] [Google Scholar]
- 27.Ramos-González, M. I., P. Godoy, M. Alaminos, A. Ben-Bassat, and J. L. Ramos. 2001. Physiological characterization of Pseudomonas putida DOT-T1E tolerance to p-hydroxybenzoate. Appl. Environ. Microbiol. 67:4338-4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramos-González, M. I., A. Ben-Bassat, M. J. Campos, and J. L. Ramos. 2003. Genetic engineering of a highly solvent-tolerant Pseudomonas putida strain for biotransformation of toluene to p-hydroxybenzoate. Appl. Environ. Microbiol. 69:5120-5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rekkert, R. F., and H. M. de Kort. 1979. The hydrophobic fragmental constant and extension to a 1,000 data point set. Eur. J. Med. Chem. 14:479-488. [Google Scholar]
- 30.Rojas, A., E. Duque, G. Mosqueda, G. Golden, A. Hurtado, J. L. Ramos, and A. Segura. 2001. Three efflux pumps are required to provide efficient tolerance to toluene in Pseudomonas putida DOT-T1E. J. Bacteriol. 183:3967-3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rojas, A., A. Segura, M. E. Guazzaroni, W. Terán, A. Hurtado, M. T. Gallegos, and J. L. Ramos. 2003. In vitro and in vivo evidence that TtgV is the local specific regulator of the TtgGHI multidrug and solvent efflux pump of Pseudomonas putida. J. Bacteriol. 185:4755-4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schoemaker, H. E., D. Mink, and M. G. Wubbolts. 2003. Dispelling the myths: biocatalysis in industrial synthesis. Science 299:1694-1697. [DOI] [PubMed] [Google Scholar]
- 33.Segura, A., A. Rojas, A. Hurtado, M. J. Huertas, and J. L. Ramos. 2003. Comparative genomic analysis of solvent extrusion pumps in Pseudomonas strains exhibiting different degrees of solvent tolerance. Extremophiles 7:371-376. [DOI] [PubMed] [Google Scholar]
- 34.Segura, A., H. Heipieper, W. Terán, M. E. Guazzaroni, A. Rojas, E. Duque, M. T. Gallegos, and J. L. Ramos. 2003. Enzymatic activation of the cis-trans isomerase and transcriptional regulation of efflux pumps in solvent tolerance in Pseudomonas putida, p. 479-508. In J. L. Ramos (ed.), Pseudomonas, vol. II, in press. Kluwer Academic/Plenum Publishers, London, United Kingdom.
- 35.Sikkema, J., J. A. de Bont, and B. Poolman. 1995. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 59:201-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Terán, W., A. Felipe, A. Segura, A. Rojas, J. L. Ramos, and M. T. Gallegos. 2003. The expression of the TtgABC efflux pump of Pseudomonas putida DOT-T1E is induced by chloramphenicol. Antimicrob. Agents Chemother. 47:3067-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Sonsbeek, H. M., H. H. Beeftink, and J. Tramper. 1993. Two-liquid-phase bioreactors. Enzyme Microb. Technol. 15:722-729. [DOI] [PubMed] [Google Scholar]
- 38.Weber, F. J., S. Isken, and J. A. M. de Bont. 1994. Cis/trans isomerization of fatty acids as a defence mechanism of Pseudomonas putida strains to toxic concentrations of toluene. Microbiology 140:2013-2017 [DOI] [PubMed] [Google Scholar]
- 39.Wery, J., D. I. Mendes da Silva, and J. A. M. de Bont. 2000. A genetically modified solvent-tolerant bacterium for optimized production of a toxic fine chemical. Appl. Microbiol. Biotechnol. 54:180-185. [DOI] [PubMed] [Google Scholar]
- 40.Williams, P. A., R. M. Jones, and G. Zylstra. 2004. Genomics of catabolic plasmids, p. 165-195. In J. L. Ramos (ed.), Pseudomonas: genomics, life style and molecular architecture, vol. I. Kluwer Academic/Plenum Publishers, London, United Kingdom.