Abstract
AIM: To study the clinical efficacy of traditional Chinese medicine (TCM) intervention “tonifying the kidney to promote liver regeneration and repair by affecting stem cells and their microenvironment” (“TTK”) for treating liver failure due to chronic hepatitis B.
METHODS: We designed the study as a randomized controlled clinical trial. Registration number of Chinese Clinical Trial Registry is ChiCTR-TRC-12002961. A total of 144 patients with liver failure due to infection with chronic hepatitis B virus were enrolled in this randomized controlled clinical study. Participants were randomly assigned to the following three groups: (1) a modern medicine control group (MMC group, 36 patients); (2) a “tonifying qi and detoxification” (“TQD”) group (72 patients); and (3) a “tonifying the kidney to promote liver regeneration and repair by affecting stem cells and their microenvironment” (“TTK”) group (36 patients). Patients in the MMC group received general internal medicine treatment; patients in the “TQD” group were given a TCM formula “tonifying qi and detoxification” and general internal medicine treatment; patients in the “TTK” group were given a TCM formula of “TTK” and general internal medicine treatment. All participants were treated for 8 wk and then followed at 48 wk following their final treatment. The primary efficacy end point was the patient fatality rate in each group. Measurements of various virological and biochemical indicators served as secondary endpoints. The one-way analysis of variance and the t-test were used to compare patient outcomes in the different treatment groups.
RESULTS: At the 48-wk post-treatment time point, the patient fatality rates in the MMC, “TQD”, and “TTK” groups were 51.61%, 35.38%, and 16.67%, respectively, and the differences between groups were statistically significant (P < 0.05). However, there were no significant differences in the levels of hepatitis B virus DNA or prothrombin activity among the three groups (P > 0.05). Patients in the “TTK” group had significantly higher levels of serum total bilirubin compared to MMC subjects (339.40 μmol/L ± 270.09 μmol/L vs 176.13 μmol/L ± 185.70 μmol/L, P = 0.014). Serum albumin levels were significantly increased in both the “TQD” group and “TTK” group as compared with the MMC group (31.30 g/L ± 4.77 g/L, 30.72 g/L ± 2.89 g/L vs 28.57 g/L ± 4.56 g/L, P < 0.05). There were no significant differences in levels of alanine transaminase among the three groups (P > 0.05). Safety data showed that there was one case of stomachache in the “TQD” group and one case of gastrointestinal side effect in the “TTK” group.
CONCLUSION: Treatment with “TTK” improved the survival rates of patients with liver failure due to chronic hepatitis B. Additionally, liver tissue was regenerated and liver function was restored.
Keywords: Clinical study, “Tonifying the kidney to promote liver regeneration and repair by affecting stem cells and their microenvironment” (“TTK”), Liver regeneration, Treatment with integrated traditional and Western medicine, Chronic hepatitis B-associated liver failure
Core tip: We conducted a randomized controlled clinical trial to observe the effects of traditional Chinese medicine intervention “tonifying the kidney to promote liver regeneration and repair by affecting stem cells and their microenvironment” (“TTK”) in treating liver failure due to chronic hepatitis B virus infection. The fatality rate in the group treated with “TTK” was significantly lower than those in the other two groups (16.67% vs 51.61%, 35.38%, P = 0.010). The mechanism for this effect may be related to promotion of liver regeneration and repair through affecting stem cells and their microenvironment.
INTRODUCTION
The fatality rate of patients with chronic hepatitis B-associated liver failure (CHBLF) can reach 70%, and survivors of this disease have very high recurrence rates[1]. In recent years, the fatality rate among CHBLF patients has been significantly reduced[2] by treatments using integrated methods of traditional and Western medicine. Our previous studies[3-5] demonstrated that the traditional Chinese medicine (TCM) methodology “TTK” (“tonifying the kidney to promote liver regeneration and repair by affecting stem cells and their microenvironment”) promotes liver regeneration and repair by regulating stem cells and their microenvironment[6]. In the present study, we conducted a randomized controlled clinical study to evaluate the efficacy of the TCM intervention “TTK” in treating CHBLF.
MATERIALS AND METHODS
Study design
We conducted this randomized controlled clinical trial for CHBLF between January 2007 and July 2013 at six different clinical sites (Hubei Provincial Hospital of TCM; Wuhan Medical Treatment Center; Wuhan No. 1 Hospital; Wuhan No. 7 Hospital; Wuhan Hospital of TCM; Zhongnan Hospital of Wuhan University) in the Hubei Province of China. The Ethics Committee of Hubei Province Hospital of Traditional Chinese Medicine reviewed and approved the protocol and patient consent form prior to initiation of the study (approval number, 2006001). All participants provided their written informed consent prior to enrollment. A total of 144 participants with a confirmed diagnosis of CHBLF were randomly assigned to three different treatment groups in a ratio of 1:2:1 using a computer-based random number generation program. The three groups consisted of a modern medicine control group (MMC group), a “tonifying qi and detoxification” (“TQD”) group, and a group designated as “tonifying the kidney to promote liver regeneration and repair by affecting stem cells and their microenvironment” (“TTK” group). A total of 12 patients (five in the MMC group and seven in the “TQD” group) did not complete the study; therefore, 132 cases (112 men and 20 women) were included in the final statistical analysis. The patients in the three groups showed similar characteristics at baseline (Table 1). The study flow diagram is shown in Figure 1.
