Abstract
AIM: to investigate the effect of Bifidobacterium infantis (B. infantis) on the T cell subsets and in attenuating the severity of experimental colitis in mice.
METHODS: Normal BALB/c mice were fed different doses of B. infantis for 3 wk, and T cell subsets and related cytokine profiles in mesenteric lymph nodes (MLNs) were detected by flow cytometry and real-time RT-PCR. Colitis was induced by administration of trinitrobenzene sulfonic acid (TNBS) in mice. Before colitis induction, mice were fed high dose B. infantis for 3 wk. Cytokine profiles in MLNs and histological changes of colonic tissue were examined 6 d after colitis induction.
RESULTS: No significant change in cytokine profiles was observed in normal mice fed low dose B. infantis. However, Th1-related cytokines (IL-2, IFN-γ, IL-12p40), Th17-related transcription factor and cytokines (RORγt, IL-21, IL-23), regulatory T cell (Treg)-related transcription factor and cytokines (Foxp3, IL-10) were increased in normal mice fed high dose B. infantis. Furthermore, flow cytometry assay showed B. infantis increased the numbers of CD4+Foxp3+ Tregs and Th17 cells in MLNs. Colitis was successfully induced by TNBS in mice, characterized by colonic inflammation and aberrant Th1 and Th17 responses. Feeding high dose B. infantis for 3 wk before colitis induction decreased the inflammatory cell infiltration and goblet cell depletion and restored the intestinal epithelium. In addition, B. infantis feeding reduced Th1-related cytokines (T-bet, IL-2 and IFN-γ) and Th17-related cytokines (IL-12p40, RORγt, IL-17A, IL-21 and IL-23), and increased Treg-related molecules (Foxp3, IL-10 and TGF-β) in colitis mice.
CONCLUSION: B. infantis effectively attenuates TNBS-induced colitis by decreasing Th1 and Th17 responses and increasing Foxp3+ Treg response in the colonic mucosa.
Keywords: Bifidobacterium, Colitis, Cytokines, Th17, Regulatory T cells
Core tip: Inflammatory bowel disease is a common autoimmune disorder characterized by chronic inflammation of the gastrointestinal tract. Abnormal immune cell responses contribute to the pathogenesis of the colitis. Probiotics are found to regulate the intestinal immune system and play a beneficial role in treating colitis. In our study, we showed that Bifidobacterium infantis (B. infantis) reduced the intestinal inflammation in TNBS-induced colitis mice though decreasing the Th1 and Th17 responses and promoting the Foxp3+ Treg response in mesenteric lymph nodes. This mechanism underlying the regulatory effect of B. infantis on the immune system may have significant clinical implications in treating inflammatory bowel disease and preventing colorectal cancer.
INTRODUCTION
Inflammatory bowel disease (IBD) is an autoimmune disorder that is characterized by chronic inflammation of the gastrointestinal tract. IBD comprises mainly Crohn’s disease (CD) and ulcerative colitis. The pathogenesis of IBD involves genetic susceptibility, gut microbiota and host immune system[1]. It is widely accepted that the abnormal gut microbiota plays a key role in the pathogenesis of IBD. Several studies showed a striking reduction of commensal microbiota including Firmicutes and Bacteroides and an overgrowth of Proteobacteria in the intestinal tissue from IBD patients[2]. Therefore, extensive research is focused on modifying the intestinal flora using probiotic bacteria.
Probiotics are live microorganisms that benefit health when supplied in adequate amounts. Among them, Bifidobacterium infantis (B. infantis) is a commensal microbe isolated from the human gastrointestinal mucosa and has been studied for its ability to treat irritable bowel syndrome[3], and to protect against inflammatory conditions including Helicobacter pylori infection[4] and IBD[5-7]. The mechanisms behind the amelioration of IBD by B. infantis are still largely unknown. IBD results from an inadequate Foxp3+ regulatory T cell (Treg) response in the face of overly exuberant Th1 and Th17 responses in CD and an abnormal Th2 response in ulcerative colitis[8]. B. infantis feeding reduced the numbers of Th1 and Th17 cells and increased the proportion of Foxp3+ Tregs within the lamina propria (LP) in DSS-induced colitis which mimics CD[5]. Moreover, B. infantis had some influence on the expression of some cytokines. For instance, B. infantis inhibited the expression of Th17 related IL-17A and induced the expression of IL-10 in DSS-induced colitis mice[7], and decreased Th1 proinflammatory cytokines (TNF-α, IL-12 and IFN-γ) and maintained the level of TGF-β in IL-10 knockout (KO) mice that are a spontaneous genetic model of CD[6]. However, the effects of single B. infantis feeding on trinitrobenzene sulfonic acid (TNBS)-induced colitis, which is an animal model of CD, and the underlying immune mechanism were still poorly understood.
We hypothesized that B. infantis may ameliorate the intestinal inflammation in TNBS-induced colitis by affecting the differentiation of CD4+ T subsets and cytokine expression.
MATERIALS AND METHODS
Animals
Female BALB/c mice (6-8-wk old) were purchased from Chongqing Medical University and maintained in standard conditions. All the experimental procedures were performed according to the guidelines of the Institutional Animal Ethics Committee.
Experiment design
In the first experiment, normal mice were randomly divided into 3 groups of five animals each: a control group, a low dose B. infantis (6 × 107 cfu/d) group (Low-B. infantis) and a high dose B. infantis (6 × 108 cfu/d) group (High-B. infantis). B. infantis (6 × 109 cfu/g) was provided by Guangzhou Baoxing Biotechnology Company (Guangzhou, China). Mice received oral administration of low or high dose B. infantis once daily for 3 wk. The control group received PBS.
For induction of colitis, 50 μL TNBS [50 mg/kg body weight (BW)] dissolved in 0.9% (w/v) NaCl/ethanol (50:50 v/v) was intrarectally administered to anesthetized mice 4 cm proximal to the anal verge through a 3.5F catheter as described previously[9]. TNBS was purchased from Sigma (St. Louis, MO, United States). In the TNBS colitis model, three groups of five animals each were utilized. Group 1 was a negative control group, which was administered with PBS, instead of TNBS. Group 2 was designated as the TBNB-3 group as these mice were sacrificed by cervical dislocation 3 d after administration of TNBS. Group 3 was designated as the TNBS-6 group in which mice were sacrificed 6 d after administration of TNBS.
In the B. infantis treated model, two groups of five animals each were used. Group 1 received daily oral administration of high dose B. infantis (6 × 108 cfu/d) for 3 wk before colitis induction (B. infantis-TNBS group). The colitis control animals were administered with PBS using the same technique before colitis induction (PBS-TNBS group). Animals were sacrificed by cervical dislocation 6 d after TNBS induction, and MLNs were selected carefully from the inflamed area of the bowel in all animals.
