Abstract
AIM: To clarify whether the performance of liver resections (LR) for incidental gallbladder carcinoma (IGBC)’s depends more on the experience of the hospitals in liver surgery than on complying with the guidelines in Germany.
METHODS: For data analysis, we used the Surgical Association of Endoscopy and Ultrasound and Minimally Invasive Surgery Central Registry of “IGBC” of the German Society of Surgery (the German Registry). In 2010, we started a second form by requesting the frequency of LR at the various hospitals in Germany. The indication for LR was irrelevant. The aim was to determine the overall frequency of liver resections at the hospitals. We divided the hospitals according to their experience in liver surgery into high- (HV), mid- (MV), and low-volume (LV) LR hospitals.
RESULTS: This study includes 487 IGBC’s from 167 centers. There were 36 high-volume, 32 mid-volume, and 99 low-volume centers. In the high-volume centers, the mean (range) number of liver resections was 101 (40-300). In the mid-volume centers, the mean (range) number of liver resections was 26 (20-39). In the low-volume centers, the mean (range) number of liver resections was 6.5 (0-19) (P < 0.001). LV’s perform LR for T2-3 gallbladder carcinomas significantly less often than high-volume or mid-volume centers (χ2 = 13.78, P = 0.001). In HV’s and MV’s, 61% of the patients with an indication for liver resection underwent LR, but in LV centers, only 41% with an indication for LR underwent LR (P < 0.001). In cases of T1b carcinomas, LR was performed significantly more often in HV’s (P = 0.009).
CONCLUSION: The central problem is that the performance of the required liver resection in IGBC in Germany depends on the hospital experience in liver surgery and not on the recommendations of the German guidelines.
Keywords: Gallbladder carcinoma, Radical cholecystectomy, High-volume center, German-registry, Volume cut-off, Hepatobiliary surgery
Core tip: The indication for radical liver resection in incidental gallbladder carcinomas depends more on the experience of the hospitals in liver surgery than on the tumor stage of the primary carcinoma. In addition, the recommendations of high quality guidelines seemed to be ignored in radical surgery in incidental gallbladder carcinoma cases.
INTRODUCTION
The 5-year survival rate for patients with gallbladder carcinoma is less than 5%[1-3]. Stage-adjusted therapy including radical surgery remains the only effective treatment[4,5]. If gallbladder carcinoma is discovered in the early stages, the 5-year survival rate can reach 75%[6]. Guidelines for radical resection vary worldwide[5,7,8]. According to the S3 Guidelines[8], the recommended treatment for gallbladder carcinoma in Germany is liver resection in the form of wedge resection of the gallbladder bed with a 3 cm margin in the liver, or a resection of liver segments 4b and 5, always combined with dissection of the regional lymph nodes along the hepatoduodenal ligament in cases of T2 or more advanced carcinomas. Similarly, a radical cholecystectomy for T1b and more advanced carcinomas is recommended by the Guidelines of the National Comprehensive Cancer Network[7].
Despite the publication of these guidelines, surgical management of gallbladder cancer remains inadequate. Data from the German Registry and recent scientific literature show an underutilization of radical resection as a critical component for the treatment of gallbladder cancer[9,10]. In the era of minimally invasive surgery, most early-stage gallbladder carcinomas are identified by laparoscopy, and radical cholecystectomy is needed in a second surgery[6,11]. The effectiveness of the wedge-resection technique and the bisegmentectomy of segments IVb/V in liver resection combined with standardized lymph node dissection have already been demonstrated based on 624 patients in the German Registry (GR)[6]. However, only 49% of the T2 and T3 carcinomas in the GR underwent a second radical surgery, despite the recommendation of the S3 Guidelines[8] to perform radical liver resection in cases of T2 and T3 carcinomas. Hepatic resection has been shown to be associated with high morbidity and mortality[12-14]. Improvements in surgical techniques and perioperative care have resulted in a reduction in morbidity, with reports of nearly no mortality in some centers[15,16]. Therefore, there may be some regional variation in treatment, whereby high-volume hospitals perform the recommended procedures in a higher portion of cases because they have accumulated enough experience to feel confident about performing these procedures.