Table 1.
Baseline characteristics of the patients n (%)
| Characteristic | MMC group (n = 31) | “TQD” group (n = 65) | “TTK” group (n = 36) | P value |
| Age, yr, mean ± SD | 43.71 ± 9.85 | 44.94 ± 12.64 | 46.69 ± 11.86 | 0.580 |
| Male | 29 (93.55) | 51 (78.46) | 32 (88.89) | 0.114 |
| Course of disease, yr, mean ± SD | 9.87 ± 10.93 | 8.74 ± 8.48 | 10.03 ± 9.21 | 0.753 |
| Chronic liver failure | 15 (48.39) | 35 (53.85) | 14 (38.89) | 0.354 |
MMC group: Modern medicine control group; “TQD” group: “Tonifying qi and detoxification” group.
Figure 1.
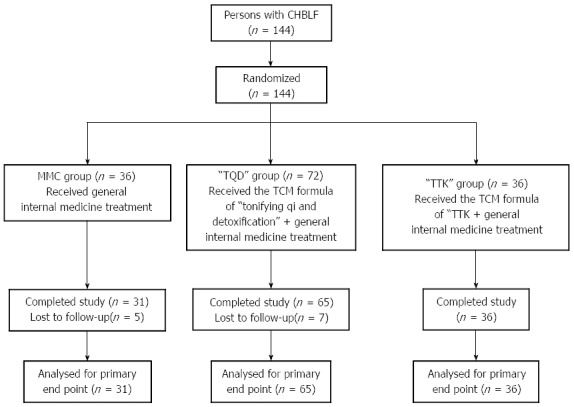
Study flow diagram. MMC group: Modern medicine control group; “TQD” group: “Tonifying qi and detoxification” group. The group was treated with the traditional Chinese medicine (TCM) formula “tonifying qi and detoxification” (“TQD”) as well as general internal medicine therapy; “TTK” group: “Tonifying the kidney to promote liver regeneration and repair by affecting stem cells and their microenvironment” group. The group was treated with the TCM formula “tonifying the kidney to promote liver regeneration and repair by affecting stem cells and their microenvironment” (“TTK”) as well as general internal medicine treatment.
Patient enrollment
All patients enrolled in the study had been diagnosed with CHBLF, and chronic liver failure (CLF) and acute-on-CLF (ACLF) were the two most common types of CHBLF. All participants had been admitted to a hospital where they could be quarantined and observed. Patients who fulfilled the following criteria were included in the study.
First, each enrolled patient was required to present with a liver disease that satisfied the following definition of CHBLF based on the diagnostic and treatment guidelines for liver failure established in 2006[7]: CLF is defined as liver function which becomes progressively dysfunctional or decompensated due to the presence of hepatic cirrhosis. For our study, patients who fulfilled the following criteria were diagnosed with CLF: (1) patients with ascites or other manifestations of portal hypertension; (2) patients with/without hepatic encephalopathy; (3) patients with TBIL levels increased and/or ALB levels decreased vs normal levels; and (4) patients with coagulation disorders (PTA ≤ 40%). ACLF is the main clinical manifestation of short-term acute hepatic decompensation following a history of chronic liver disease, and can be divided into categories of early stage, middle stage, and late stage. Patients who fulfilled the following criteria were diagnosed with early stage ACLF: patients with extreme fatigue and serious digestive symptoms (significant anorexia, vomiting or abdominal distension); patients with progressive deepening jaundice (TBIL ≥ 171 μmol/L, or daily TBIL increases ≥ 17.1 μmol/L); patients with a tendency to bleed (30% < PTA < 40%); patients without hepatic encephalopathy or significant ascites. Patients who satisfied one of the following criteria as well as symptoms of early stage were diagnosed with middle stage ACLF: patients with hepatic encephalopathy (≤ degree II) and/or significant ascites; patients with a tendency to bleed (hemorrhagic spot or ecchymosis) and 20% < PTA ≤ 30%. Patients who satisfied one of the following criteria as well as symptoms of middle stage were diagnosed with late stage ACLF: patients with intractable complications (hepatorenal syndrome, upper gastrointestinal bleeding, serious infections, serious electrolyte imbalance, etc.); patients with hepatic encephalopathy ≥ degree III); patients with a strong tendency to bleed (e.g., ecchymoses at injection sites) and a PTA ≤ 20%. The second criterion for inclusion was that the patient must have volunteered to sign an informed consent document.
Patients who fulfilled any of the following criteria were excluded from the study: patients with acute hepatic failure; patients with chronic hepatic failure concurrent with a disease other than chronic hepatitis B; patients who were lactating or pregnant; patients with primary hepatocellular carcinoma; patients with a history of chronic illegal drug use; patients complicated with other severe systematic diseases or mental diseases; patients showing a positive HIV test; patients complicated with cytomegalovirus, EB virus, or some other hepatotropic virus infection; patients who had participated in another clinical study within the previous three months; patients who had previously demonstrated poor compliance, or could not guarantee completing the protocol; patients complicated with severe cerebral edema, a severe infection, type 1 hepatorenal syndrome or massive hemorrhage of the gastrointestinal tract, etc.