RNA extraction and real-time PCR
Total RNA was extracted from MLNs using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. Total RNA (2 μg) was transcribed to cDNA using a reverse-transcription kit (TaKaRa). Primer sequences were as follows: T-bet (forward) 5′-CAACAA CCCCTTTGCCAAAG-3′ and (reverse) 5′-TCCCCCAAGCAGTTGACAGT-3′, IL-2 (forward) 5′-CCTGAGCAGGATGGAGAATTACA-3′ and (reverse) 5′-TCCAGA ACATGCCGCAGAG-3′, IFN-γ (forward) 5′-TCAAGTGGCATAGATGTGGAA, GAA-3′ and (reverse) 5′-TGGCTTGCAGGATTTTCATG-3′, IL-12p4 (forward) 5′-GGAAGCACGGCAGCAGAATA-3′ and (reverse) 5′-AACTTGAGGGAGAAG TAGGAATGG-3′, GATA3 (forward) 5′-CCTACCGGGTTCGGATGTAA-3′ and (reverse) 5′-CACACACTCCCTGCCTTCTGT-3′, IL-4 (forward) 5′-ACAGGAGAA GGGACGCCAT-3′ and (reverse) 5′-GAAGCCCTACAGACGAGCTCA-3′, RORγt (forward) 5′-GCTGTCAAAGTGATCTGGAG-3′ and (reverse) 5′-GGTGGAACT TATGGGAAATC-3′, IL-21 (forward) 5′-GAGGACCCTTGTCTGTCTGG-3′ and (reverse) 5′-TCATCTTTTGAAGGAGCCATTT-3′, IL-23 (forward) 5′-GAGGTG GACTGGACTACCGA-3′ and (reverse) 5′-GGAACTGCTACTGCTCTTGA-3′, Foxp3 (forward) 5′-CCCATCCCCAGGAGTCTTG-3′ and (reverse) 5′-CCATGACTA GGGGCACTGTA-3′, IL-10 (forward) 5′-ATAACTGCACCCACTTCCCA-3′ and (reverse) 5′-TCATTTCCGATAAGGCTTGG-3′, TGF-β (forward) 5′-GAAGGCAGA GTTCAGGGTCTT-3′ and (reverse) 5′-GGTTCCTGTCTTTGTGGTGAA-3′, GAPDH (forward) 5′-CATCACTGCCACCCAGAAGA-3′ and (reverse) 5′-TGAAGT CGCAGGAGACAACC-3′. Real-time reverse transcription polymerase chain reaction (RT-PCR) was performed with the ABI 7300 system. The reaction mixture consisted of 5.0 μL of cDNA, 12.5 μL of SYBR Green qPCR SuperMix-UDG/ROX (Invitrogen) and 6.5 μL of ddH20. All the PCR experiments were performed under the same condition as follows: 95 °C for 10 min, 95 °C for 15 s and 60 °C for 1 min, up to 40 cycles. GAPDH was used as an internal control. Gene expression was calculated relative to GAPDH.
Flow cytometry analysis
Single cell suspensions were prepared from MLNs of each group. In order to identify Tregs in MLNs, cells were first surface labeled with FITC labeled anti-mouse CD4 (eBioscience) and PE labeled anti-mouse CD25 antibodies (eBioscience). After that, cells were fixed, permeated and intracellularly stained with APC labeled anti-Foxp3 antibody (eBioscience). To measure Th17 cells, cells were pre-stimulated for 4 h with PMA (50 ng/mL, Sigma) and ionomycin (500 ng/mL, Sigma) in the presence of Brefeldin A (1 mg/mL, eBioscience) at 37 °C and 5% CO2. Then, cells were washed in PBS and surface labeled with CD3-FITC and CD4-PE-Cy5. For intracellular labeling of IL-17A, these cells were permeabilized with IL-17A fixation/permeabilization buffer (eBioscience) and stained with anti-IL-17A-PE (eBioscience). Cells were incubated with affinity purified anti-mouse CD16/32 to block non-specific staining. IgG isotypes (BD pharmingen) were used as a control in all FACS experiments. Data were acquired on a FACS Calibur flow cytometer with CELLQuest software.
Histological analysis
For histological examination, samples of colonic tissue located precisely 3 cm above the anal canal was obtained from the mice of all groups. The colonic tissues were fixed in 10% neutral buffered formalin and embedded in paraffin for histological analysis. Four-micrometer-sections were deparaffinized with xylene and stained with hematoxylin and eosin using routine techniques.
Statistical analysis
Statistical analyses were carried out using GraphPad Prism for Windows (Version 5.0). Significant differences between different doses of B. infantis groups were assessed by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test. Differences among experimental colitis groups induced by TNBS for 3 d or 6 d were also evaluated using ANOVA followed by Dunnett’s multiple comparison test. The difference between PBS-TNBS group and B. infantis-TNBS group was measured by a paired t test. Data are given as mean ± SE. Differences were considered significant at P < 0.05.
RESULTS
B. infantis augments the expression of Th1-, Th17- and Treg-related cytokines in MLNs in normal mice
To investigate the effect of B. infantis on T cell cytokine expression, we studied the relative expression of different cytokines in MLNs from normal mice by real-time RT-PCR. Low dose B. infantis feeding (6 × 107 cfu/d) caused no significant changes in the expression of all cytokines tested, but high dose B. infantis feeding (6 × 108 cfu/d) augmented the expression of Th1-, Th17- and Treg-related cytokines in MLNs (Figure 1). After high dose B. infantis feeding, the mRNA expression of Th1 cytokines IL-2 and IL-12p40 increased significantly in the MLNs when compared with PBS control (IL-2, P < 0.05; IL-12p40, P < 0.005 ) (Figure 1A and C). The mRNA level of proinflammatory cytokine IFN-γ also seemed to elevate but did not reach the significance in comparison with the control group (Figure 1B). Th17-related cytokine IL-23 and transcription factor RORγ increased more than 2-fold in MLNs after B. infantis feeding compared with the control group (P < 0.005) (Figure 1E and G). IL-21 also tended to rise. Moreover, mice receiving B. infantis had a significant increase in Foxp3 and IL-10 mRNA expression in MLNs in comparison with PBS control (Foxp3, P < 0.005; IL-10, P < 0.05) (Figure 1H and I). However, no difference in the mRNA expression of Th2-related cytokine IL-4 was detected after B. infantis feeding (Figure 1D).
Figure 1.

mRNA expression of T cell-related cytokines from mesenteric lymph nodes of normal BALB/c mice fed different doses of Bifidobacterium infantis. A: IL-2; B: IFN-γ; C: IL-12p40; D: IL-4; E: RORγt; F: IL-21; G: IL-23; H: Foxp3; I: IL-10. Control: Mice from control group; Low: Mice treated with low dose Bifidobacterium infantis (B. infantis); High: Mice treated with high dose B. infantis. Real-time RT-PCR was employed to examine the expression of Th1, Th17 and Foxp3+ Treg related cytokines. Data are from 5 mice per group. Data are expressed as relative expression and the control group was chosen as the calibrator. Data represent mean ± SE. aP < 0.05 vs the control group; bP < 0.01 vs the control group. IL: Interleukin; IFN: Interferon.
B. infantis increases the levels of CD4+T cells, Th17 cells and CD4+Foxp3+ Tregs in the MNLs in normal mice
We measured the numbers of CD4+T cells and Th17 cells in the MLNs in normal mice by flow cytometry to determine if they were altered after B. infantis feeding. In order to identify CD4+T and Th17 cells, we utilized antibodies against CD3, CD4, and IL-17A to detect them. Flow cytometric analyses revealed that high dose B. infantis slightly increased the percentage of CD4+T cells in MLNs compared with PBS control (Figure 2A and C). B. infantis also up-regulated the level of Th17 cells (Figure 2B and C). However, both the increase of CD4+T and Th17 cells did not reach significance (P > 0.05). For deciding the change of the population of Tregs after B. infantis feeding, CD4, CD25 and Foxp3 were measured by flow cytometry. B. infantis feeding slightly increased the percentage of CD4+Foxp3+ Tregs compared with control (9.99% ± 0.26% vs 9.40% ± 0.38%) (Figure 3D). The increased CD4+Foxp3+ Tregs by B. infantis feeding were also mostly derived from CD4+CD25+T cells, not from CD4+CD25-T (Figure 3B and C).