The question is whether the performance of liver resection for gallbladder carcinoma cases depends more on the experience of the different hospitals with liver surgery than on complying with the recommendations of the S3 Guidelines in Germany[8]. According to the literature, there is an association between mortality rate and volume of liver resection surgery, but arbitrary cut-offs have been used to differentiate high-volume from low-volume hospitals[17-19]. Thresholds ranging from 15 to 50 cases per year have been used to define a high-volume center[17-19]. The volume cut-off for a high-volume center was 20 or more cases. Volume cut-offs of 15 and 50 cases has also been previously examined[17,18]. The aim is to determine whether clinics with more experience in liver surgery, represented by means of so-called high-volume centers, more often follow the S3 Guidelines regarding liver resection or radical cholecystectomy in gallbladder cancer surgery. A further question addresses the definition of high-volume in liver surgery. Especially in cancers without a high incidence, the definition of high volume in the literature is often inconsistent. “High-volume centers” in liver and pancreas surgery was defined by Fong et al[20] as more than 25 cases per year. However, they also mentioned that there is no exact science concerning the cut off thresholds, so there is no major break at that point. Regarding the more seldom type of cancers, data are sparse concerning what is high and low volume and most cut-offs are arbitrarily. Langer[21] provided a list of articles defining “high-volume” in pancreas resection differing from 2 to 20 resections per year. A Report by the Cancer Care Ontario task force on the regionalization of pancreatic cancer surgery in Ontario in 1999[22], defined the volumes of major pancreatic surgery with at least 10 cases per year and total hepatobiliary surgery with at least 25 cases per year as high-volume centers for this type of cancer surgery. In 2006, an expert panel, “Cancer Care Ontario, hepatic, pancreatic and biliary (HPB) Surgical Oncology Standards”[23], published a report entitled ”HPB Surgical Oncology Standards”. According to this document, the volume targets were 20 major pancreatic cases and 50 major HPB cases per year for a high-volume center. Glasgow et al[24] states that in general surgical practice, standards for the minimum experience necessary to perform highly complex and risky procedures (i.e., major hepatic, pancreatic, or esophageal resections for neoplasia) do not exist, which again indicates that a clear definition for cut-offs in the more rare cancers seems to be complicated. Finlayson et al[25] defined three groups: low-, mid-, and high-volume. In pancreatic resection, the mid-volume group was designated by 3-13 operations, whereas the mid-volume group for the more frequent colon resection procedures was designated by 61-116 cases, similar to the analysis of Schrag et al[26] in JAMA 2000. According to Schrag et al[26], the definition of different case volumes for colon cancer were as follows: 1 to 57 low volume; 58 to 112 mid-volume; 113 to 165 high-volume; and 166 to 383, very high-volume. For the types of carcinomas with higher incidence, the cut-offs seem to be more consistent. Based on a systematic review of 12 studies with a total of 19688 patients, van Heek et al[27] were not able to define a clear cut-off volume for pancreas resection.
MATERIALS AND METHODS
Data collection
For data analysis, we used the Surgical Association of Endoscopy, currently called the Surgical Association of Endoscopy and Ultrasound (CAE, currently CAES), and the Surgical Association of Minimally Invasive Surgery (CAMIC) Central Registry of “incidental gallbladder carcinoma (IGBC)” of the German Society of Surgery [(the German Registry (GR)]. The GR was established in 1997[28], and is supported by CAES/CAMIC, which are both part of the German Society of Surgery. Cases of IGBC in Germany are registered in the GR. Patients who underwent a radical re-resection were treated under the conditions of the S3 guidelines for the diagnosis and treatment of gallstones from the German Society for Digestive and Metabolic Diseases and the German Society for Surgery of the Alimentary Tract[8]. The patients underwent a liver resection that involved either a wedge resection of the gallbladder bed with a 3 cm margin in the liver or a liver resection of segments 4b and 5, always in addition to standardized lymph node dissection of the hepatoduodenal ligament. According to the S3 Guidelines, liver resection is recommended in cases of T2 tumors and more advanced carcinomas.
Questionnaire
A standardized form was sent periodically (every 3 mo) to all surgical hospitals to collect data for the GR. This form also contained questions about whether immediate re-resection was performed, and if so by which technique.
Study design
In 2010, we implemented a second form requesting data on the frequency of liver resections per year at the various hospitals with approval of the German Society of General and Visceral Surgery. The indication for liver resections was irrelevant. We were not only interested in the number of liver resections for gallbladder carcinoma.
The aim was to determine the overall frequency of liver resections at the different clinics to determine a conclusion regarding the experience in liver resection of the different clinics. The form sought to determine whether the indication for liver resection in gallbladder carcinoma depends on the experience of the clinics in liver surgery more than on the recommendations of the S3 Guidelines.