Treatment
All patients in the three treatment groups received eight weeks of treatment and were followed at 48 wk after their final treatment.
Patients in the MMC group were treated with general internal medicine techniques which included basic, symptomatic, and supportive treatment plus antiviral therapy. The drugs used to treat CHBLF in the MMC group included compound glycyrrhizin for injection (80-160 mg, once daily, intravenous drip), reduced glutathione for injection (1.2 g, once daily, intravenous drip), N-acetylcysteine (4.0 g, once daily, intravenous drip), and hepatocyte growth-promoting factor for injection (100-160 mg, once daily, intravenous drip). Efforts were also made to prevent and treat complications such as hepatic encephalopathy, cerebral edema, hepatorenal syndrome, infection, and hemorrhage of the digestive tract, etc.). The need for antiviral therapy was determined by attending physicians. Patients who tested positive for hepatitis B virus (HBV) DNA were administered nucleoside drugs such as lamivudine tablets, adefovir dipivoxil tablets, entecavir tablets or telbivudine tablets as antiviral therapy. There were no differences among the three groups of patients regarding treatment with nucleoside drugs (P = 0.153) (Table 2).
Table 2.
Antiviral therapy in groups n (%)
| Nucleoside drugs | MMC group (n = 31) | “TQD” group (n = 65) | “TTK” group (n = 36) |
| Not using nucleoside drugs | 21 (67.74) | 55 (84.62) | 27 (75.00) |
| Using nucleoside drugs | 10 (32.26) | 10 (15.38) | 9 (25.00) |
| Lamivudine | 12 (38.71) | 30 (46.15) | 12 (33.33) |
| Adefovir | 0 (0.00) | 5 (7.69) | 4 (11.11) |
| Telbivudine | 2 (6.45) | 6 (9.23) | 1 (2.78) |
| Entecavi | 6 (19.35) | 12 (18.46) | 5 (13.89) |
| Lamivudine combined with adefovir | 1 (3.23) | 1 (1.54) | 5 (13.89) |
| Entecavir combined with adefovir | 0 (0.00) | 1 (1.54) | 0 (0.00) |
There was no difference among the three groups in using nucleoside drugs in the study (P = 0.153).
Patients in the “TQD” group were given the TCM formula “tonifying qi and detoxification” (“TQD”) as well as general internal medicine therapy. The composition of the TCM formula of “TQD” (Table 3) was as follows: zhihuangqi (Astmgali Radix Praeparata cum Melle) (30 g), huzhang (Polygoni Cuspidati Rhizoma et Radix) (30-60 g), fuling (Poria) (30 g), danshen (Salviae Miltiorrhizae Radix et Rhizoma) (30 g), yimucao (Leonuri Herba) (30 g), zhuling (Polyporus) (20 g), chaobaizhu (stir-baked Atractylodis Macrocephalae Rhizoma) (30 g), yinchen (Artemisiae Scopariae Herba) (30-60 g), zhizi (Gardeniae Fructus) (12 g), huangqin (Scutellariae Radix) (6 g), dahuang (Rhei Radix et Rhizoma) (10 g), and gancao (Glycyrrhizae Radix et Rhizoma) (6 g).
Table 3.
Traditional Chinese medicine formula of “tonifying qi and detoxification”
| English translation | Chinese pinyin | Dosage |
| Astmgali Radix Praeparata cum Melle | Zhihuangqi | 30 g |
| Polygoni Cuspidati Rhizoma et Radix | Huzhang | 30-60 g |
| Poria | Fuling | 30 g |
| Salviae Miltiorrhizae Radix et Rhizoma | Danshen | 30 g |
| Leonuri Herba | Yimucao | 30 g |
| Polyporus | Zhuling | 20 g |
| Stir-baked Atractylodis Macrocephalae Rhizoma | Chaobaizhu | 30 g |
| Artemisiae ScopariaeHerba | Yinchen | 30-60 g |
| Gardeniae Fructus | Zhizi | 12 g |
| Scutellariae Radix | Huangqin | 6 g |
| Rhei Radix et Rhizoma | Dahuang | 10 g |
| Glycyrrhizae Radix et Rhizoma | Gancao | 6 g |
The TCM formula “TQD” was slightly altered for patients with different symptoms. “TQD” formula supplemented with 6 g of chenxiang (Aquilariae Lignum Resinatum) and 30 g of laifuzi (Raphani Semen) were used to treat patients with severe abdominal distension. Jiaomaiya (charred Hordei Fructus Germinatus) (10 g), jiaoshanzha (charred Crataegi Fructus) (10 g), jiaoshenqu (charred Medicated Leaven) (10 g) or jineijin (Gigeriae Galli Endothelium Corneum) (20 g) was added to “TQD” for treating patients with poor appetite. Jiangbanxia (Pinelliae Rhizoma Praeparatum cum Zingibere et Alumine) (15 g), chenpi (Citri Reticulatae Pericarpium) (15 g) or zhuru (Bambusae Caulis in Taenias) (15 g) was added to “TQD” for treating patients with nausea and vomiting. Chaoyiyiren (stir-baked Coicis Semen) (30 g) was added for patients with diarrhea or loose stools. Mudanpi (Moutan Cortex) (20 g) and qinjiao (Gentianae Macrophyllae Radix) (20 g) were added for patients with skin itch. Baimaogen (Imperatae Rhizoma) (15 g) and zicao (Arnebiae Radix) (30 g) were added for patients with epistaxis, bleeding gums or skin ecchymosis. The Chinese patent drug “chidantuihuang soluble granules” (SFDA Approval No. Z20010176, Hunan Jiuzhitang Co., Ltd.) (10 g dissolved in tepid water) was added for patients with severe jaundice.