Figure 2.
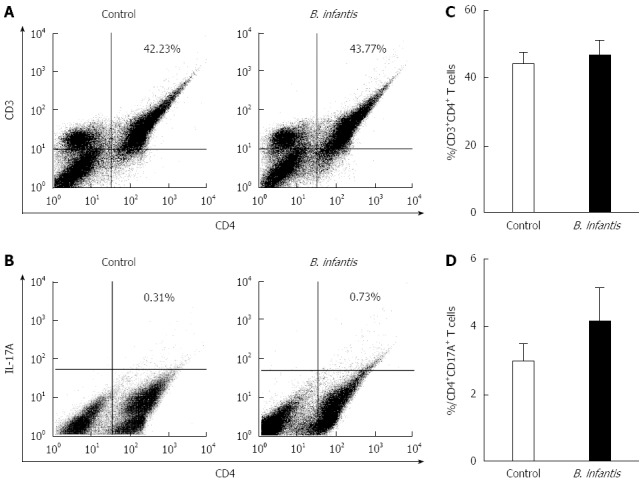
Representative flow plots and levels of CD4+ T and CD4+IL-17A+ T cells in mesenteric lymph nodes from normal mice fed high dose Bifidobacterium infantis. A: Levels of mesenteric lymph node (MLN) CD4+T cells in PBS-control and Bifidobacterium infantis (B. infantis) treated groups; B: Levels of CD4+IL-17A+T cells in PBS-control and B. infantis treated groups; C: The percentage of MLN CD4+T cells; D: The percentage of CD4+IL-17A+T cells. Results are either shown as mean ± SE from three independent experiments, n = 5 (bar graphs) or as a representative flow cytometry graph.
Figure 3.
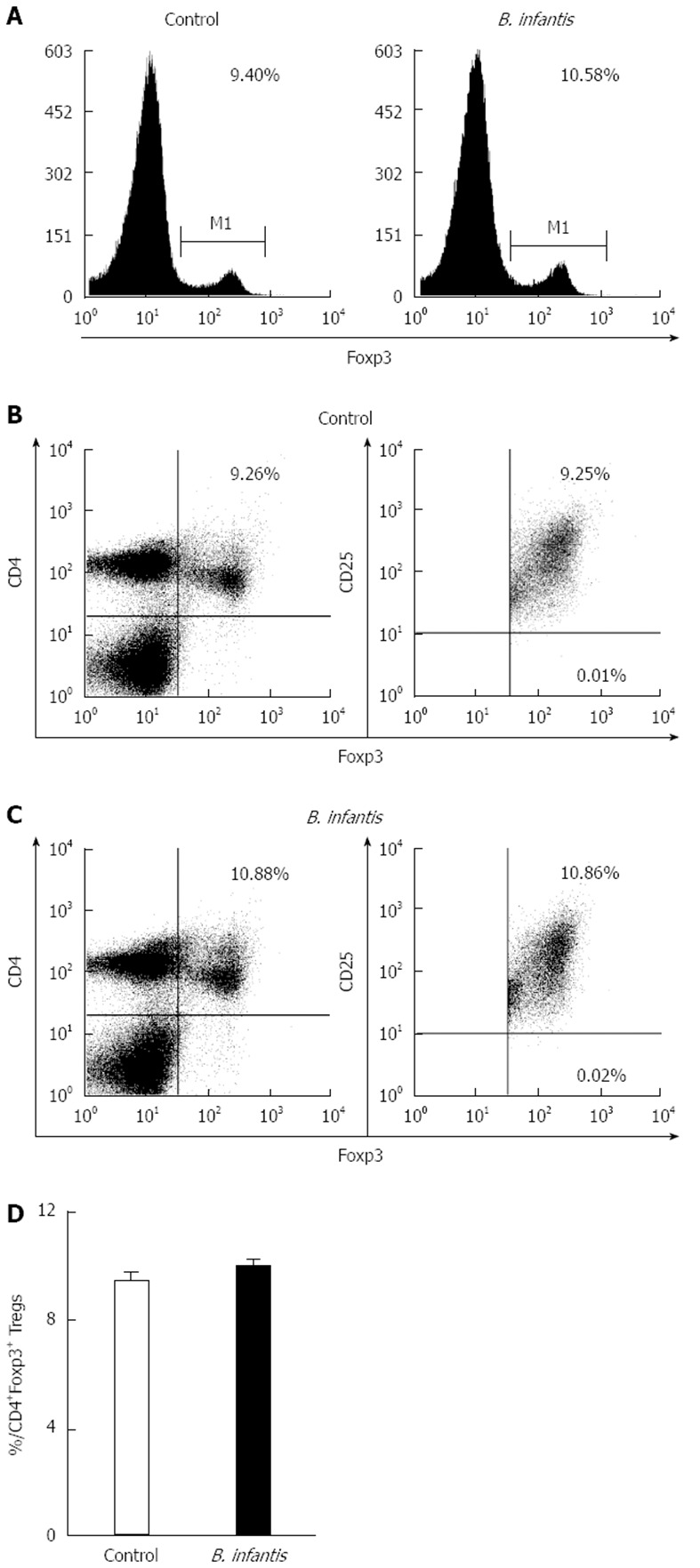
Representative flow plots and levels of Foxp3+ cells in mesenteric lymph nodes from normal mice fed high dose Bifidobacterium infantis. A: Levels of Foxp3+ cells in mesenteric lymph nodes from PBS-control group and Bifidobacterium infantis (B. infantis) group; B: Levels of CD4+Foxp3+ Tregs from PBS-control group; C: Levels of CD4+Foxp3+ Tregs from B. infantis group; D: The percentage of the CD4+Foxp3+ Tregs. Data are mean ± SE with 5 mice per group from three independent experiments (bar graphs) or as a representative flow cytometry graph.
Colonic damage in TNBS-induced colitis mice and upregulation of Th1- and Th17-responses
TNBS-induced colitis is a well-characterized animal model of Th1- and Th17-mediated colitis, and it mimics human CD. However, the appropriate time when the pathological change of the colon was dramatic was not known. Therefore we decided to perform the histological analysis in colitis control mice on days 3 and 6 after intrarectal instillation of TNBS. Hematoxylin and eosin staining was used to evaluate the severity of TNBS-induced colitis. Integral colonic epithelium and no inflammatory cell infiltration were observed in the slices from the negative control mice (Figure 4A). Sections of colonic tissues taken from mice on day 3 after instillation of TNBS showed extensive inflammatory cell infiltration (Figure 4B). In contrast, mice on day 6 after the instillation of TNBS exhibited goblet cell depletion, colonic epithelial loss and more inflammatory cells infiltrated in the colon (Figure 4C). These microscopic evaluations of colons from mice with colitis induced by TNBS revealed pathologic correlations with CD and longer duration after colitis induction was linked to more severe inflammation in the colon.
