Abstract
Renal aspergillosis (RAsp) is a rare complication in liver transplant (LT) recipients. Here we report RAsp in two LT recipients. In both patients, RAsp occurred more than 90 d after allogenetic orthotropic LT, and all the clinical findings were unspecific. RAsp involved unilateral kidney in Case one and bilateral kidneys in Case two. Both computed tomography (CT) and magnetic resonance imaging (MRI) revealed renal abscesses, with progressively enhanced walls and separations and unenhanced alveolate areas after contrast agent administration. On unenhanced CT images they showed inhomogeneous hypo-attenuation. On fat-suppressed T2-weighted images (T2WIs), the walls and separations of the abscesses showed slightly low signal intensity and the central parts of the lesions showed slightly high signal intensity. Both on CT and MRI, there were some hints of renal infarction or chronic ischemia. Both cases were treated by radical nephrectomy followed by adjuvant antifungal treatment. They all recovered well.
Keywords: Liver transplantation, Kidney, Aspergillus infection, Computed tomography, Magnetic resonance imaging, Treatment
Core tip: This paper reports renal aspergillosis (RAsp) in two liver transplant (LT) recipients more than 90 d after allogenetic orthotropic LT, and describes the computed tomography and magnetic resonance imaging manifestations of renal lesions, including the abscesses and surroundings. The findings in this report may help improve the diagnosis of RAsp. After receiving radical nephrectomy followed by adjuvant antifungal treatment, both patients recovered well without mortality from RAsp.
INTRODUCTION
Although advances in immunosuppressive therapy have led to an increased survival rate of transplant recipients, the great risk of developing life-threatening fungal infection still is a serious problem[1,2]. Behind Candida albicans, aspergillus is the second most common cause of opportunistic fungal infections after liver transplant (LT), with an incidence rate around 0.7%-8%[3,4]. Although nearly all the organs and tissues could be infected[5-7], renal infection is very rare. Due to unspecific clinical manifestations, the diagnosis of aspergillosis was always delayed, and when the disseminated infection developed, the mortality rate can approach to 100%[8,9]. However, some studies have proved that effective anti-fungal treatment or complete removal of the original lesions prior to the dissemination could reduce the risk of death[4,10-12], so early diagnosis of aspergillosis is crucial for saving the lives.
Abdominal imaging examinations, including computed tomography (CT) and magnetic resonance imaging (MRI), have been proved to be the effective means in monitoring the complications after LT. However, up to now, the imaging manifestations of renal aspergillosis (RAsp) in LT recipients have not been described in detail.
From October 2003 to October 2013, two patients suffered from RAsp after allogenetic orthotropic LT (AOLT) at our hospital, and both were confirmed by histopathology with or without cultures of abscess aspirate. In this paper, we retrospectively analyzed the clinical and imaging manifestations of RAsp and reviewed the relevant literatures. This research has been approved by the ethics committee of our hospital.
CASE REPORT
Case one
A 47-year-old man with diabetes mellitus and renal calculus received AOLT in September 2005 due to end-stage liver cirrhosis and chronic hepatitis B. After the operation, FK506 (Tacrolimus capsules; Astellas, Kerry, Ireland) and methylprednisolone (Medrol; Pharmacia Italia, Marino, Italy) were used for the immunosuppressive treatment. The dose of FK506 was adjusted according to the serum drug level. The patient recovered well. Neither acute rejection nor other important complications were detected.
However, in the 9th mo after operation, this patient was admitted again with intermittent low-grade fever and lumbodynia, without chills, urinary frequency or urgency, or dysuria. Obvious tenderness in the left kidney area was detected. Urinalysis revealed mild leucocyturia (58 leucocytes/μL) and erythrocyturia (6 erythrocytes/μL). Other laboratory tests showed elevated erythrocyte sedimentation rate (95 mm/h), alanine aminotransferase (57 U/L), direct bilirubin (14.7 μmol/L) and creatinine (120.3 μmol/L). Both blood and urine cultivations were negative for fungi and bacteria. The serum drug level of FK506 was 8-10 ng/mL. No active lesion was detected in his chest films.