Patients in the “TTK” group were given the TCM formula “TTK” as well as general internal medicine therapy. The TCM formula of “TTK” (Table 4) was mainly composed of following herbs: shudihuang (Rehmanniae Radix Praeparata) (15-30 g), yinchen (Artemisiae Scopariae Herba) (30-60 g), wuweizi (Schisandrae Chinensis Fructus) (10-15 g), jianghuang (Curcumae Longae Rhizoma) (3-6 g), gancao (Glycyrrhizae Radix et Rhizoma) (9-12 g), shanyao (Rhizoma Dioscoreae) (15 g), gouqizi (Fructus Lycii) (15 g), shanzhuyu (Fructus Corni) (15 g), tusizi (Cuscutae Semen) (10 g), fuling (Poria) (30 g), mudanpi (Moutan Cortex) (10 g), and zexie (Alismatis Rhizoma) (10 g).
Table 4.
Traditional Chinese medicine formula of “tonifying the kidney to promote liver regeneration and repair by affecting stem cells and their microenvironment”
| English translation | Chinese Pinyin | Dosage |
| Rehmanniae Radix Praeparata | Shudihuang | 15-30 g |
| Artemisiae Scopariae Herba | Yinchen | 30-60 g |
| Schisandrae Chinensis Fructus | Wuweizi | 10-15 g |
| Curcumae Longae Rhizoma | Jianghuang | 3-6 g |
| Glycyrrhizae Radix et Rhizoma | Gancao | 9-12 g |
| Rhizoma Dioscoreae | Shanyao | 15 g |
| Fructus Lycii | Gouqizi | 15 g |
| Fructus Corni | Shanzhuyu | 15 g |
| Cuscutae Semen | Tusizi | 10 g |
| Poria | Fulin | 30 g |
| Moutan Cortex | Mudanpi | 10 g |
| Alismatis Rhizoma | Zexie | 10 g |
The TCM formula “TTK” was modified for patients with different symptoms. Binglang (Arecae Semen) (10 g) and dafupi (Arecae Pericarpium) (10 g) were added for patients with abdominal distension. Jiaoshenqu (charred Medicated Leaven) (10 g), dangshen (Codonopsis Radix) (15 g), or chaobaizhu (stir-baked Atractylodis Macrocephalae Rhizoma) (10 g) was added for patients with poor appetite. Jiangbanxia (Pinelliae Rhizoma Praeparatum cum Zingibere et Alumine) (15 g) or zhuru (Bambusae Caulis in Taenias) (15 g) was added for patients with nausea and vomiting. Ganjiang (Zingiberis Rhizoma) (10 g), huanglian (Coptidis Rhizoma) (6 g), and Huangqin (Scutellariae Radix) (10 g) were added for patients with diarrhea or loose stools. Qiancao (Rubiae Radix et Rhizoma) (15 g) was added for patients with epistaxis, bleeding gums or skin ecchymosis. Shudihuang (Rehmanniae Radix Praeparata) was removed from “TTK”, and dahuang (Rhei Radix et Rhizoma) (6 g) plus zhizi (Gardeniae Fructus) (10 g) were added for treating patients with a thick and greasy yellow coating on their tongue.
All Chinese medicines used in the study were provided by Hubei Tianji Chinese Herbal Sliced Medicine Co.,Ltd. The medicines were tested for quality, and met the standards used in China. When using the traditional TCM decoction method, each unit of TCM formula yielded 260 mL of decoction. An oral dose of warm decoction (130 mL) was administered to patients twice daily.
Study assessments
The primary efficacy end point in this study was patient fatality rate in the different treatment groups during the time period starting from randomization and ending at the follow-up visit. Secondary endpoints were values for certain virological and biochemical indicators at baseline and after 8 wk of treatment; these included levels of HBV DNA, prothrombin activity (PTA), serum total bilirubin (TBIL), albumin (ALB), and alanine transaminase (ALT). Real-time PCR was used to test for the presence of HBV DNA. Specialized technicians used an AMAX-200 automatic coagulation analyzer (paramagnetic particle method; Trinity Biotech, Germany) and the corresponding control sera and reagents to measure PTA. A Toshiba 120 automatic biochemical analyzer and ancillary reagents were used to measure levels of TBIL, ALB, and ALT.