Figure 4.

Representative histological images of colon samples from trinitrobenzene sulfonic acid-induced colitis mice. A: Colon image from a negative control mouse; B: Colon image from a mouse on day 3 after trinitrobenzene sulfonic acid (TNBS) induction; C: Colon image from a mouse on day 6 after TNBS induction. All images are at the same original magnification (× 40).
TNBS-induced colitis was associated with excessive Th1- and Th17-responses. Therefore we measured the relative mRNA expression of different T cell cytokines in MLNs by real-time RT-PCR on days 3 and 6 after TNBS induction (Figure 5). Compared with the negative control group, the mRNA expression of Th1 cytokines (IL-2, IFN-γ and IL-12p40) displayed no change in colonic tissues from mice on day 3 after TNBS induction (TNBS-3) (Figure 5A-C). However, IL-2 and IL-12p40 increased significantly in mice on day 6 after TNBS induction (TNBS-6) compared with PBS control group (IL-2, P < 0.05; IL-12p40, P < 0.05) (Figure 5A and C), and the IFN-γ also increased a little but insignificantly. Mice in the TNBS-6 group had significantly higher expression of Th17 related molecules (RORγt and IL-17A) in the colonic tissue than control mice (RORγt, P < 0.01; IL-17A, P < 0.005) (Figure 5E and H). The expression of other Th17-related cytokines (IL-21 and IL-23) increased dramatically in the TNBS-3 group and in the TNBS-6 group compared to the control group, though mice in the TNBS-3 group had higher expression of both cytokines than mice in the TNBS-6 group (Figure 5F and G). However, TNBS did not induce the change of IL-4 mRNA representing Th2 (Figure 5D). Thus, we established the TNBS colitis model successfully and the colon damage was associated with increased activity of Th1 and Th17 cells. Besides, we determined the appropriate time, on day 6 after colitis induction, to evaluate the histological change and Th1- and Th17-responses.
Figure 5.
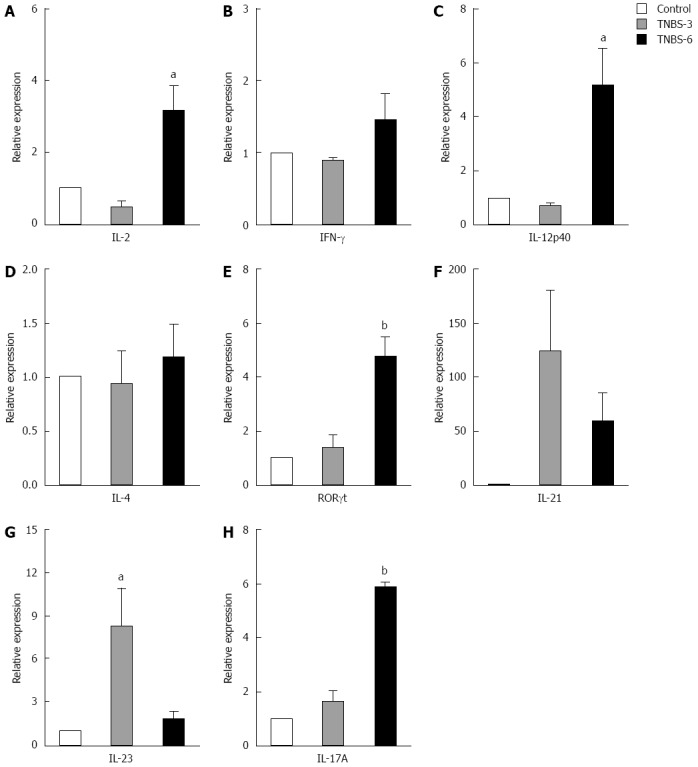
mRNA expression of T cell-related cytokines from mesenteric lymph nodes of trinitrobenzene sulfonic acid-induced colitis mice. A: IL-2; B: IFN-γ; C: IL-12p40; D: IL-4; E: RORγt; F: IL-21; G: IL-23; H: IL-17A. Real-time RT-PCR was employed to examine the expression of Th1, Th17 and Foxp3+ Treg related cytokines. Control: In the control group, the animals were administered with PBS; Trinitrobenzene sulfonic acid (TNBS)-3: In the TNBS-3 group, the animals were sacrificed 3 d after TNBS induction; TNBS-6: In the TNBS-6 group, the animals were sacrificed 6 d after TNBS induction. Data are from 5 mice per group. Data are expressed as relative expression and the control group was chosen as the calibrator. Data represent mean ± SE. aP < 0.05 vs the control group; bP < 0.01 vs the control group. IL: Interleukin; IFN: Interferon.
Attenuation of experimental colitis by B. infantis is associated with downregulation of Th1- and Th17-responses
We then investigated whether B. infantis reduced the severity of experimental colitis. Mice were daily administrated with high dose B. infantis (6 × 108 cfu/d) for 3 wk before induction of colitis by the instillation of TNBS. On day 6 after TNBS induction, sections of colonic tissues from mice in the PBS-TNBS group showed extensive goblet cell depletion, epithelial loss as well as dense chronic inflammatory infiltrates in the LP (Figure 6A). In contrast, administration of B. infantis drastically diminished goblet cell depletion and the intensity of inflammatory infiltrates and restored the intestinal epithelium (Figure 6B). These results revealed that B. infantis can attenuate the intestinal inflammation in the experimental colitis model.
Figure 6.
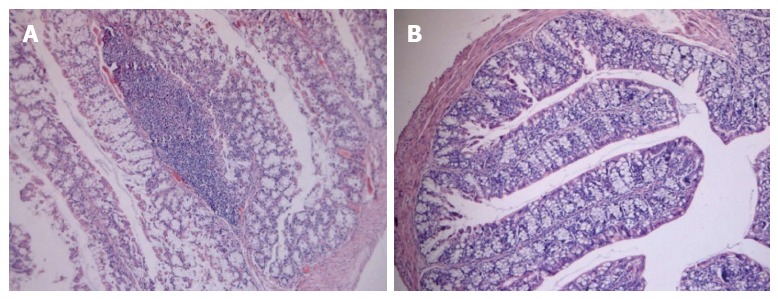
Representative histological images of colon samples from trinitrobenzene sulfonic acid-induced colitis mice fed high dose Bifidobacterium infantis. A: Colon image from a trinitrobenzene sulfonic acid (TNBS)-induced colitis mouse fed PBS; B: Colon image from a TNBS-induced mouse fed high dose Bifidobacterium infantis. All images are at the same original magnification (× 40).
Next we tested whether Th1/Th17 responses were downregulated after B. infantis feeding in experimental colitis mice. As shown in Figure 7, analyses of cytokine mRNA expression showed that Th1 cytokines (IFN-γ and IL-12p40) and Th17 cytokines (IL-17A and IL-23 ) were significantly decreased in the colons of mice receiving B. infantis before induction of colitis in comparison with mice receiving PBS before induction of colitis (IFN-γ, P < 0.05; IL-12p40, P < 0.05; IL-17A < 0.05; IL-23, P < 0.01) (Figure 7C, D, I and J). Th1 cytokine IL-2 seemed to fall down but not significantly (Figure 7B and J). In addition, B. infantis feeding led to lower levels of Th1 transcription factor T-bet and Th17 transcription factor RORγt compared with PBS control (T-bet, P < 0.05) (Figure 7A and G). No significant difference in mRNA expression of Th2-related GATA3 and IL-4 was measured between the PBS-TNBS group and B. infantis-TNBS group. However, surprisingly, B. infantis feeding increased the mRNA expression of Foxp3, IL-10 and TGF-β in colitis mice significantly when compared with the PBS-TNBS group (Foxp3, P < 0.01; IL-10, P < 0.05; TGF-β, P < 0.05) (Figure 7K-M). Taken together, these results show that B. infantis feeding attenuates the development of colonic inflammation in TNBS-induced colitis model in association with downregulation of both Th1- and Th17-responses.