Unenhanced and tri-phase contrast-enhanced CT scans were performed to search for potential abdominal lesions. After 100 mL of contrast agent was administrated through bolus injection, tri-phase contrast-enhanced CT scans were obtained in order, including cortical nephrographic phase (20 s delay), parenchymal nephrographic phase (50 s delay) and excretory phase (> 150 s delay). CT revealed the enlarged left kidney and two round lesions in the upper-middle part (68 mm × 62 mm × 55 mm) and the lower pole (29 mm × 24 mm × 20 mm) of the left kidney, respectively. Both lesions involved the renal cortex and medulla without an obvious mass effect. On unenhanced CT images, both lesions showed inhomogeneous hypo-attenuation with ill-defined margins. After contrast agent administration, the peripheral parts of the lesions, which looked like walls, were clearly displayed with slight enhancement in cortical nephrographic phase and moderate progressive enhancement during the following two phases. In the central parts of the lesions, there were unenhanced alveolate areas and irregular separations. The separations presented with synchronous enhancement with the walls. The inner margins of the walls and separations were rough. Partially around the large focus, a relatively lowly enhanced thin layer appeared in the cortical nephrographic phase and parenchymal nephrographic phase, and disappeared in the excretory phase. Corticomedullary differentiation disappeared in the involved renal parenchyma. The adjacent calices were involved with coarse margins and moderate enhancement. Besides these, a wedge-shaped relatively lowly enhanced area with reduced corticomedullary differentiation was detected around the large focus, which showed iso-attenuation on unenhanced CT images. Following contrast agent administration, there was a gradually increased density difference between this area and the surrounding parenchyma. The adjacent renal contour was blunt. Flocculent infiltration, fat planes and stranding appeared in the left perinephric space. The right kidney and renal pelvis, bilateral ureters and bladder were normal. Several lymph nodes were seen in the left retroperitoneal space (Figure 1).
Figure 1.
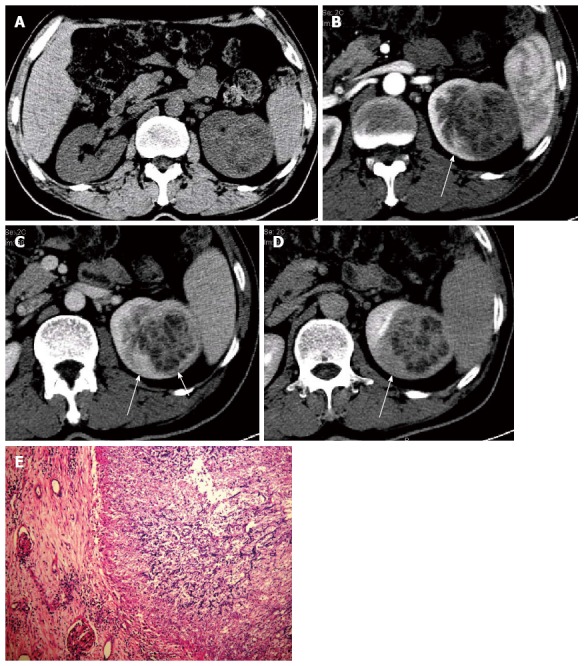
Renal aspergillosis in a 47-year-old allogenetic orthotropic liver transplantation recipient (Case one). A: Unenhanced transverse CT image reveals a large inhomogeneous hypo-attenuation focus in the left kidney; B: Transverse image in the contrast-enhanced cortical nephrographic phase; C: Transverse image in the parenchymal nephrographic phase; D: Transverse image in the excretory phase. The focus shows progressively enhanced abscess walls and separations with unenhanced necrotic areas. A wedge-shaped relatively lowly enhanced area (long arrow) is detected around the focus in B, C and D, which presents with a gradually increased density difference with the surrounding parenchyma and is confirmed to be the secondary change to chronic ischemia by histopathology. A relatively lowly enhanced thin layer (short arrow) is clearly detected in the parenchymal nephrographic phase, which is dense fibrous connective tissues in the abscess wall; E: Pathological findings under a light microscope (HE, × 100), which shows the abscesses with necrosis and amounts of aspergillus hyphae, spores and neutrophils. CT: Computed tomography; HE: Hematoxylin-eosin.
The left kidney lesions were suspected to be malignant. Without needle biopsy, this patient received radical nephrectomy for the left kidney and regional lymph node dissection in July, 2006. The gross specimen verified two yellowish abscesses, filled with necrotic tissues and amounts of aspergillus hyphae, spores and neutrophils. Abscess walls and separations were composed of dense fibrous connective tissues with inflammatory cell infiltration. Mixed with the abscesses, granulomatous inflammation was also detected. Neither inflammatory edema area nor congestive hemorrhage in the surrounding was detected. Around the large abscess, chronic inflammatory infiltration, atrophied renal tubules, thickened and hyalinized glomerular capillary basement membrane appeared at the same location as the wedge-shaped relatively lowly enhanced area on CT images, which was considered to be the secondary change to chronic ischemia. However, neither renal infarction nor vascular embolus was detected. The involved renal calices presented with mucosal erosion and ulcer. Left renal capsule and perirenal fat space presented with chronic inflammatory infiltration and fibrotic adhesion. The lymph nodes, extirpated from the left retroperitoneal space, also presented with chronic inflammation.