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 19.0 (Armonk, NY; IBM Corp). Data are expressed as mean ± SD. The χ2 test was used for analysis of numeration data. Differences between groups were analyzed using one-way analysis of variance and the Student’s t-test. P-values < 0.05 were considered statistically significant.
Safety
The two TCM formulas used in the current study had been frequently used before, and had not been found to be associated with serious adverse events. Only two patients in our study experienced an unexpected effect of medication. One patient in the “TQD” group reported a stomachache, and a patient in the “TTK” group reported vomiting.
RESULTS
Fatality rate
At the 48-wk follow-up, fatality rates in the MMC, “TQD”, and “TTK” groups were 51.61%, 35.38%, and 16.67%, respectively; and the differences between groups were statistically significant (P < 0.05) (Figure 2). Fatality was lowest in the “TTK” group (16.67%), and the fatality rate in the “TTK” group was significantly lower than those in the MMC group (16.67% vs 51.61%, P = 0.002) and “TQD” group (16.67% vs 35.38%, P = 0.046).
Figure 2.
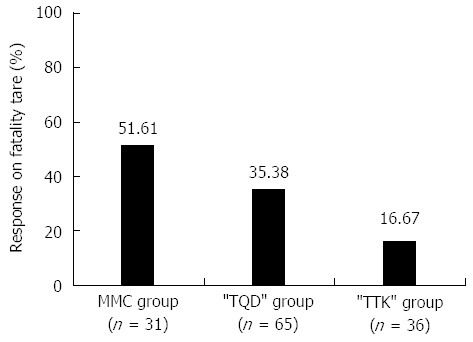
Fatality rates after 8-wk treatment and at 48-wk follow-up%. The figure showed the fatality rates of three groups: the fatality rate was lowest in the “TTK” group (16.67%); the fatality rate in the “TTK” group was significantly lower than those in the MMC group (16.67% vs 51.61%, P = 0.002) and “TQD” group (16.67% vs 35.38%, P = 0.046).
HBV DNA
Patient test results showing that HBV DNA was absent or had decreased by ≥ 2 logarithms were considered to be positive clinical results. However, after 8 wk of treatment, there were no significant differences in levels of HBV DNA among the three groups (P > 0.05) (Table 5).
Table 5.
Hepatitis B virus DNA after 8-wk treatment n (%)
| Group | n | Negative | Positive |
| MMC | 6 | 3 (50.0) | 3 (50.0) |
| “TQD” | 17 | 4 (23.5) | 13 (76.5) |
| “TTK” | 6 | 2 (33.3) | 4 (66.7) |
Normal range of HBV DNA (FQ-PCR): < 1.0 × 103 copies/mL. Positive result: HBV DNA was absent or had decreased by ≥ 2 logarithms. HBV: Hepatitis B virus.
Biochemical results
Table 6 showed biochemical results. After 8 wk of treatment, there were no statistically significant differences in PTA levels among the three groups (P > 0.05). However, the “TTK” group and MMC group showed a statistically significant difference in their TBIL levels following 8 wk of treatment (339.40 μmol/L ± 270.09 μmol/L vs 176.13 μmol/L ± 185.70 μmol/L, t = -2.552, P = 0.014). Additionally, ALB levels in the “TQD” group and “TTK” group were significantly higher compared with those in the MMC group (31.30 g/L ± 4.77 g/L vs 28.57 g/L ± 4.56 g/L, t = -2.389, P = 0.019 and 30.72 g/L ± 2.89 g/L vs 28.57 g/L ± 4.56 g/L, t = -2.378, P = 0.021). There were no significant differences in ALT levels among the three groups after treatment (P > 0.05).
Table 6.
Biochemical indicators after 8-wk treatment (mean ± SD)
| Group | Time | n | PTA (%) | n | TBIL (µmol/L) | n | ALB (g/L) | n | ALT (IU/L) |
| MMC | Before treatment | 28 | 26.80 ± 10.91 | 30 | 326.29 ± 210.47 | 28 | 28.07 ± 4.56 | 30 | 202.00 ± 249.20 |
| After treatment | 18 | 43.14 ± 18.60 | 21 | 176.13 ± 185.70 | 21 | 28.57 ± 4.56 | 21 | 39.90 ± 30.19 | |
| “TQD” | Before treatment | 61 | 32.50 ± 12.15 | 65 | 314.04 ± 160.20 | 65 | 29.54 ± 4.75 | 65 | 254.80 ± 424.32 |
| After treatment | 55 | 43.59 ± 22.08 | 63 | 242.54 ± 229.05 | 63 | 31.30 ± 4.77c | 63 | 78.70 ± 161.80 | |
| “TTK” | Before treatment | 36 | 28.63 ± 11.28 | 36 | 369.13 ± 198.06 | 36 | 27.82 ± 4.52 | 36 | 189.52 ± 241.97 |
| After treatment | 30 | 32.55 ± 15.58 | 33 | 339.40 ± 270.09a | 33 | 30.72 ± 2.89e | 33 | 70.12 ± 82.23 |
Normal range: PTA: 80%-120%; TBIL: 3.4-20.5 μmol/L; ALB: 35-55 g/L; ALT: 0-46 IU/L.