Based on results from the literature and the statistical analysis we divided the hospitals into three groups according to their frequency of liver resections (LR) per year. High-volume LR hospitals were defined as hospitals performing 40 or more resections per year, mid-volume LR hospitals were defined as hospitals performing 20-39 resections per year, and low-volume LR hospitals are defined as hospitals performing less than 20 resections per year. We choose to use three groups because a binary cut-off consisting of high- and low-volume hospitals seemed to be too sharp a limit that does not reflect the reality.
Staging
Clinical and histopathological staging was based on the 6th edition of the UICC/AJCC classification of 2002[29].
Statistical analysis
SPSS version 11.5 (SPSS Inc., Chicago, IL, United States) was used. We used ANOVA-analysis (analysis of variance), Box plots and χ2 tests, and a P-value < 0.05 was considered statistically significant.
RESULTS
This study includes 487 gallbladder carcinoma patients from 167 centers. There were 36 high-volume, 32 mid-volume, and 99 low-volume centers.
A total of 31.5% of patients who underwent re-resection of the liver underwent the liver resection at a high-volume center, whereas 23% of patients who underwent re-resection of the liver underwent the liver resection at a mid-volume center, and 45.5% of patients who underwent re-resection of the liver underwent the liver resection at a low- volume center.
At high-volume centers, 60% of patients with an indication for liver resection (T2-3 carcinomas) underwent the required liver resection, and at mid-volume centers, 61% did. In contrast, only 41% of patients with an indication for liver resection (T2-3 carcinomas) underwent the required liver resection at low-volume centers. The Chi-square test indicates that low-volume centers perform liver resection (for T2-3 carcinomas) significantly less often than high-volume or mid-volume centers (χ2 = 13.78, P = 0.001) (Table 1).
Table 1.
χ2 test
| High-volume | Mid-volume | Low-volume | Total | |
| HV vs MV vs LV1 | ||||
| No ReOP | 37 | 26 | 117 | 180 |
| ReOP | 56 (31.5) | 41 (23) | 81 (45.5) | 178 |
| Total | 93 (60) | 67 (61) | 198 (41) | 358 |
| High + medium vs low2 | ||||
| No ReOP | 63 | - | 117 | 180 |
| ReOP | 97 (54.5) | - | 81 (45.5) | 178 |
| Total | 160 (61) | - | 198 (41) | 358 |
| HV vs MV vs LV3 | ||||
| No ReOP | 6 | 11 | 17 | 34 |
| ReOP | 14 (70) | 4 (26) | 7 (29) | 25 |
| Total | 20 | 15 | 24 | 59 |
1χ2 test of 358 patients (T2-3)/volume groups [high-volume (HV) vs mid-volume (MV) vs low-volume (LV)] vs re-resection (χ2 = 13.78, P = 0.001/Cramer’s V of 0.2);
χ2 test of 358 patients (T2-3)/volume groups (high + medium vs low) vs re-resection (χ2 = 13.76, P < 0.001/Cramer’s V of 0.2);
χ2 test of 59 T1b patients with the corresponding volume groups (HV vs MV vs LV) vs re-resection (χ2 = 9.48, P = 0.009).
The direct internal comparison of the three volume groups with boxplots (Figure 1) shows that low-volume centers ignore the indications for liver resection significantly more often than the guidelines stipulate; however, mid-volume centers perform liver resections significantly more often than not. High-volume centers show the same trend as mid-volume centers, but the difference was not statistically significant.
Figure 1.
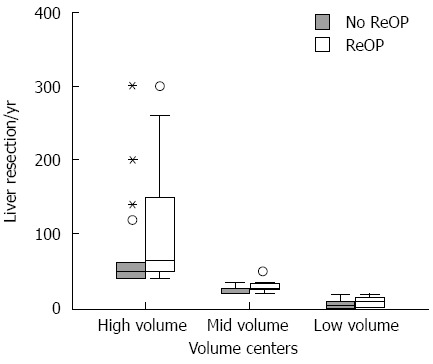
Box plots of the annual volume of liver resections, separated according to volume strata and occurrence of re-resection (T2-3 carcinomas). The middle bar of the box plot represents the median, while the lower and upper bars of the box represent the 25th and 75th quartiles, respectively. The dots and asterisks represent outliers.