After the operation, as the patient can not afford the price of voriconazole, fluconazole (Diflucan; Pfizer Roerig, Liaoning, China) was given for prophylactic antifungal therapy for 5 d. The patient recovered well. Until now, more than 7 years after the operation, he is doing well and there is no evidence of recurrence.
Case two
A 53-year-old man received AOLT in December, 2006 due to end-stage liver cirrhosis and hepatocellular carcinoma. After the operation, FK506 (Tacrolimus capsules; Astellas, kerry, Ireland) and methylprednisolone (Medrol; Pharmacia Italia, Marino, Italy) were given for the immunosuppressive treatment. The dose of FK506 was adjusted according to the serum drug level. The patient recovered well without acute rejection.
In the 15th mo after transplantation, he was admitted again due to left flank pain and dysuria after he received fulguration for urethral meatus condyloma. No fever, macroscopic hematuria, increased frequency or urgency of urination occurred. Physical examination revealed severe urethral stenosis (2 mm in diameter), mild tenderness and sensitivity to percussion in left kidney area. Urinalysis revealed erythrocyturia (32 erythrocytes/μL) and leucocyturia (170 leucocytes/μL). Serum creatinine (198.6 μmol/L) and the percentage of mononuclear cells (8.1%) were elevated. Both blood and urine cultivations were negative for fungi and bacteria. The serum drug level of FK506 was 6-8 ng/mL. There was no active lesion in his chest film. Intravenous urography showed mild bilateral hydronephrosis.
MRI was performed on a 1.5 T scanner with an 8-channel torso phased array coil. Gadopentetic acid dimeglumine salt injection (Magnevist, Bayer Schering Pharma AG, Berlin, Germany) was administrated for obtaining the multiphase contrast-enhanced MR sequences, including the cortical nephrographic phase (20 s delay), parenchymal nephrographic phase (50 s delay) and excretory phase (> 150 s delay). MRI revealed the enlarged left kidney and bilateral multiple lesions, including three in the left and one in the right. The largest was about 37 mm × 33 mm × 52 mm. The smallest focus was limited in the renal cortex; the other three large ones involved both the cortex and medulla without an obvious mass effect. On dual-echo chemical shift T1-weighted images (T1WIs), all the lesions presented with iso- or slightly low-signal intensity with ill-defined margins, and no signal loss on out-of-phase images. On fat-suppressed T2WIs and tri-phase contrast-enhanced T1WIs, all the lesions presented with inhomogeneous signals and enhancements. The peripheral parts of the lesions revealed slightly low signal intensity on T2WIs and moderate progressive enhancement on contrast-enhanced T1WIs, which looked as walls with uneven thickness and rough inner rims. Alveolate unenhanced areas with irregular separations appeared in the central part of the lesions. Alveolate areas showed inhomogeneous slightly high signal intensity on T2WIs, without any enhancement on the tri-phase contrast-enhanced T1WIs; the irregular separations revealed slightly low signal intensity on T2WIs and isochronous enhancement with the peripheral walls. On the coronal images, most lesions were wedge-shaped with the top pointing to the renal hilum and the bottom towards the cortical surface. Corticomedullary differentiation disappeared in the involved renal parenchyma. In the largest focus, renal calices were involved with coarse margins and thickened walls. The left renal pelvis and superior ureter were involved with the thickened walls. MRI also demonstrated peri- and para-nephric extension around the left kidney, with obvious fat planes and stranding, and thickening of Gerota’s fascia. The left psoas major muscle showed stripped high signal on T2WIs and slightly low signal on T1WIs with obvious progressive enhancement. The right renal pelvis, peri- and para-nephric spaces, ureter and bladder were normal. Based on these, the bilateral renal lesions were suspected to be abscesses due to chronic infection, which involved the left ureter, peri- and para-nephric spaces and left psoas major muscle (Figure 2).
Figure 2.