Figure 7.
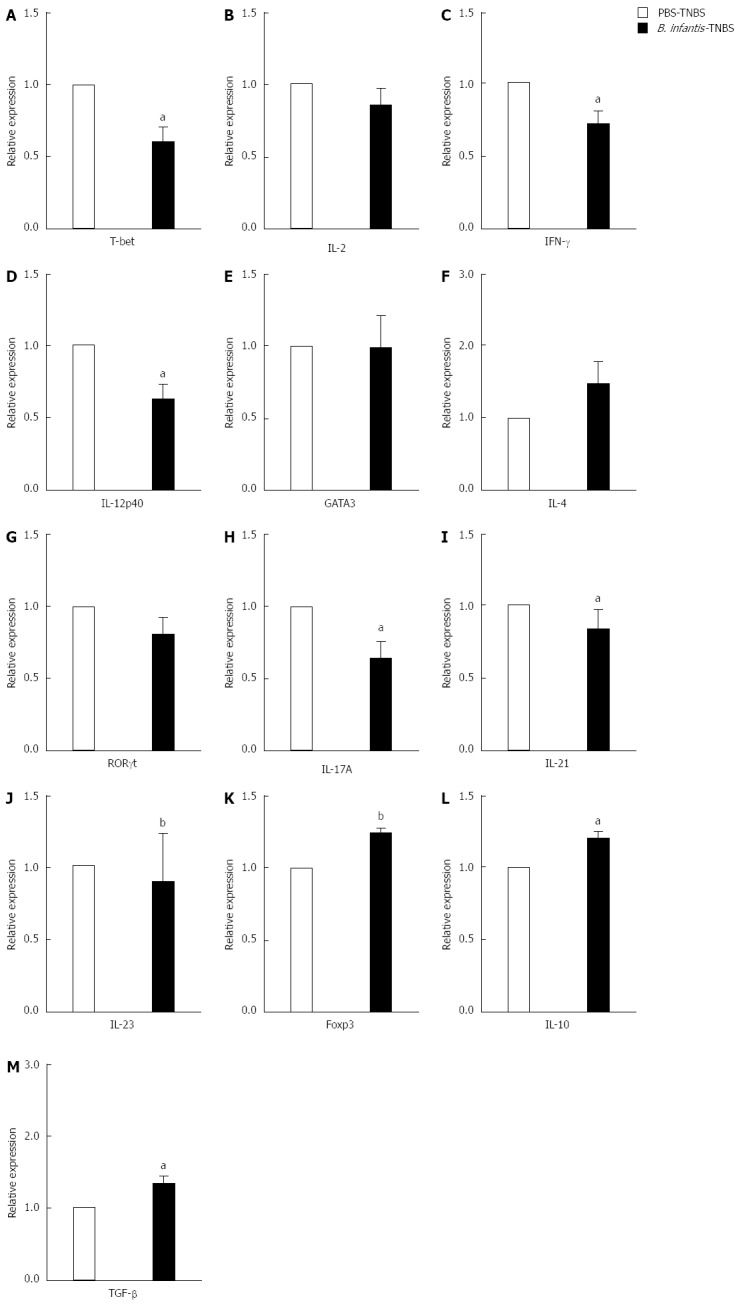
mRNA expression of T cell-related cytokines from mesenteric lymph nodes of trinitrobenzene sulfonic acid-induced colitis mice fed high dose Bifidobacterium infantis. A: T-bet; B: IL-2; C: IFN-γ; D: IL-12p40; E: GATA3; F: IL-4; G: RORγt; H: IL-17A; I: IL-21; J: IL-23; K: Foxp3; L: IL-10; M: TGF-β. Real-time RT-PCR was employed to examine the expression of Th1, Th17 and Foxp3+ Treg related cytokines. PBS-TNBS: In the PBS-TNBS group, the animals were fed PBS before TNBS induction; Bifidobacterium infantis (B. infantis)-TNBS: In the B. infantis-TNBS group, the animals were fed high dose B. infantis before TNBS induction. Data are expressed as relative expression and the control group was chosen as the calibrator. Data are mean ± SE (n = 5, per group). aP < 0.05 vs PBS-TNBS, bP < 0.01 vs the control group. IL: Interleukin; IFN: Interferon; TNBS: Trinitrobenzene sulfonic acid.
DISCUSSION
This study showed that B. infantis feeding to mice reduced the severity of TNBS-induced colitis, which was associated with suppression of Th1 and Th17 responses and promotion of Foxp3+ Treg response within MLNs. This revealed that the attenuation of TNBS-induced colitis by B. infantis feeding is mediated by regulating the differentiation of CD4+ T subsets and cytokine expression.
We found that B. infantis feeding reduced the severity of TNBS-induced colitis. TNBS instillation resulted in extensive goblet cell depletion, epithelial loss and dense inflammatory cell infiltrations in the LP. However, colitis mice with B. infantis feeding showed a noticeable resolution of inflammatory infiltrates and goblet cell depletion and restored intestinal epithelium. Next, the study explored the relation between the attenuation of colitis by B. infantis and the change of T cell subset responses, given that immune cell responses play a crucial role in the pathogenesis of colitis. Mucosal dendritic cells present gut antigens to the adaptive immune system and then direct the polarization of naïve CD4+ T cells towards different effector T-helper cells (Th1, Th2 and Th17) and Tregs[10]. These effector Th cells are important for combating pathogen infections; when aberrant they release enormous proinflammatory cytokines and initiate excessive inflammation, resulting in chronic inflammatory diseases. Nonetheless, the gut immune system has mechanisms of tolerance that avoid uncontrolled Th1, Th2 or Th17 responses. Foxp3+ Tregs displayed immunosuppressive function by suppressing Th cells and secreting anti-inflammatory cytokines IL-10 and TGF-β[11]. It is well known that aberrant Th1 and Th17 responses and deficient Treg response contribute to IBD.