Combining the high- and mid-volume centers into one group and comparing them to the low volume centers produced the following results (Figure 2). Based on an ANOVA, 358 patients at tumor stage T2-3 were analyzed: 160 patients at high/mid-volume centers and 198 patients at low-volume centers. The mean of re-resections at high-/mid-volume centers was 61 vs 41 at low volume centers (P < 0.001). According to the means, the high-/mid-volume centers perform liver resection significantly more often for T2-3 carcinomas than low-volume centers.
Figure 2.
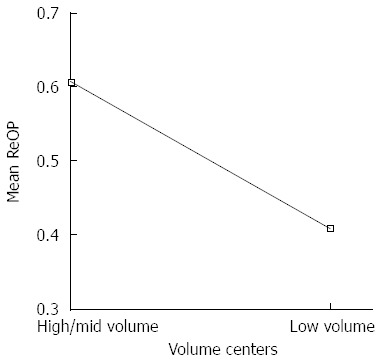
ANOVA of high/mid-volume vs low-volume centers according to the mean rate of liver resection.
The corresponding analysis (combining the high- and mid-volume groups into one group) using a χ2 test shows similar results (Table 1). A total of 54.5% of patients who underwent liver resection (in the T2-3 tumor stage) underwent liver surgery at high- or mid-volume centers, whereas 45.5% underwent liver surgery at low-volume centers. At high- and mid-volume centers, 61% of the patients with an indication for liver resection underwent liver resection, but at low-volume centers, only 41% of patients with an indication for liver resection actually underwent a liver resection (χ2 = 13.76, P < 0.001).
Figure 3 shows the results of an ANOVA of the number of liver resections in the 487 patients with Tis-T4 carcinomas at all volume centers separated according to high-, mid-, and low-volume centers. At high-volume centers, the mean (range) number of liver resections was 101 (40-300), whereas at mid-volume centers, the mean (range) number of liver resections was 26 (20-39) and at low-volume centers, the mean (range) number of liver resections was 6.5 (0-19) (P < 0.001).
Figure 3.
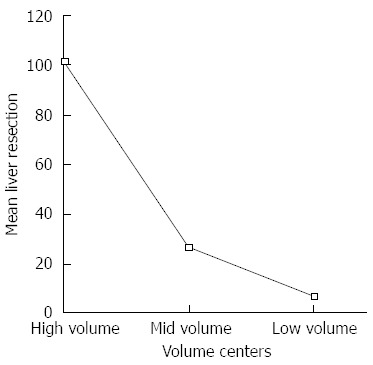
Mean rate of liver resection in the 3 volume groups including all patients/all volume centers.
Figure 4 shows the distribution of the number of resections at high-, mid-, and low- volume centers for patients at all T stages/all volume centers.
Figure 4.
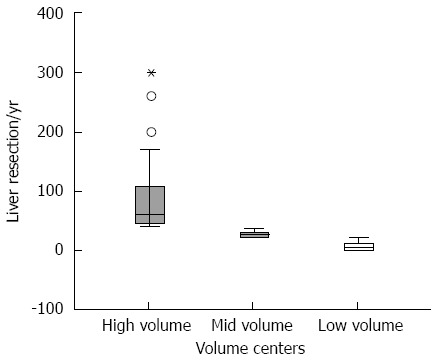
Medians of liver resections according to the 3 volume groups (Tis-T4 carcinomas/all patients). The middle bar of the box plot represents the median, whereas the lower and upper bars of the box represent the 25th and 75th quartiles, respectively. The dots and asterisks represent outliers. The boxplot-test of all patients/volume-centers shows that the median of liver resection at high-volume centers was 60, that at mid-volume centers was 28 and that at low-volume centers was 5.
A non-parametric χ2 showed that only 178 of 358 patients (49%) with T2-3 stage disease underwent liver resection (P = 0.011).
In addition, 9 patients (2.5%) with T2-3 carcinomas were referred from a low- volume to a high-volume center. Three of these patients have already been reported by a high-volume center; three patients were referred by one hospital, and the remaining 3 patients were referred by 3 different hospitals.
In cases of T1b carcinomas, liver resection was performed significantly more often at high-volume centers than at mid or low volume centers (Table 1, χ2 = 9.48, P = 0.009).