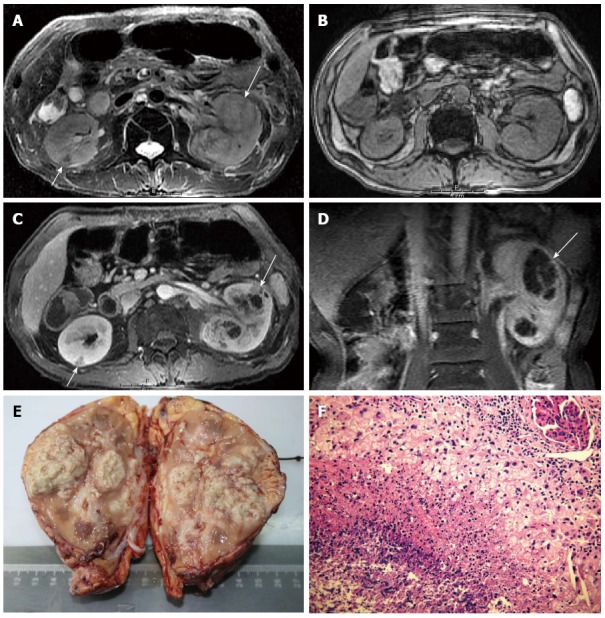
Renal aspergillosis in a 53-year-old allogenetic orthotropic liver transplantation recipient (Case two). A: Fat-suppressed T2WI, which shows two large foci in the left kidney and one small focus in the right. The large foci in the left kidney present slightly high signal centers with slightly low signal walls and separations (long arrow). The wedge-shaped small focus in the right renal cortex presents low signal intensity (short arrow); B: Unenhanced out-of-phase dual-echo chemical shift T1WI; C: Contrast-enhanced T1WI on the parenchymal nephrographic phase. All foci present iso- or slightly low signal intensity and ill-defined margins on the unenhanced T1WI. On the contrast-enhanced T1WI, the large foci present enhanced irregular abscess walls and separations with unenhanced necrotic areas (long arrow); and the small focus (short arrow) presents decreased enhancement; D: Contrast-enhanced coronal T1WI, on which the large focus is typically wedge-shaped (long arrow); E: Macroscopic aspect of the left kidney with multiple foci in it; F: Pathological findings under light microscope (HE, × 40), which shows amounts of fungal hyphae and spores in the necrosis. RAsp: Renal aspergillosis; T2WI: T2-weighted image; T1WI: T1-weighted image; HE: Hematoxylin-eosin.
Aspergillus infection was finally confirmed by cultures of abscess aspiration, which revealed branching septated hyphae and aspergillus fumigatus. In order to avoid distant dissemination, left nephrectomy was performed in May 2008. The left ureter and the involved left psoas major muscle were also removed. The gross specimen revealed multiple lesions in the left kidney, with a plenty of necrotic areas. Some small foci coalesced into large foci. Under a microscope, amounts of inflammatory lesions were detected, including multiple abscesses, granulomas and chronic inflammatory infiltration. There were amounts of aspergillus hyphae and spores in the necroses, with infiltration of neutrophils and foreign body multinucleated giant cells. Dense fibrous connective tissues constituted the abscess walls and separations, with lymphocyte and plasma cell infiltration. Among the lesions, fibroplasia was obvious; however, neither inflammatory edema nor congestive hemorrhage was detected. Besides these, mucosal erosion and ulcers were detected in the pelvis. There was no evidence for blood vessel obstruction due to aspergillus embolus. The left renal capsule and perirenal fat space presented with severe fibrotic adhesion. The specimen from the left psoas major muscle also presented with aspergilloma.
After the operation, voriconazole (Vfend; Pfizer Roerig, Germany) was administrated for 2 mo for anti-fungal treatment. Besides this, cefoperazone sodium and tazobactam sodium for injection (General Pharmaceutical Sanyo, Hainan, China) were also used for anti-inflammatory treatment. The patient recovered well. In October 2009, the patient died of extensive metastasis. Until then, there is no evidence of aspergillosis progression or dissemination.
DISCUSSION
Although aspergillus is a ubiquitous saprophytic fungus seldom pathogenic for normal hosts, aspergillus infections in organ transplant recipients demonstrate high morbidity and mortality[3,5,13]. In most cases, aspergillosis occurs within 90 d after the transplantation, however the late onset cases also have been noticed[5,6,14,15]. Singh et al[5] observed a 100% mortality rate for late-onset (more than 90 d after retransplantation) and 76.9% for early-onset (within 90 d of retransplantation) aspergillosis after liver retransplantation. In this study, both of the cases developed RAsp more than 90 d after AOLT. Therefore, the potential possibility of aspergillosis should be considered even in the late stage after liver transplantation.
According to previous reports[16-18], renal aspergillus infection may be due to haematogenous spreading, ascending from the urinary tract infection or secondary to obstructive uropathy, which may cause renal failure, renal enlargement, unilateral or bilateral localized renal masses, or urinary obstruction[16-22]. The former usually spreads in the peripheral renal cortex initially, and then involves the medulla; the latter two usually occur in the pelvicalyceal system. In this study, RAsp may be related to haematogenous spreading in Case one who had no obvious risk factors besides AOLT and immunosuppressive therapy, and secondary to obstructive uropathy in Case two who had severe urethral stenosis and mild bilateral hydronephrosis besides AOLT and immunosuppressive therapy.