Classically, CD has been associated with Th1 response. In our study, levels of Th1 cytokines (IL-2, IL-12p40 and IFN-γ) were up-regulated in the MLNs from TNBS-induced colitis mice. Th1 cells secrete a high level of IFN-γ, a proinflammatory cytokine responsible for macrophage activation. IFN-γ is essential for IBD pathogenesis as the administration of anti-IFN-γ antibody to mice can prevent the development of colitis[12] and the treatment with fontolizumab, an anti-IFN-γ antibody, improved clinical response and remission in patients with active CD compared with placebo[13]. Consistent with these results, our colitis mice were detected to have a reduction of IFN-γ mRNA in MLNs after B. infantis feeding. IL-12 has two subunits, p40 and p35, and it is mainly produced by macrophages/monocytes and can be efficiently induced by intracellular bacteria. IL-12 plays a pivotal role in the Th1 differentiation and induces naïve T cells to produce IFN-γ. Administration of antibody against its subunit IL-12p40 attenuated TNBS colitis with decreased levels of Th1 cells and IFN-γ in the LP[14]. In patients with active CD, anti-IL-12p40 treatment reduced Th1-mediated inflammatory cytokines and promoted clinical remission[15]. In this experiment, colitis mice showed down-regulated mRNA of IL-12p40 following B. infantis feeding. Further, the study revealed that Th1 transcription factor T-bet was up-regulated in colitis mice but was down-regulated by B. infantis feeding. T-bet is a member of the T-box transcription factor family that is essential for Th1 development as Th1 cells cannot be generated either in vitro or in vivo without it[16]. These outcomes showed B. infantis seemed to weaken the Th1 cell response via regulating its differentiation-related factors and cytokine expression in TNBS-induced colitis mice.
In the recent decade, the discovery of the Th17 subset has rapidly expanded the understanding of IBD. High levels of Th17 and its cytokine IL-17 were detected in inflamed colons of mice and patients with IBD[17]. IL-17A is a potent mediator of inflammatory responses as it induces proinflammatory cytokines such as IL-6, IL-8, and monocyte chemotactic protein-1 (MCP-1)[18]. Increased IL-17A mRNA was measured in our colitis model, though B. infantis reduced it. Likely, in DSS-induced colitis, B. infantis decreased Th17 cells and IL-17A[5]. Interestingly, IL-17A was also verified to induce production of IL-12 and promote the Th1 response[19], implying that IL-17A downregulation by B. infantis may facilitate the inhibition of Th1 response in IBD. IL-21, a CD4+ T cell-derived cytokine produced in excess in IBD was found to promote the differentiation of Th17 cells from naïve CD4+ T cells and prevent the TGF-β-dependent expression of Foxp3 by naïve CD4+ T cells[20]. IL-21-deficient mice have less Th17 cells with reduced severity of colitis after induction by either DSS or TNBS[21]. However, we found that B. infantis feeding led to the reduction of IL-21 in colitis mice. Orphan nuclear receptor RORgammat (RORγt) is constitutively expressed by Th17 cells and is the key transcription factor to Th17 differentiation[22]. In addition, RORγt can regulate the production of IL-17A and IL-17F to induce chronic colitis[23]. The animal experiment showed a reduction in RORγt mRNA in colitis mice fed B. infantis. Another key cytokine, IL23 secreted by DCs potently promoted Th17 amplification, sustained and strengthened the Th17 phenotype. Several groups concurrently showed that IL-23 played a crucial role in development of IBD in different colitis models partly because IL-23 induced proinflammatory cytokines such as TNF-α, IFN-γ and IL-6 in the colon[24]. Moreover, other work has shown that IL-23 inhibited Foxp3+ Tregs to induce colitis[25]. It is, therefore, of great importance to find that B. infantis feeding reduced the IL-23 mRNA in the colitis model. Taken together, B. infantis suppressed Th17 responses in TNBS-induced colitis mice.
Foxp3+ Tregs are a specialized class of lymphocytes characterized by the expression of the forkhead transcription factor Foxp3. Foxp3+ Tregs are essential for normal immunohomeostasis by suppressing Th effector cells[11]. The importance of Tregs in the prevention of colitis has been confirmed by previous studies showing that transfer of naïve CD4+ T cells without Tregs into immunodeficient mice resulted in inflammation and colitis, while co-transfer of Tregs ameliorated or reversed pathology[26]. In IBD patients, reduced numbers of Foxp3+ Tregs were observed in the peripheral blood and greater numbers of Foxp3+ Tregs in the intestinal mucosa, although the increase in the intestine of IBD patients was lower compared with non-IBD inflammatory conditions[11]. Given the lower Tregs, the expansion of Tregs seems to be a therapeutic tool for IBD. There is some evidence that probiotic administration is able to ameliorate the colitis by increasing Foxp3+ Tregs in the colonic tissue, such as probiotic mixtures (lactobacillus, bifidobacterium and streptococcus; lactobacillus and bifidobacterium; bifidobacterium, lactobacillus and enterococcus)[27-29]. In our normal mice, single B. infantis feeding increased the Foxp3+ Tregs in the MLNs. Similarly, B. infantis feeding increased Foxp3 mRNA in colitis mice. Consistent with our results, human volunteers taking B. infantis had elevated levels of Foxp3 expression in PBMCs[30]. The expression of Foxp3 is critical because it controls the development of Tregs and the immunosuppressive function of Tregs requires a high level of Foxp3 expression[31]. Decreased Foxp3 expression could lead to impaired Treg function and be causal for immune disorders including human immunodysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome[32]. Hence, the increased Foxp3 mRNA induced by B. infantis feeding may enhance the immunosuppressive activities in colitis mice. Besides, B. infantis increased Treg-related IL-10 and TGF-β in the colonic tissue in our colitis model. IL-10 is a potent anti-inflammatory cytokine, produced by both nonimmune and immune cells including Tregs. IL-10 has particular significance in IBD because IL-10-deficient mice develop colitis[33] and Treg-specific deletion of IL-10 resulted in spontaneous colitis[34]. Apart from IL-10, Tregs produce large amounts of TGF-β. TGF-β is a potent regulatory cytokine that inhibits Th cell proliferation, differentiation and activation, and decreases secretion of harmful cytokines[35]. Application of TGF-β blocking antibody eliminated the ability of Tregs to prevent colitis and TGF-β-deficient Tregs failed to suppress colitis[36]. What’s more, TGF-β is involved in the induction of Foxp3 and drives the differentiation of naïve T cells to a Treg phenotype[37]. Overall, B. infantis promoted Foxp3+ Treg responses in association with increased levels of Foxp3, L-10 and TGF-β in the MLNs in TNBS-induced colitis mice.
However, B. infantis feeding had some different effects on the immune cells in different gut environments. In normal gut environment a high dose elicited some Th1, Th17 and Treg responses with slight increases of Th17 cells and Tregs and their-related cytokines, though this did not cause pathological inflammation. One reason why B. infantis feeding resulted in a little high immune response is that B. infantis, which is derived from the human gut, is regarded as foreign antigen, thereby triggering weak responses. But this immune response did not lead to pathological inflammation, which indicated that there was a proper balance among Th1, Th17 and Tregs in maintaining gut homeostasis. This was confirmed by evidence that a good balance between Tregs and Th17 is essential for both eliminating potential pathogens and suppressing intestinal inflammation[38]. In some way, proper increases in Th1 and Th17 responses may enhance the mucosal defense against pathogens and thus be beneficial to the promotion of the host intestinal immunity.