DISCUSSION
Only 49% of patients in the German registry with an indication for liver resection (T2-3 IGBCs) underwent a liver resection as recommended by the German S3 Guidelines[8]. Thus, it appears that the surgical recommendations of the German S3 Guidelines are often not being followed. The aim of this study was not to discuss the quality of liver resection at the different types of hospitals but rather to determine whether the indication for radical resection depends more on the experience of the hospital in liver surgery than on objective factors such as tumor stage. Therefore, we have analyzed mainly IGBCs in T2 and T3 tumor stages, where liver resection is recommended by the German S3 Guidelines[8]. Additionally, liver resection is recommended for up to T1b gallbladder carcinomas according to the NCCN Guidelines[7]. In addition, there is evidence in the GR that T1b IGBC cases benefit from liver resection[30,31]. Therefore, we have also analyzed the T1b carcinomas. For completeness, data from all other T stages were also added.
A substantial portion of patients in this study with incidental gallbladder carcinoma was treated at low-volume centers. This seems to be logical because most hospitals in Germany that perform cholecystectomy are low-volume centers regarding liver surgery.
Table 1 shows that 31.5% of T2-3 carcinomas with re-resection of the liver underwent liver resection at a high-volume center, whereas 23% underwent resection at a mid-volume center and 45.5% underwent resection at a low-volume center because the majority of the centers the perform cholecystectomy are low-volume centers.
At high- and mid-volume centers, respectively, 60% and 61% of patients with an indication for liver resection (T2-3 carcinomas) underwent the required liver resection. However, at low-volume centers, only 41% of the patients with an indication for liver resection (T2-3 carcinomas) underwent the required resection. Therefore, a substantial portion of gallbladder carcinomas was treated at low-volume centers, but low-volume centers perform liver resection significantly less often than high- or mid-volume centers in patients with T2-3 carcinomas, a tumor stage where liver resection is recommended by the S3 Guidelines and the literature[7,8,11,32-37]. The direct comparison using boxplots (Figure 1) of the three volume groups regarding T2-3 carcinomas shows that low-volume centers ignore the indication for liver resection significantly more often than not. In contrast, mid-volume centers perform liver resection significantly more often than not. High-volume centers show the same trend as mid-volume centers. To clarify these results, we have combined high- and mid-volume centers into one group and compared them to low-volume centers (Table 1, Figure 2). According to the mean values (Figure 2), high/mid-volume centers perform liver resection for T2-3 carcinomas significantly more often than low-volume centers. Table 1 shows that at high/mid-volume centers, 61% of patients with an indication for liver resection underwent liver resection, but at low-volume centers, significantly fewer patients (41%) with an indication for liver resection (T2-3 carcinomas) actually underwent the required liver resection.
We have previously shown the positive effect of liver-resection for T1b gallbladder carcinomas[30,31]. The corresponding analysis in the present patient cohort regarding volume centers and T1b carcinomas (n = 59) (Table 1) shows that 70% of the cases with T1b carcinoma at high-volume centers underwent liver resection, but less than 30% of T1b IGBCs at mid- or low-volume centers underwent liver surgery (P = 0.009). The results are more remarkable for Tb carcinomas, showing that only hospitals that are more comfortable performing liver surgery based on the larger number of liver resections per year perform radical cholecystectomy. Nevertheless, there is no clear recommendation by the German S3 Guidelines[8] to perform liver resection for T1b carcinomas, despite evidence in the literature[30,31] and guidelines from abroad[7].
The results show that the referral of patients from a low-volume center to a high- volume one has no practical relevance in the GR.
The division into high-, mid-, and low-volume centers with the cut-offs used here seems reasonable for German hospitals, and the results seem even more precise by combining high- and mid-volume centers into one group. However, there is definitely no clear data regarding the defining of the cut-offs.
The central problem is that the performance of a liver resection in IGBC patients in Germany depends much more on the hospital’s volume of liver surgery than on complying with the S3 Guidelines[8].
ACKNOWLEDGMENTS
The authors would like to thank the following: the CAMIC (surgical working group of minimally invasive surgeons) and CAES (surgical working group of endoscopy) of the German Society of Surgery as well as the clinics and colleagues supporting the Registry, as listed under www.ketteler-krankenhaus.de/register. The authors also would like to thank STATWORX-statistics Frankfurt Germany for statistical- analysis.