The common complaints of patients with RAsp include fever, lumbodynia, hematuria, pyuria, perineal or suprapubic discomfort, and all of which are unspecific for the diagnosis. Final confirmation depended on the detection of aspergillus either by culture or histopathology. CT and MRI are of greater value in detecting, localizing and differentiating renal lesions, but the imaging manifestations of RAsp in LT recipients still have not been described in detail. In this study, misdiagnosis occurred in Case one because we were unfamiliar to the imaging manifestations of Rasp at that time. In this report, we focus on analyzing the imaging findings of RAsp in the two cases, aiming to help for its diagnosis.
According to the literature[12,20-24], the most common feature of RAsp is abscess. On unenhanced CT images, renal abscesses always reveal ill-defined iso-attenuation or inhomogenous hypo-attenuation, relying on the degree of liquefaction in necrotic tissues. Tri-phase contrast-enhanced CT images could reflect the hemodynamic changes of the lesions, on which the irregular abscess walls and reticular separations demonstrate moderate, progressive enhancement due to the fibrous connective tissues and granulomatous inflammation. Vascular occlusion and subsequent renal infarction due to fungal hyphae clumps have been reported to be the pathological characteristics of hematogenous RAsp[8,24]. Although we did not find any renal vascular embolus in Case one, the wedge-shaped relatively lowly enhanced area highly suggested the decreased blood supply and normal excretion of the involved renal parenchyma, which presented with gradually increased density difference with normal parenchyma during the tri-phase contrast-enhanced CT scan, and was finally confirmed to be the secondary change to chronic ischemia by histopathology. Fungus ball was not detected in the pyelocaliceal system in our cases, but it could appear as a nonspecific irregular filling defect[20,21], which should be differentiated from filling defects of other causes, such as blood clots or sloughed papillae[25]. Thickened pelvicalyceal wall, peri- or para-nephric fat planes, stranded and thickened Gerota’s fascia do not help for the differentiation, which also could present in other inflammatory or neoplastic lesions. Besides these, CT also plays important roles in guiding aspiration biopsy[21] and demonstrating the involvement of other organs, such as the paranasal sinuses and lung[22].
On MRI examination, signal changes reflect the distribution of water within lesions, so it bears greater advantages in characterizing renal masses[26]. However, up to now, only one report supplied a detailed description of MRI manifestations of RAsp[21]. Our Case two presented with similar manifestations as the previous report, and the abscess walls and separations revealed low-signal intensity on T2WIs with progressive enhancement on contrast-enhanced T1WIs, due to the less water content and blood supply of dense fibrous connective tissues; but on T2WIs, the signal intensity of the central parts in Case two was lower than that in Heussel’s case[21], which may be due to the less liquefaction of the necrotic tissues. Although intravascular emboli also were not detected in this case, on coronal MR images, the peripheral distribution of the small focus and the typically wedge-shaped manifestation of the foci are useful clues which support the hints of previous ischemia or infarction. MRI also could clearly demonstrate the involvement of the pelvicalyceal wall, the peri- and para-nephric extension, and the involvement of the adjacent organs, which provided important information for lesion clearance in operation.
However, delayed enhancement scan, beyond 30 min from contrast-agent administration, was not obtained in any of the two cases. Thus, we could not describe the manifestations of RAsp in the delayed phase.
The imaging manifestations of RAsp should be differentiated from those of several other diseases. Clear cell renal cell carcinoma (RCC) is the most common renal tumor, with a high chance of hemorrhage, necrosis and cystic degeneration, which could be very similar to the manifestations of RAsp on unenhanced CT and MR images. However, on contrast-enhanced CT and MR images, most of the clear cell RCCs tend to present with inhomogeneous hypervascular enhancement with fast opacification and fast clearance, which is different from the manifestations of RAsp. Besides these, not like the aspergillus abscesses in the two cases, most of the RCCs have obvious mass effects, which cause the displacement and deformation of the adjacent structures. Bacterial renal abscess also could present with central necrosis. The edema zone of the surrounding parenchyma, which represents the infected but non-necrotic tissues[27-29], could supply a useful hint for its diagnosis. It did not appear around the foci of RAsp both in Heussel’s[21] and our cases. Advanced renal tuberculosis with cavities also should be considered. Calcification, diffuse pelvicalyceal and ureter involvements are of great value for its diagnosis. Besides the acute and typical clinical signs, the striated nephrogram is useful for the identification of acute pyelonephritis, which represents obstructed tubules with intervening normal tubules[30] and could be detected on T2WIs due to the increased water content[31]. Sometimes focal renal infarct with central necrosis can be very similar to RAsp, since both of them could appear as wedge-shaped renal lesions. The typical clinical syndrome of acute flank pain without fever may help for the diagnosis of renal infarct[32].