It is of great importance for the potential of B. infantis to suppress IBD because the risk of colorectal cancer is increased in patients with ulcerative colitis and CD[39]. Animal models revealed that the setting of intestinal inflammation contributed to the tumorigenesis through T cell and TLR-mediated inflammation[40]. IL-17 immunoreactive cells were significantly expressed in the normal mucosa of colon cancer patient[41]. However, a high density of Foxp3+ Tregs in tumor tissue was associated with improved prognosis in colorectal cancer patients in early T stage[42]. Thus reducing chronic intestinal inflammation appears to be a good strategy in preventing the incidence of colorectal cancer. The microbial dysbiosis was found in colon cancer patients[41], indicating that improvement of intestinal flora environment may benefit these patients. Studies showed that supplementation of viable Bifidobacteria before surgery can reduce infection complication of surgery in patients with colorectal cancer[43]. More importantly, probiotics can delay or block the development of tumor from inflammation disorders by reducing inflammation. Pretreatment with the probiotic VSL#3, a mixture of lactobacillus, bifidobacterium and streptococcus, can attenuate the inflammation in TNBS-induced colitis, delaying transition to dysplasia and cancer[44]. Bifidobacterium lactis prevents DSS-induced acute colitis and DSS colitis-associated cancer in mice[45]. B. infantis has the potential to attenuate intestinal inflammation through suppressing Th1 and Th17 cell responses as well as promoting Foxp3+ Tregs. Therefore, B. infantis may reduce risk of the transition from IBD to colorectal cancer, even slow down the existing cancer progression and improve the prognosis.
However, the major drawback of this study is that we failed to measure the frequencies of Th17 cells and Foxp3+ Tregs and identify their origin in colitis mice and B. infantis-fed colitis mice. The expression of Foxp3 can be induced in naturally thymic-drived CD4+CD25+ Tregs or in mature CD4+ “conventional” T cells in vitro and in vivo[11]. Nevertheless, Foxp3+ Tregs from CD4+CD25+ Tregs or from CD4+CD25- conventional T cells both have similar immunosuppressive functions. In normal mice, we found that increased Foxp3+ Tregs by B. infantis were mostly derived from the CD4+CD25+ Tregs in the MLNs. But whether the origin of the increased Foxp3 mRNA in MLNs in our colitis mice and B. infantis-treated colitis mice was also derived from CD4+CD25+ Tregs or from peripheral CD4+CD25- T was not known. Previous studies showed that feeding mixed probiotics (bifidobacterium, lactobacillus and enterococcus) or (lactobacillus and bifidobacterium) led to elevated levels of CD4+CD25+Foxp3+ cells in MLNs or intraepithelial in TNBS colitis mice[28,29]. However, in the same model, probiotic IRT5 administration increased the CD4+CD25−Foxp3+ cells in MLNs[27]. For this discrepancy, one explanation is that the individual component of probiotic mixtures had different function on the population of Tregs in the intestine. In order to elucidate the origin of elevated levels of Foxp3 mRNA in our B. infantis-treated colitis mice, further studies will be done in the future.
In conclusion, we showed that B. infantis attenuated intestinal inflammation in TNBS-induced colitis mice by suppressing the Th1 and Th17 responses and increasing the Foxp3+ Treg response in MLNs. This mechanism underlying the regulatory effect of B. infantis on the immune system may have significant clinical implications in treating IBD and preventing colorectal cancer.
ACKNOWLEDGMENTS
The authors thank Guangzhou Baoxing Biotechnology Company for providing the Bifidobacterium infantis.
COMMENTS
Background
Crohn’s disease (CD), one type of inflammatory bowel disease, is characterized by chronic inflammation of intestinal tract. Abnormal microbiota and mucosal immune cell responses contribute to the pathogenesis of CD.
Research frontiers
Alterations of gut microbiota cause immune dysregulation, leading to autoimmune attacks, as a result of serious damage of the intestinal mucosal barrier. CD involves aberrant Th1 and Th17 responses and deficient T regulatory cell response. This suggested that regulating immune cell responses could potentially attenuate intestinal inflammation.
Innovations and breakthroughs
In this study, the authors found that Bifidobacterium infantis (B. infantis) had the potential to effectively attenuate the colitis induced by 2,4,6-trinitrobenzene sulfonic acid. These beneficial effects were associated with the role of B. infantis in the suppression of Th1 and Th17 cell responses, the promotion of Foxp3+ T regulatory cell response, and restoring the balance of T cell subsets responses.
Applications
Understanding the mechanism underlying the effects of B. infantis in treating experimental colitis has significant clinical implications in effectively and safely treating inflammatory bowel disease and further preventing colorectal cancer associated with intestinal inflammation.
Peer review
The authors investigated effects of B. infantis in treating experimental colitis which mimics CD and explored its potential mechanism by measuring levels of Th1, Th17 and Foxp3+ T regulatory cells and expression of their related cytokines and transcription factors in mesenteric lymph nodes. They revealed that B. infantis effectively relieved the colitis, which was associated with reducing the Th1, Th17 responses and increasing the T regulatory cell response. The results provide valuable evidence for understanding the mechanism of probiotics for treating inflammatory bowel disease such as CD.
Footnotes
Supported by Guangzhou Baoxing Biotechnology Company
P- Reviewer: Ciccone MM, Foligne B, Lee I S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Wang CH
References
- 1.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whorwell PJ, Altringer L, Morel J, Bond Y, Charbonneau D, O’Mahony L, Kiely B, Shanahan F, Quigley EM. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:1581–1590. doi: 10.1111/j.1572-0241.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 4.Dajani AI, Abu Hammour AM, Yang DH, Chung PC, Nounou MA, Yuan KY, Zakaria MA, Schi HS. Do probiotics improve eradication response to Helicobacter pylori on standard triple or sequential therapy? Saudi J Gastroenterol. 2013;19:113–120. doi: 10.4103/1319-3767.111953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konieczna P, Ferstl R, Ziegler M, Frei R, Nehrbass D, Lauener RP, Akdis CA, O’Mahony L. Immunomodulation by Bifidobacterium infantis 35624 in the murine lamina propria requires retinoic acid-dependent and independent mechanisms. PLoS One. 2013;8:e62617. doi: 10.1371/journal.pone.0062617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCarthy J, O’Mahony L, O’Callaghan L, Sheil B, Vaughan EE, Fitzsimons N, Fitzgibbon J, O’Sullivan GC, Kiely B, Collins JK, et al. Double blind, placebo controlled trial of two probiotic strains in interleukin 10 knockout mice and mechanistic link with cytokine balance. Gut. 2003;52:975–980. doi: 10.1136/gut.52.7.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanabe S, Kinuta Y, Saito Y. Bifidobacterium infantis suppresses proinflammatory interleukin-17 production in murine splenocytes and dextran sodium sulfate-induced intestinal inflammation. Int J Mol Med. 2008;22:181–185. [PubMed] [Google Scholar]
- 8.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 9.Foligné B, Nutten S, Steidler L, Dennin V, Goudercourt D, Mercenier A, Pot B. Recommendations for improved use of the murine TNBS-induced colitis model in evaluating anti-inflammatory properties of lactic acid bacteria: technical and microbiological aspects. Dig Dis Sci. 2006;51:390–400. doi: 10.1007/s10620-006-3143-x. [DOI] [PubMed] [Google Scholar]