COMMENTS
Background
Generally incidental gallbladder carcinomas (IGBC) are identified after laparoscopy, and radical cholecystectomy including liver resection (LR) is needed in a second surgery. Stage-adjusted surgery including radical liver resection remains the only effective oncologic treatment for IGBC because of lack of effective alternatives such as chemo- or radiation therapy. It is not known whether the performance of the required LR for IGBC cases depends more on the experience of the hospitals in liver surgery than on complying with the recommendations of the guidelines.
Research frontiers
The current article addresses the adequate oncologic therapy for gallbladder carcinomas and its implementation into practice.
Innovations and breakthroughs
Guidelines for radical resection of gallbladder carcinoma vary worldwide. According to the current literature and the NCCN guidelines, radical cholecystectomy (RC) is recommended for up to T1b gallbladder carcinomas. According to the effective guidelines in Germany, RC is recommended for T2 carcinomas and more advanced stages. Unfortunately, the implementation of the guidelines into practice in Germany occurs in less than 50% of cases. The effort of a more radical surgery in IGBC cases in earlier stages based on the research results seems to be highly questionable if implementation of the guidelines is ignored to the detriment of the patients.
Applications
It is important that further research seeks to define high and low volume in liver surgery for implantation of cut offs because precise data are lacking. Additional articles are needed to address the problematic cut offs in liver surgery and the implementation of guidelines or innovations of research results into practice.
Terminology
High- and low- volume centers in surgery are defined according to the frequency of certain surgical techniques. It is difficult to define cut offs because there is no exact science. Large cohorts of patients (such as the German registry) are required to define such values and correlate such values with clinical results and their impact on clinical practice.
Peer review
These results based on the data from the Surgical Association of Endoscopy and Ultrasound and Minimally Invasive Surgery Central Registry of “IGBC” of the German Society of Surgery (the German Registry) and the liver resection study, in which the authors analyze the effect of centralization in surgery in implementing guidelines in oncological stage adjusted therapy for gallbladder carcinomas.
Footnotes
P- Reviewer: Tan CH, Zheng X S- Editor: Gou SX L- Editor: A E- Editor: Wang CH
References
- 1.Varshney S, Butturini G, Gupta R. Incidental carcinoma of the gallbladder. Eur J Surg Oncol. 2002;28:4–10. doi: 10.1053/ejso.2001.1175. [DOI] [PubMed] [Google Scholar]
- 2.Wullstein C, Woeste G, Barkhausen S, Gross E, Hopt UT. Do complications related to laparoscopic cholecystectomy influence the prognosis of gallbladder cancer? Surg Endosc. 2002;16:828–832. doi: 10.1007/s00464-001-9085-7. [DOI] [PubMed] [Google Scholar]
- 3.Benoist S, Panis Y, Fagniez PL. Long-term results after curative resection for carcinoma of the gallbladder. French University Association for Surgical Research. Am J Surg. 1998;175:118–122. doi: 10.1016/s0002-9610(97)00269-9. [DOI] [PubMed] [Google Scholar]
- 4.Kohya N, Miyazaki K. Hepatectomy of segment 4a and 5 combined with extra-hepatic bile duct resection for T2 and T3 gallbladder carcinoma. J Surg Oncol. 2008;97:498–502. doi: 10.1002/jso.20982. [DOI] [PubMed] [Google Scholar]
- 5.Shih SP, Schulick RD, Cameron JL, Lillemoe KD, Pitt HA, Choti MA, Campbell KA, Yeo CJ, Talamini MA. Gallbladder cancer: the role of laparoscopy and radical resection. Ann Surg. 2007;245:893–901. doi: 10.1097/SLA.0b013e31806beec2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goetze TO, Paolucci V. Adequate extent in radical re-resection of incidental gallbladder carcinoma: analysis of the German Registry. Surg Endosc. 2010;24:2156–2164. doi: 10.1007/s00464-010-0914-4. [DOI] [PubMed] [Google Scholar]