Generally, once RAsp has been diagnosed, reversal of immunosuppression is crucial for the treatment. Corticosteroids and immunosuppressive agents should be stopped immediately. Then, systemic antifungal therapy is routinely administrated. However, for the recipients after solid organ transplantation, we are obliged to deliberate over the risk of using the conservative treatment as the main treatment strategy, due to the high mortality rate of invasive aspergillosis. In 2009, Ju et al[33] reported a high mortality rate of 55.6% among recipients with invasive pulmonary aspergillosis treated by antifungal agents after solid organ transplantation. In 2010 Badiee et al[34] reported a further higher mortality rate of 63.2% among liver transplant recipients with aspergillosis. However, according to previous studies, for patients with a local focus or unilateral multi-foci, radical or partial nephrectomy could be effective for reducing the risk of dissemination and mortality[17,23]; and for patients with bilateral multi-foci or a focus in the solitary kidney, surgical drainage with long-term antifungal therapy might be an acceptable alternative to bilateral or solitary kidney nephrectomy[12,23]. In this study, both of the cases were successfully treated by surgical resection followed by adjuvant antifungal treatment. They all recovered well and did not present with any evidence of aspergillosis progression or dissemination more than one year after the operation, although bilateral RAsp occurred in Case two and only the left kidney was removed. Therefore, we recommend surgical resection first followed by adjuvant antifungal treatment for RAsp in liver transplant recipients.
In conclusion, liver transplant recipients might suffer RAsp in the late stage after LT. Clinical findings are often inadequate for the diagnosis. Although imaging features of renal abscesses and hints of renal infarction or chronic ischemia might help for the diagnosis of RAsp, misdiagnosis is still not uncommon. However, both CT and MRI are important in the discovery and localization of the lesions. For LT recipients, early removal of the RAsp lesion by radical or partial nephrectomy is advisable for reducing the risk of dissemination and mortality.
COMMENTS
Case characteristics
Two liver transplant (LT) recipients suffered fever, lumbodynia or flank pain, perineal or suprapubic discomfort.
Clinical diagnosis
Both of them presented with tenderness and sensitivity to percussion in diseased kidney area.
Differential diagnosis
Renal cell carcinoma, bacterial renal abscess, focal renal infarct, advanced renal tuberculosis and acute pyelonephritis.
Laboratory diagnosis
Erythrocyturia, leucocyturia and elevated serum creatinine in two patients; elevated mononuclear cells in one patient; neither blood nor urine cultivation was positive for fungi or bacteria.
Imaging diagnosis
Both computed tomography (CT) and magnetic resonance imaging (MRI) revealed renal abscesses with unenhanced alveolate areas and progressively enhanced walls and separations.
Pathological diagnosis
The gross specimen after radical nephrectomy revealed yellowish abscesses filled with necrotic tissues and amounts of aspergillus hyphae, spores and neutrophils.
Treatment
Both of the patients were treated by radical nephrectomy first followed by adjuvant antifungal treatment (fluconazole for Case one; voriconazole for Case two).
Related reports
The imaging manifestations of renal aspergillosis (RAsp) in LT recipients still have not been described in detail.
Experiences and lessons
This case report not only gives a detailed description of the CT and MR manifestations of RAsp in the late stage after allogenetic orthotropic LT, but also provides successful treatments by radical nephrectomy followed by adjuvant antifungal treatment.
Peer review
Aspergillosis is fatal for immunocompromised patients and is very rare. This paper reports this rare infection in two LT recipients and gives a detailed description of the imaging features, which is very useful in clinical settings.
Footnotes