P < 0.05 vs MMC group;
P < 0.05 vs MMC group,
P < 0.05 vs MMC group.
DISCUSSION
While drugs and alcohol are the major pathogenic factors associated with liver failure in Western countries[8], the hepatitis B virus is the major cause of liver failure in China.CLF and ACLF are two common types of CHBLF. The pathogenesis of CHBLF is complex and has not been clarified clear until now. Currently, it is believed that interactions among different viral factors and host factors stimulate development of CHBLF. Viral factors mainly include the virus genotype, virus replication level, and the presence of viral mutations, etc. Host factors include genetic factors, mechanisms of immunopathological injury, and abnormal liver regeneration[9,10].
CHBLF is often accompanied by various complications and has a high rate of morality. To date, there is no effective modern medical therapy for treating CHBLF, and symptomatic and supportive therapies constitute the major methods of treatment. However, with the development of new medical treatments and antiviral drugs, the rates of patient fatality due to CHBLF have decreased by 30% to 50%[2]. All patients in our study received the same general internal medicine treatment, and some patients were also given antiviral drugs. However, there were no significant differences in the usage rate of antiviral drugs among the three groups (P > 0.05) (Table 2). Regarding the primary efficacy endpoint in this clinical study (patient fatality), we found that fatality due to CHBLF in the group treated with the integrated traditional and Western medicine program and the TCM formula “TTK” was significantly lower compared with morality rates in the MMC and “TQD” groups (16.67% vs 51.61%, 35.38%, P < 0.05). Additionally, ALB levels in the “TTK” group were significantly increased compared with those in the MMC group (30.72 ± 2.89 vs 28.57 ± 4.56, P = 0.021). All of the above results suggest the beneficial effects of “TTK” in treatment of CHBLF.
We believe that an imbalance between liver damage and liver regeneration (heavy damage and insufficient regeneration) is an important mechanism for CHBLF. Liver regeneration is the vital aspect of recovery in patients with liver failure. If damaged liver tissue is not replaced with normal tissue in a timely manner, the patient will die. However, if damaged liver tissue can regenerate in sufficient time, normal liver function can be restored, and the patient will survive[11,12].
Although the “TQD” group and “TTK” group were both treated with integrated traditional and Western medicine programs, the TCM formulae used in the two groups were different. We found that the formula “TTK” was more efficacious for treating CHBLF. The formula “TQD” is conventionally used in TCM for treating CHBLF, while “TTK” is a new formula of TCM, and can be used for treating CHBLF and reducing liver damage. At the same time, the TCM formula “TTK” can promote regeneration of normal tissue, inhibit regeneration of abnormal tissue, and thus restore the balance between damage and regeneration by affecting stem cells (liver stem cells[13,14], bone marrow stem cells[3,5,15-18], brain marrow stem cells[19-22], etc.) and their microenvironments. Finally, “TTK” stimulates reconstruction of damaged liver tissue to partially restore tissue function, and thereby reduces patient fatality and improves the patient’s quality of life.
Our previous studies showed that “TTK” can stimulate the transformation of bone marrow stem cells into liver cells, and may act by affecting the expression of genes in liver tissue[3,5,15,23]. We used the “MSG-regeneration-rat” model[24] to explore relevant connections between liver regeneration and various activities of the central nervous system/hypothalamus-hypophysis-liver axis/nerve-endocrine-immune network. Our results revealed that “TTK” has a bidirectional regulatory role in liver regeneration, as well as a beneficial role in orderly recovery from liver damage[20,21,25-29].
Our current multi-center clinical study did not use a double-blind design; therefore, the results may have been affected by observer bias. A large-scale, multi-center, randomized, controlled, double-blind clinical trial will be required to confirm our findings.
COMMENTS
Background
Chronic hepatitis B liver failure (CHBLF) is a major cause of death in patients with viral hepatitis. However, the authors found that the fatality in CHBLF was significantly reduced by treating patients with an integrated approach using both traditional and Western medicine.
Research frontiers
Recently, the Ministry of Science of China established the “Key Projects in the National Science and Technology Pillar Program during the Eleventh Five-year Plan Period” and the “Key Projects in the National Science and Technology Pillar Program during the Twelfth Five-year Plan Period” for purposes of organizing nationally recognized experts in Chinese and Western medicine to conduct joint research programs. These programs will focus on reducing patient fatality and also explore various methods of using integrative medicine in disease prevention and treatment. This area of research will be of paramount importance in studying the prevention and treatment of major infectious diseases.
Innovations and breakthroughs
The fatality rate among different groups of CHBLF patients served as the primary efficacy end point in this evidence-based medical study. The authors found that CHBLF patients in the “TTK” group showed a significantly lower rate of fatality compared with patients in the MMC and “TQD” groups (16.67% vs 51.61%, 35.38%, P < 0.05). Liver regeneration and repair are thought to be the key pathophysiological mechanisms underlying the survival of CHBLF patients. In the past, the academic community has consistently emphasized the importance of promoting the liver regeneration process. However, in a previous study, the authors found that dysfunctions of liver regeneration processes were also important in the pathogenesis of CHBLF, and could hinder the reconstruction and recovery of liver tissue and function. Furthermore, treatment with “TTK” demonstrated a bidirectional regulatory action in the process of liver regeneration, and may have affected an important mechanism involved in reducing the fatality rate of CHBLF patients.