- 7.Benson AB, Abrams TA, Ben-Josef E, Bloomston PM, Botha JF, Clary BM, Covey A, Curley SA, D’Angelica MI, Davila R, et al. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Canc Netw. 2009;7:350–391. doi: 10.6004/jnccn.2009.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lammert F, Neubrand MW, Bittner R, Feussner H, Greiner L, Hagenmüller F, Kiehne KH, Ludwig K, Neuhaus H, Paumgartner G, et al. [S3-guidelines for diagnosis and treatment of gallstones. German Society for Digestive and Metabolic Diseases and German Society for Surgery of the Alimentary Tract] Z Gastroenterol. 2007;45:971–1001. doi: 10.1055/s-2007-963437. [DOI] [PubMed] [Google Scholar]
- 9.Jensen EH, Abraham A, Habermann EB, Al-Refaie WB, Vickers SM, Virnig BA, Tuttle TM. A critical analysis of the surgical management of early-stage gallbladder cancer in the United States. J Gastrointest Surg. 2009;13:722–727. doi: 10.1007/s11605-008-0772-8. [DOI] [PubMed] [Google Scholar]
- 10.Wright BE, Lee CC, Iddings DM, Kavanagh M, Bilchik AJ. Management of T2 gallbladder cancer: are practice patterns consistent with national recommendations? Am J Surg. 2007;194:820–85; discussion 820-85;. doi: 10.1016/j.amjsurg.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 11.Goetze TO, Paolucci V. Benefits of reoperation of T2 and more advanced incidental gallbladder carcinoma: analysis of the German registry. Ann Surg. 2008;247:104–108. doi: 10.1097/SLA.0b013e318154bf5d. [DOI] [PubMed] [Google Scholar]
- 12.Nagasue N, Yukaya H. Liver resection for hepatocellular carcinoma: results from 150 consecutive patients. Cancer Chemother Pharmacol. 1989;23 Suppl:S78–S82. doi: 10.1007/BF00647246. [DOI] [PubMed] [Google Scholar]
- 13.Fan ST, Lai EC, Lo CM, Ng IO, Wong J. Hospital mortality of major hepatectomy for hepatocellular carcinoma associated with cirrhosis. Arch Surg. 1995;130:198–203. doi: 10.1001/archsurg.1995.01430020088017. [DOI] [PubMed] [Google Scholar]
- 14.Thompson HH, Tompkins RK, Longmire WP. Major hepatic resection. A 25-year experience. Ann Surg. 1983;197:375–388. doi: 10.1097/00000658-198304000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vauthey JN, Pawlik TM, Abdalla EK, Arens JF, Nemr RA, Wei SH, Kennamer DL, Ellis LM, Curley SA. Is extended hepatectomy for hepatobiliary malignancy justified? Ann Surg. 2004;239:722–30; discussion 730-2. doi: 10.1097/01.sla.0000124385.83887.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torzilli G, Makuuchi M, Inoue K, Takayama T, Sakamoto Y, Sugawara Y, Kubota K, Zucchi A. No-mortality liver resection for hepatocellular carcinoma in cirrhotic and noncirrhotic patients: is there a way? A prospective analysis of our approach. Arch Surg. 1999;134:984–992. doi: 10.1001/archsurg.134.9.984. [DOI] [PubMed] [Google Scholar]
- 17.Asiyanbola B, Chang D, Gleisner AL, Nathan H, Choti MA, Schulick RD, Pawlik TM. Operative mortality after hepatic resection: are literature-based rates broadly applicable? J Gastrointest Surg. 2008;12:842–851. doi: 10.1007/s11605-008-0494-y. [DOI] [PubMed] [Google Scholar]
- 18.Csikesz NG, Simons JP, Tseng JF, Shah SA. Surgical specialization and operative mortality in hepato-pancreatico-biliary (HPB) surgery. J Gastrointest Surg. 2008;12:1534–1539. doi: 10.1007/s11605-008-0566-z. [DOI] [PubMed] [Google Scholar]
- 19.Eppsteiner RW, Csikesz NG, Simons JP, Tseng JF, Shah SA. High volume and outcome after liver resection: surgeon or center? J Gastrointest Surg. 2008;12:1709–116; discussion 1716. doi: 10.1007/s11605-008-0627-3. [DOI] [PubMed] [Google Scholar]
- 20.Fong Y, Gonen M, Rubin D, Radzyner M, Brennan MF. Long-term survival is superior after resection for cancer in high-volume centers. Ann Surg. 2005;242:540–54; discussion 540-54;. doi: 10.1097/01.sla.0000184190.20289.4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langer B. Role of volume outcome data in assuring quality in HPB surgery. HPB (Oxford) 2007;9:330–334. doi: 10.1080/13651820701611234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hillner BE, Smith TJ. Report of the Cancer Care Ontario task force on regionalization of pancreatic cancer surgery in Ontario. Criteria for the Delivery of Pancreatic Cancer Surgery, March 1999. Available from: http://www.iom.edu/~/media/Files/Activity Files/Disease/NCPF/mcvqual.pdf.