Supported by Forty-third Batch of the Scientific Research Foundation for Returned Oversea Scholars from the Ministry of Education; National Natural Science Foundation of China, No. 81201090, No. 81371554, No. 81371655 and No. 81071206; Guangdong Natural Science Foundation, No. S2012010008367; and Guangdong Science and Technology Project, No. 2010B031600053
P- Reviewer: Hori T, Tao R, Wakiyama S, Wu TJ S- Editor: Nan J L- Editor: Wang TQ E- Editor: Wang CH
References
- 1.Patel G, Huprikar S. Infectious complications after orthotopic liver transplantation. Semin Respir Crit Care Med. 2012;33:111–124. doi: 10.1055/s-0032-1301739. [DOI] [PubMed] [Google Scholar]
- 2.Ohkubo T, Sugawara Y, Takayama T, Kokudo N, Makuuchi M. The risk factors of fungal infection in living-donor liver transplantations. J Hepatobiliary Pancreat Sci. 2012;19:382–388. doi: 10.1007/s00534-011-0423-4. [DOI] [PubMed] [Google Scholar]
- 3.Minari A, Husni R, Avery RK, Longworth DL, DeCamp M, Bertin M, Schilz R, Smedira N, Haug MT, Mehta A, et al. The incidence of invasive aspergillosis among solid organ transplant recipients and implications for prophylaxis in lung transplants. Transpl Infect Dis. 2002;4:195–200. doi: 10.1034/j.1399-3062.2002.t01-2-02002.x. [DOI] [PubMed] [Google Scholar]
- 4.Teisseyre J, Kaliciński P, Markiewicz-Kijewska M, Szymczak M, Ismail H, Drewniak T, Nachulewicz P, Broniszczak D, Teisseyre M, Pawłowska J, et al. Aspergillosis in children after liver transplantation: Single center experience. Pediatr Transplant. 2007;11:868–875. doi: 10.1111/j.1399-3046.2007.00754.x. [DOI] [PubMed] [Google Scholar]
- 5.Singh N, Pruett TL, Houston S, Muñoz P, Cacciarelli TV, Wagener MM, Husain S. Invasive aspergillosis in the recipients of liver retransplantation. Liver Transpl. 2006;12:1205–1209. doi: 10.1002/lt.20756. [DOI] [PubMed] [Google Scholar]
- 6.Singh N, Paterson DL. Aspergillus infections in transplant recipients. Clin Microbiol Rev. 2005;18:44–69. doi: 10.1128/CMR.18.1.44-69.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Latgé JP. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999;12:310–350. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marañés A, Portolés J, Blanco J, Torrente J, Herrero J, Coronel F, Marrón B, Barrientos A. Aspergillus infection of a renal allograft without evidence of a site of origin. Nephrol Dial Transplant. 1996;11:1639–1642. [PubMed] [Google Scholar]
- 9.Singh N, Arnow PM, Bonham A, Dominguez E, Paterson DL, Pankey GA, Wagener MM, Yu VL. Invasive aspergillosis in liver transplant recipients in the 1990s. Transplantation. 1997;64:716–720. doi: 10.1097/00007890-199709150-00009. [DOI] [PubMed] [Google Scholar]
- 10.Ergin F, Arslan H, Azap A, Demirhan B, Karakayali H, Haberal M. Invasive aspergillosis in solid-organ transplantation: report of eight cases and review of the literature. Transpl Int. 2003;16:280–286. doi: 10.1007/s00147-002-0522-x. [DOI] [PubMed] [Google Scholar]
- 11.Rabagliati R, Santolaya ME. [Prophylaxis against fungal infections in solid organ and hematopoietic stem cells transplantation] Rev Chilena Infectol. 2012;29 Suppl 1:S11–S18. doi: 10.4067/S0716-10182012000500002. [DOI] [PubMed] [Google Scholar]
- 12.Avkan-Oguz V, Ozkardesler S, Unek T, Ozbilgin M, Akan M, Firuzan E, Kose H, Astarcioglu I, Karademir S. Risk factors for early bacterial infections in liver transplantation. Transplant Proc. 2013;45:993–997. doi: 10.1016/j.transproceed.2013.02.067. [DOI] [PubMed] [Google Scholar]
- 13.Marik PE. Fungal infections in solid organ transplantation. Expert Opin Pharmacother. 2006;7:297–305. doi: 10.1517/14656566.7.3.297. [DOI] [PubMed] [Google Scholar]
- 14.Husain S, Alexander BD, Munoz P, Avery RK, Houston S, Pruett T, Jacobs R, Dominguez EA, Tollemar JG, Baumgarten K, et al. Opportunistic mycelial fungal infections in organ transplant recipients: emerging importance of non-Aspergillus mycelial fungi. Clin Infect Dis. 2003;37:221–229. doi: 10.1086/375822. [DOI] [PubMed] [Google Scholar]
- 15.Singh N, Avery RK, Munoz P, Pruett TL, Alexander B, Jacobs R, Tollemar JG, Dominguez EA, Yu CM, Paterson DL, et al. Trends in risk profiles for and mortality associated with invasive aspergillosis among liver transplant recipients. Clin Infect Dis. 2003;36:46–52. doi: 10.1086/345441. [DOI] [PubMed] [Google Scholar]