- 10.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayne CG, Williams CB. Induced and natural regulatory T cells in the development of inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1772–1788. doi: 10.1097/MIB.0b013e318281f5a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 13.Hommes DW, Mikhajlova TL, Stoinov S, Stimac D, Vucelic B, Lonovics J, Zákuciová M, D’Haens G, Van Assche G, Ba S, et al. Fontolizumab, a humanised anti-interferon gamma antibody, demonstrates safety and clinical activity in patients with moderate to severe Crohn’s disease. Gut. 2006;55:1131–1137. doi: 10.1136/gut.2005.079392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neurath MF, Fuss I, Kelsall BL, Stüber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281–1290. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mannon PJ, Fuss IJ, Mayer L, Elson CO, Sandborn WJ, Present D, Dolin B, Goodman N, Groden C, Hornung RL, et al. Anti-interleukin-12 antibody for active Crohn’s disease. N Engl J Med. 2004;351:2069–2079. doi: 10.1056/NEJMoa033402. [DOI] [PubMed] [Google Scholar]
- 16.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 17.Niess JH, Leithäuser F, Adler G, Reimann J. Commensal gut flora drives the expansion of proinflammatory CD4 T cells in the colonic lamina propria under normal and inflammatory conditions. J Immunol. 2008;180:559–568. doi: 10.4049/jimmunol.180.1.559. [DOI] [PubMed] [Google Scholar]
- 18.Andoh A, Takaya H, Makino J, Sato H, Bamba S, Araki Y, Hata K, Shimada M, Okuno T, Fujiyama Y, et al. Cooperation of interleukin-17 and interferon-gamma on chemokine secretion in human fetal intestinal epithelial cells. Clin Exp Immunol. 2001;125:56–63. doi: 10.1046/j.1365-2249.2001.01588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng T, Qin H, Wang L, Benveniste EN, Elson CO, Cong Y. Th17 cells induce colitis and promote Th1 cell responses through IL-17 induction of innate IL-12 and IL-23 production. J Immunol. 2011;186:6313–6318. doi: 10.4049/jimmunol.1001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fantini MC, Rizzo A, Fina D, Caruso R, Becker C, Neurath MF, Macdonald TT, Pallone F, Monteleone G. IL-21 regulates experimental colitis by modulating the balance between Treg and Th17 cells. Eur J Immunol. 2007;37:3155–3163. doi: 10.1002/eji.200737766. [DOI] [PubMed] [Google Scholar]
- 21.Fina D, Sarra M, Fantini MC, Rizzo A, Caruso R, Caprioli F, Stolfi C, Cardolini I, Dottori M, Boirivant M, et al. Regulation of gut inflammation and th17 cell response by interleukin-21. Gastroenterology. 2008;134:1038–1048. doi: 10.1053/j.gastro.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 22.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 23.Leppkes M, Becker C, Ivanov II, Hirth S, Wirtz S, Neufert C, Pouly S, Murphy AJ, Valenzuela DM, Yancopoulos GD, et al. RORgamma-expressing Th17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology. 2009;136:257–267. doi: 10.1053/j.gastro.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 24.Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, McKenzie BS, Powrie F, Maloy KJ. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203:2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Izcue A, Hue S, Buonocore S, Arancibia-Cárcamo CV, Ahern PP, Iwakura Y, Maloy KJ, Powrie F. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity. 2008;28:559–570. doi: 10.1016/j.immuni.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrissey PJ, Charrier K. Induction of wasting disease in SCID mice by the transfer of normal CD4+/CD45RBhi T cells and the regulation of this autoreactivity by CD4+/CD45RBlo T cells. Res Immunol. 1994;145:357–362. doi: 10.1016/s0923-2494(94)80200-9. [DOI] [PubMed] [Google Scholar]
- 27.Kwon HK, Lee CG, So JS, Chae CS, Hwang JS, Sahoo A, Nam JH, Rhee JH, Hwang KC, Im SH. Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc Natl Acad Sci USA. 2010;107:2159–2164. doi: 10.1073/pnas.0904055107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roselli M, Finamore A, Nuccitelli S, Carnevali P, Brigidi P, Vitali B, Nobili F, Rami R, Garaguso I, Mengheri E. Prevention of TNBS-induced colitis by different Lactobacillus and Bifidobacterium strains is associated with an expansion of gammadeltaT and regulatory T cells of intestinal intraepithelial lymphocytes. Inflamm Bowel Dis. 2009;15:1526–1536. doi: 10.1002/ibd.20961. [DOI] [PubMed] [Google Scholar]
- 29.Zhao HM, Huang XY, Zuo ZQ, Pan QH, Ao MY, Zhou F, Liu HN, Liu ZY, Liu DY. Probiotics increase T regulatory cells and reduce severity of experimental colitis in mice. World J Gastroenterol. 2013;19:742–749. doi: 10.3748/wjg.v19.i5.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konieczna P, Groeger D, Ziegler M, Frei R, Ferstl R, Shanahan F, Quigley EM, Kiely B, Akdis CA, O’Mahony L. Bifidobacterium infantis 35624 administration induces Foxp3 T regulatory cells in human peripheral blood: potential role for myeloid and plasmacytoid dendritic cells. Gut. 2012;61:354–366. doi: 10.1136/gutjnl-2011-300936. [DOI] [PubMed] [Google Scholar]
- 31.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 32.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 33.Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 34.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 35.Wan YY, Flavell RA. ‘Yin-Yang’ functions of transforming growth factor-beta and T regulatory cells in immune regulation. Immunol Rev. 2007;220:199–213. doi: 10.1111/j.1600-065X.2007.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26:579–591. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 37.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eastaff-Leung N, Mabarrack N, Barbour A, Cummins A, Barry S. Foxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. J Clin Immunol. 2010;30:80–89. doi: 10.1007/s10875-009-9345-1. [DOI] [PubMed] [Google Scholar]
- 39.Rutter MD, Saunders BP, Wilkinson KH, Rumbles S, Schofield G, Kamm MA, Williams CB, Price AB, Talbot IC, Forbes A. Thirty-year analysis of a colonoscopic surveillance program for neoplasia in ulcerative colitis. Gastroenterology. 2006;130:1030–1038. doi: 10.1053/j.gastro.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 40.Sussman DA, Santaolalla R, Strobel S, Dheer R, Abreu MT. Cancer in inflammatory bowel disease: lessons from animal models. Curr Opin Gastroenterol. 2012;28:327–333. doi: 10.1097/MOG.0b013e328354cc36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sobhani I, Tap J, Roudot-Thoraval F, Roperch JP, Letulle S, Langella P, Corthier G, Tran Van Nhieu J, Furet JP. Microbial dysbiosis in colorectal cancer (CRC) patients. PLoS One. 2011;6:e16393. doi: 10.1371/journal.pone.0016393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–192. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 43.Zhang JW, Du P, Gao J, Yang BR, Fang WJ, Ying CM. Preoperative probiotics decrease postoperative infectious complications of colorectal cancer. Am J Med Sci. 2012;343:199–205. doi: 10.1097/MAJ.0b013e31823aace6. [DOI] [PubMed] [Google Scholar]
- 44.Appleyard CB, Cruz ML, Isidro AA, Arthur JC, Jobin C, De Simone C. Pretreatment with the probiotic VSL#3 delays transition from inflammation to dysplasia in a rat model of colitis-associated cancer. Am J Physiol Gastrointest Liver Physiol. 2011;301:G1004–G1013. doi: 10.1152/ajpgi.00167.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim SW, Kim HM, Yang KM, Kim SA, Kim SK, An MJ, Park JJ, Lee SK, Kim TI, Kim WH, et al. Bifidobacterium lactis inhibits NF-kappaB in intestinal epithelial cells and prevents acute colitis and colitis-associated colon cancer in mice. Inflamm Bowel Dis. 2010;16:1514–1525. doi: 10.1002/ibd.21262. [DOI] [PubMed] [Google Scholar]