Applications
This clinical study contributes to evidence-based medicine, and its results are of clinical value. In the future, a large-scale, multi-center, double-blind, randomized, controlled study should be conducted to confirm these findings. Additionally, the mechanism for preventing and treating CHBLF by regulating liver regeneration requires further investigation.
Terminology
“TTK”: “Tonifying the kidney to promote liver regeneration and repair by affecting stem cells and their microenvironment”; “TQD”: “Tonifying qi and detoxification”; CHBLF: Chronic hepatitis B liver failure; Bidirectional regulation: Promoting normal liver regeneration and inhibiting abnormal liver regeneration.
Peer review
The study is well designed, the methods of statistical analyses are appropriate, and the data collection and interpretation are sound. It is an important finding that rates of fatality due to CHBLF can be decreased by regulating the mechanisms of liver regeneration and repair. These important scientific and clinical results should be further investigated in future studies.
Footnotes
Supported by National Science and Technology Key Projects on “Major Infectious Diseases such as HIV/AIDS, Viral Hepatitis Prevention and Treatment”, No. 2008ZX10005-007; Research Projects of Key Disease of National Traditional Chinese Medicine (Hepatopathy) Clinical Research Center (Hubei Province), No. JDZX2012054; National Natural Science Foundation of China, No. 81373513, No. 90709041, No. 30672590, No. 30271562, No. 30371787, No. 81102531 and No. 81274147; Key Projects of Natural Science Foundation of Hubei Province, No. 2011CDB463; Specialized Research Fund for the Doctoral Programs in Institution of Higher Education, No. 20124230110001; Key Subjects of Department of Science and Technology of Wuhan City, No. 201260523199
P- Reviewer: Hwang SG, Naser SA, Tomizawa M S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Wang CH
References
- 1.Li FY, Zhang L, Wang LF, Zhang XF, Zhuang YL, Li J. Establishment and application of prognosis research database of retrospective cases of chronic severe hepatitis B. Shijie Zhongxiyi Jiehe Zazhi. 2011;6:324–326. [Google Scholar]
- 2.Zhou SN, Zhang N, Wang LF, Li J. Clinical research of hepatitis B-related liver failure by tcm and wm combination therapy. Zhonghua Ganzang Zazhi. 2012;17:36–38. [Google Scholar]
- 3.Li HM, Gao X, Yan XS, Ming AP, Peng YQ, Li JJ. Promotion effect of Zuoguiwan on bone marrow cells’ forming into liver cells in mice. Shijie Huaren Xiaohua Zazhi. 2005;13:2818–2822. [Google Scholar]
- 4.Li HM, Yan XS, Ming AP, Peng YQ, Luo JJ, Lan SB, Gao X. The differentiation of marrow stromal cells into hepatocyte by conditioned medium of liver cells in vitro. Zhongxiyi Jiehe Ganbing Zazhi. 2005;15:28–30. [Google Scholar]
- 5.Li HM, Yan XS, Luo JJ, Li JJ, Gao X, Ming AP, Peng YQ. Effect of Zuogui Wan drug serum on the differentiation of bone marrow mesenchymal stem cells into hepatocytes. Zhongguo Zuzhi Gongcheng Yanjiu and Linchuang Kangfu. 2007;11:5465–5468. [Google Scholar]
- 6.Li HM. Therapy rule of “nourishing kidney to produce marrow and form liver”. Zhonghua Zhongyiyao Xuekan. 2012;30:937–940. [Google Scholar]
- 7.Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association; Severe Liver Diseases and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Diagnostic and treatment guidelines for liver failure. Zhonghua Gan Zang Bing Zazhi. 2006;14:643–646. [PubMed] [Google Scholar]
- 8.Ning Q. [Prospect and progression on study associated with severe acute exacerbation of chronic hepatitis B] Zhonghua Gan Zang Bing Zazhi. 2010;18:81–84. doi: 10.3760/cma.j.issn.1007-3418.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Ning Q, Zhu L, Yan WM. [Prewarning of severe acute aggravation of chronic hepatitis B] Zhonghua Gan Zang Bing Zazhi. 2010;18:805–807. doi: 10.3760/cma.j.issn.1007-3418.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Tan XM, Ning Q. The progress in molecular mechanism of severe hepatitis. Guoji Liuxingbingxue Chuanranbingxue Zazhi. 2004;31:150–157. [Google Scholar]