- 16.Flechner SM, McAninch JW. Aspergillosis of the urinary tract: ascending route of infection and evolving patterns of disease. J Urol. 1981;125:598–601. doi: 10.1016/s0022-5347(17)55119-8. [DOI] [PubMed] [Google Scholar]
- 17.Viale P, Di Matteo A, Sisti M, Voltolini F, Paties C, Alberici F. Isolated kidney localization of invasive Aspergillosis in a patient with AIDS. Scand J Infect Dis. 1994;26:767–770. doi: 10.3109/00365549409008651. [DOI] [PubMed] [Google Scholar]
- 18.Martinez-Jabaloyas J, Osca JM, Ruiz JL, Beamud A, Blanes M, Jimenez-Cruz JF. Renal aspergillosis and AIDS. Eur Urol. 1995;27:167–169. doi: 10.1159/000475151. [DOI] [PubMed] [Google Scholar]
- 19.Munõz-Bustillo E, Tejedor D, García A, Noguerado A. Acute nephritis by Aspergillus in a patient with AIDS. Nephrol Dial Transplant. 1996;11:194–196. [PubMed] [Google Scholar]
- 20.Zirinsky K, Auh YH, Hartman BJ, Rubenstein WA, Morrison HS, Sherman SJ, Kazam E. Computed tomography of renal aspergillosis. J Comput Assist Tomogr. 1987;11:177–178. doi: 10.1097/00004728-198701000-00041. [DOI] [PubMed] [Google Scholar]
- 21.Heussel CP, Kauczor HU, Heussel G, Thelen M, Jahn B. Multiple renal aspergillus abscesses in an AIDS patient: contrast-enhanced helical CT and MRI findings. Eur Radiol. 1999;9:616–619. doi: 10.1007/s003300050719. [DOI] [PubMed] [Google Scholar]
- 22.Oosten AW, Sprenger HG, van Leeuwen JT, Meessen NE, van Assen S. Bilateral renal aspergillosis in a patient with AIDS: a case report and review of reported cases. AIDS Patient Care STDS. 2008;22:1–6. doi: 10.1089/apc.2007.0051. [DOI] [PubMed] [Google Scholar]
- 23.Poll LW, Koch J, Medve M, May P, Sarbia M, Engelbrecht V, Mödder U. CT appearance of a renal aspergilloma in a patient with the acquired immunodeficiency syndrome. Urol Int. 1999;62:110–113. doi: 10.1159/000030369. [DOI] [PubMed] [Google Scholar]
- 24.Lisson SW, Hellinger WC, Parra RO. Primary bilateral parenchymal renal Aspergillus infection. Urology. 2002;60:345. doi: 10.1016/s0090-4295(02)01746-6. [DOI] [PubMed] [Google Scholar]
- 25.Kawashima A, Sandler CM, Goldman SM, Raval BK, Fishman EK. CT of renal inflammatory disease. Radiographics. 1997;17:851–866; discussion 867-868. doi: 10.1148/radiographics.17.4.9225387. [DOI] [PubMed] [Google Scholar]
- 26.Israel GM, Hindman N, Bosniak MA. Evaluation of cystic renal masses: comparison of CT and MR imaging by using the Bosniak classification system. Radiology. 2004;231:365–371. doi: 10.1148/radiol.2312031025. [DOI] [PubMed] [Google Scholar]
- 27.Soulen MC, Fishman EK, Goldman SM, Gatewood OM. Bacterial renal infection: role of CT. Radiology. 1989;171:703–707. doi: 10.1148/radiology.171.3.2655002. [DOI] [PubMed] [Google Scholar]
- 28.Kawashima A, Sandler CM, Goldman SM. Imaging in acute renal infection. BJU Int. 2000;86 Suppl 1:70–79. doi: 10.1046/j.1464-410x.2000.00578.x. [DOI] [PubMed] [Google Scholar]
- 29.Kenney PJ. Imaging of chronic renal infections. AJR Am J Roentgenol. 1990;155:485–494. doi: 10.2214/ajr.155.3.2117344. [DOI] [PubMed] [Google Scholar]
- 30.Papanicolaou N, Pfister RC. Acute renal infections. Radiol Clin North Am. 1996;34:965–995. [PubMed] [Google Scholar]
- 31.Majd M, Nussbaum Blask AR, Markle BM, Shalaby-Rana E, Pohl HG, Park JS, Chandra R, Rais-Bahrami K, Pandya N, Patel KM, et al. Acute pyelonephritis: comparison of diagnosis with 99mTc-DMSA, SPECT, spiral CT, MR imaging, and power Doppler US in an experimental pig model. Radiology. 2001;218:101–108. doi: 10.1148/radiology.218.1.r01ja37101. [DOI] [PubMed] [Google Scholar]
- 32.Suzer O, Shirkhoda A, Jafri SZ, Madrazo BL, Bis KG, Mastromatteo JF. CT features of renal infarction. Eur J Radiol. 2002;44:59–64. doi: 10.1016/s0720-048x(01)00476-4. [DOI] [PubMed] [Google Scholar]
- 33.Ju MK, Joo DJ, Kim SJ, Chang HK, Kim MS, Kim SI, Kim YS. Invasive pulmonary aspergillosis after solid organ transplantation: diagnosis and treatment based on 28 years of transplantation experience. Transplant Proc. 2009;41:375–378. doi: 10.1016/j.transproceed.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 34.Badiee P, Alborzi A, Malekhosseini SA, Nikeghbalian S, Shakiba E. Determining the incidence of aspergillosis after liver transplant. Exp Clin Transplant. 2010;8:220–223. [PubMed] [Google Scholar]