- 23.Cancer Care Ontario. Hepatic, Pancreatic and Biliary (HPB) Surgical Oncology Standards, June 2006. [accessed July 20, 2007] Available from: http://www.cancercare.on.ca/index_SurgicalOncology.htm.
- 24.Glasgow RE, Showstack JA, Katz PP, Corvera CU, Warren RS, Mulvihill SJ. The relationship between hospital volume and outcomes of hepatic resection for hepatocellular carcinoma. Arch Surg. 1999;134:30–35. doi: 10.1001/archsurg.134.1.30. [DOI] [PubMed] [Google Scholar]
- 25.Finlayson EV, Goodney PP, Birkmeyer JD. Hospital volume and operative mortality in cancer surgery: a national study. Arch Surg. 2003;138:721–75; discussion 726. doi: 10.1001/archsurg.138.7.721. [DOI] [PubMed] [Google Scholar]
- 26.Schrag D, Cramer LD, Bach PB, Cohen AM, Warren JL, Begg CB. Influence of hospital procedure volume on outcomes following surgery for colon cancer. JAMA. 2000;284:3028–3035. doi: 10.1001/jama.284.23.3028. [DOI] [PubMed] [Google Scholar]
- 27.van Heek NT, Kuhlmann KF, Scholten RJ, de Castro SM, Busch OR, van Gulik TM, Obertop H, Gouma DJ. Hospital volume and mortality after pancreatic resection: a systematic review and an evaluation of intervention in the Netherlands. Ann Surg. 2005;242:781–78, discussion 781-78. doi: 10.1097/01.sla.0000188462.00249.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schumpelick V, Manegold BC, Paolucci V. Zentralregister “laparoskopische Tumordissemination und Portmetastasen”. Dtsch Gesellsch Chirurg Mitt. 1997;2:133. [Google Scholar]
- 29.Sobin LH, Wittekind C. TNM classification of malignant tumors (UICC), 6th ed. Wiley-Blackwell, New York: UICC; 2002. [Google Scholar]
- 30.Goetze TO, Paolucci V. Immediate re-resection of T1 incidental gallbladder carcinomas: a survival analysis of the German Registry. Surg Endosc. 2008;22:2462–2465. doi: 10.1007/s00464-008-9747-9. [DOI] [PubMed] [Google Scholar]
- 31.Goetze TO, Paolucci V. [Immediate Radical Re-Resection of Incidental T1b Gallbladder Cancer and the Problem of an Adequate Extent of Resection (Results of the German Registry “Incidental Gallbladder Cancer”).] Zentralbl Chir. 2011:Epub ahead of print. doi: 10.1055/s-0030-1262698. [DOI] [PubMed] [Google Scholar]
- 32.Shirai Y, Yoshida K, Tsukada K, Muto T. Inapparent carcinoma of the gallbladder. An appraisal of a radical second operation after simple cholecystectomy. Ann Surg. 1992;215:326–331. doi: 10.1097/00000658-199204000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fong Y, Jarnagin W, Blumgart LH. Gallbladder cancer: comparison of patients presenting initially for definitive operation with those presenting after prior noncurative intervention. Ann Surg. 2000;232:557–569. doi: 10.1097/00000658-200010000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ouchi K, Owada Y, Matsuno S, Sato T. Prognostic factors in the surgical treatment of gallbladder carcinoma. Surgery. 1987;101:731–737. [PubMed] [Google Scholar]
- 35.Kondo S, Nimura Y, Hayakawa N, Kamiya J, Nagino M, Uesaka K. Regional and para-aortic lymphadenectomy in radical surgery for advanced gallbladder carcinoma. Br J Surg. 2000;87:418–422. doi: 10.1046/j.1365-2168.2000.01384.x. [DOI] [PubMed] [Google Scholar]
- 36.Ogura Y, Mizumoto R, Isaji S, Kusuda T, Matsuda S, Tabata M. Radical operations for carcinoma of the gallbladder: present status in Japan. World J Surg. 1991;15:337–343. doi: 10.1007/BF01658725. [DOI] [PubMed] [Google Scholar]
- 37.Mekeel KL, Hemming AW. Surgical management of gallbladder carcinoma: a review. J Gastrointest Surg. 2007;11:1188–1193. doi: 10.1007/s11605-007-0115-1. [DOI] [PubMed] [Google Scholar]