- 11.Li HM. The comprehensive,systematic and in-depth study on Traditional Chinese Medicine for regulating liver regeneration. Zhongxiyi Jiehe Ganbing Zazhi. 2007;17:129–132. [Google Scholar]
- 12.Li HM. Evolution rule of jaundice syndromes in chronic sever hepatitis. Zhongxiyi Jiehe Ganbing Zazhi. 2009;19:148–150. [Google Scholar]
- 13.Li HM, Yang ML, Mei JJ, Zhang LT, Qiu XF. [The effects of Zuogui Pill on expression of TGF-alpha, beta and it’s receptor in ARN and regenerative liver of the MSG-regeneration-rat] Zhonghua Gan Zang Bing Zazhi. 2004;12:307–308. [PubMed] [Google Scholar]
- 14.Li HM. The unbalance of EMT/MET and “marrow cells cannot differentiate into liver cells”. Zhongxiyi Jiehe Ganbing Zazhi. 2012;22:1–4. [Google Scholar]
- 15.Li HM, Gao X, Yan XS, Ming AP, Peng YQ. Study on Molecular Mechanism of Zuoguiwan in Promoting Bone Marrow Cells to Form Hepatic Cells. Zhongyi Zazhi. 2006;47:778–780. [Google Scholar]
- 16.Li HM, Gui WJ, Li JJ, Gao X, Yan XS, Cheng Y. Effects of Zuoguiwan on the liver regeneration related gene signaling pathway in female mice with male mice bone marrow transplant. Zhongguo Zuzhi Gongcheng Yanjiu and Linchuang Kangfu. 2008;12:6069–6073. [Google Scholar]
- 17.Li HM, Gao X, Yan XS. Effects of Zuoguiwan on the Wnt signaling pathway in female mice with male mice bone marrow transplant. Zhongxiyi Jiehe Ganbing Zazhi. 2010;20:29–31. [Google Scholar]
- 18.Li HM, Gao X, Yan XS. Studies of Zuogui Wan medicated serum pharmacology based on the co-culture system of bone marrow stem cells and hepatocytes. Zhongguo Zuzhi Gongcheng Yanjiu and Linchuang Kangfu. 2010;14:3527–3532. [Google Scholar]
- 19.Li HM, Gao X, Yang ML, Mei JJ, Zhang LT, Qiu XF. Effects of Zuogui Wan on neurocyte apoptosis and down-regulation of TGF-beta1 expression in nuclei of arcuate hypothalamus of monosodium glutamate -liver regeneration rats. World J Gastroenterol. 2004;10:2823–2826. doi: 10.3748/wjg.v10.i19.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li HM, Yang ML, Mei JJ, Zhang LT, Qiu XF. Apoptosis of ARN neural cell in MSG-regeneration-rat and genetic expression of apoptotic related gene TGF-β1. Zhongguo Yingyong Xinlixue Zazhi. 2003;19:46–47, 93. [Google Scholar]
- 21.Yang ML, Li HM. The expression of TGF-β1 mRNA in the hypothalamic ARN of rat with MSG-liver regeneration using in situ hybridization. Zhongguo Zuzhi Huaxue and Xibao Huaxue Zazhi. 2002;11:22–24. [Google Scholar]
- 22.Song HL, Li HM, Lin LS, Gao X, Zhao BB, Zhang J, Wu Y, Yan XS, Xiao L. Effects of Diwuyanggan capsule on liver regeneration of rat with deficiency of kidney essence and liver blood. Zhongxiyi Jiehe Ganbing Zazhi. 2013;23:90–92. [Google Scholar]
- 23.Li HM, Gao X, Yan XS, Ming AP, Peng YQ. Analysis of gene expression profile of bone marrow cells forming liver cells. Zhongxiyi Jiehe Ganbing Zazhi. 2006;16:212–214, 217. [Google Scholar]
- 24.Li HM, Gao X, Zhou MS. Gene expression profile of liver regeneration induced by monosodium L-glutamate in rat. Shijie Huaren Xiaohua Zazhi. 2005;13:448–451. [Google Scholar]
- 25.Li HM, Gao X, Zhou MS. Effects of Zuogui Pill on Gene Expression Profile of Regenerate Hepatic Tissue in MSG-Liver Regeneration-Rat. Zhonghua Zhongyiyao Zazhi. 2006;21:104–106. [Google Scholar]
- 26.Li HM, Gao X, Zhou MS. Zuogui Pill Regulating Gene Expression Profile in Liver of MSG-induced Liver Regeneration in Rats. Zhongguo Zhongyi Jichu Yixue Zazhi. 2005;11:595–598. [Google Scholar]
- 27.Xiao L, Li HM, Ye ZH, Gao X, Yan XS. Effect of tonifying kidney to regulate stem cells for transforming liver on HBV Pre-C gene mutation. Zhongxiyi Jiehe Ganbing Zazhi. 2013;23:217–218. [Google Scholar]
- 28.Li HM. Objective and Quantitative Study on the Syndrome of “Liver-Kidney Essence Deficiency” in Chronic Liver Diseases. Shijie Kexue Jishu-Zhongyiyao Xiandaihua. 2013;15:1429–1432. [Google Scholar]
- 29.Li HM. Fundamental and Clinical Application for Liver Disease Treatment with Therapeutic Principle of “Tonifying the Kidney to Promote Liver Regeneration and Repair”. Shijie Kexue Jishu-Zhongyiyao Xiandaihua. 2013;15:1425–1428. [Google Scholar]


