Abstract
Background
Anaplastic large cell lymphoma (ALCL) is uncommon in children, accounting for approximately 15% of all cases of childhood non-Hodgkin lymphoma. Despite many studies attempting new treatment strategies, treatment outcomes have not significantly improved, and the optimal treatment for pediatric ALCL has not been established.
Methods
The records of newly diagnosed ALCL patients at our institute between July 1998 and April 2013 were reviewed. We evaluated the general characteristics of the patients, chemotherapy regimens, overall survival (OS) rates, and event-free survival (EFS) rates.
Results
Twenty-eight ALCL patients were eligible. The median age at diagnosis was 10.8 years. Lymph node involvement was the most common presentation (79%). CCG-5941, a multi-agent T-cell lineage chemotherapy, was the predominant treatment regimen (57%). The five-year OS and EFS rates were 88% and 69%, respectively. Stage, the presence of B symptoms, lung involvement, and bone marrow involvement were significant prognostic factors for EFS (P=0.02, 0.01, 0.01, and 0.02, respectively). Eight patients relapsed, and three died during the study period. Four of the eight patients who relapsed were treated with high-dose chemotherapy and autologous stem cell transplantation (HDCT-ASCT). Two of the four who had undergone HDCT-ASCT developed secondary relapses and were subsequently treated with allogeneic SCT or brentuximab.
Conclusion
We found that treatment outcomes with multi-agent chemotherapy in children with ALCL were similar to those of previous reports, and that relapsed patients could be salvaged with HDCT-ASCT or allogeneic SCT. A prospective, larger cohort study is warranted to define the optimal treatment for pediatric ALCL.
Keywords: Anaplastic large cell lymphoma, Childhood, Prognosis, Relpase
INTRODUCTION
Anaplastic large cell lymphoma (ALCL), which is characterized by the proliferation of anaplastic cells of the T or null phenotype [1], is a rare disease in children accounting for about 15% of childhood non-Hodgkin lymphomas (NHL) [2]. ALCL has a broad morphological spectrum characterized by the infiltration of pleomorphic cells in a sinusoidal pattern and co-expression of Ki-1 or CD30 epithelial membrane antigen and the interleukin-2 receptor [3, 4]. ALCL is referred to as "large cell anaplastic lymphoma" in the revised Kiel classification, and it is a subgroup of the peripheral T-cell lymphomas in the Revised European-American Lymphoma REAL) classification [5, 6]. The definition of ALCL has recently been further refined; in the 2008 World Health Organization (WHO) classification of lymphoma, ALCL is classed as a subtype of the mature T-cell and NK-cell neoplasms [7].
Despite recent advances in its characterization, the optimal treatment of ALCL has not been established, and the efficacy and safety of treatments are still under investigation [8]. Most European pediatric oncology groups report successful outcomes for ALCL patients treated with an intensive short-pulse chemotherapy regimen based on B-cell NHL-type therapy [9, 10, 11]. In contrast, several other pediatric oncology groups treat ALCL patients with less-intensive but prolonged, repeated-pulse therapy [1, 12, 13, 14]. In a number of studies, the event-free survival (EFS) rate ranged from 60% to 75%, and relapse occurred in up to 35% of patients [1, 10, 12, 14, 15]. For relapsed patients, various second-line treatments ranging from vinblastine alone to high-dose chemotherapy with hematopoietic stem cell transplantation (HSCT) have been investigated, but there is currently no consensus on the optimal treatment strategy [16, 17, 18]. Approximately 50% of patients with refractory disease will relapse again, and they have limited treatment options, such as palliative chemotherapy, radiotherapy, allogeneic SCT, or experimental approaches [19, 20]. Recently, new, targeted therapies such as brentuximab or crizotinib have shown impressive results in clinical trials treating relapsed patients with ALCL [21, 22, 23].
Although the first study regarding the clinical features and treatment outcomes of Korean pediatric patients with ALCL (N=5) was published in 1995 [24], there have been very few studies reported since. We have, therefore, analyzed the clinical characteristics and treatment outcomes of children newly diagnosed with ALCL at a single center in Korea.
PATIENTS AND METHODS
Patients
The medical records of 28 patients who were diagnosed and treated with ALCL at the Asan Medical Center in Seoul, Korea between July 1998 and April 2013 were reviewed retrospectively. Demographic data, clinical history, clinical characteristics, and treatment outcome were analyzed. Patients were diagnosed with ALCL by a combination of routine histology and immunohistochemistry. The anaplastic lymphoma kinase (ALK) status of the tumor was investigated in 18 patients.
Staging
All patients underwent a physical examination, a complete blood cell count, biochemical profiling, serum lactate dehydrogenase (LDH) level measurement, bone marrow examination, and computed tomography (CT) to determine the extent of the disease. Staging was defined according to the St. Jude's classification of childhood NHL [25]. Bone marrow involvement was defined as the presence of tumor cells on a bone marrow smear, and central nervous system (CNS) involvement as the presence of intracranial lesions on CT and/or the presence of tumor cells on cerebrospinal fluid (CSF) analysis.
Treatment and response evaluation
All 28 patients received chemotherapy. As an initial treatment, 16 patients (57%) received the Children's Cancer Group (CCG)-5941 protocol [14]; seven (25%) received AD-COMP/COMP (daunomycin-cyclophosphamide, vincristine, methotrexate, and prednisone); two (7%) received the Pediatric Oncology Group (POG) 9315 protocol [13]; one (3%) received the Modified Berlin-Frankfurt-Münster (BFM) protocol [15]; one (3%) received the Modified CCG-1882 protocol [26]; and one (3%) received the CCG-1901 protocol [27]. The physician decided the treatment regimens. Response was assessed on the basis of clinical examination, imaging, and bone marrow examination.
Statistical analysis
Overall survival (OS) and EFS rates were estimated using the Kaplan-Meier method. OS rates were measured using the time from the date of diagnosis to the date of death from any cause or to the date of the last follow-up visit for patients who were still alive. EFS rates were estimated using the time from the date of diagnosis to the date of first relapse or the date of death. Patients who experienced EFS were censored at the last follow-up date. Prognostic variables were tested in a log-rank test. Follow-up information was updated in February 2014. The statistical analysis was performed with SPSS version 18.0 (Statistical Package for the Social Sciences; IBM, Armonk, NY, USA).
RESULTS
Clinical characteristics
The demographic and clinical characteristics of the 28 patients are detailed in Table 1. Of the 28 patients, 19 (68%) were male. The median age of the patients was 10.8 years (range, 1.4-16 years). According to the St Jude's classification, 2 (7%) patients had stage I disease, 6 (21%) had stage II, 15 (53%) had stage III, and 5 (18%) had stage IV. Lymph node involvement was present in 79% of patients at diagnosis. Of the 18 (64%) patients presenting with extranodal involvement, mediastinal involvement was the most common (N=9). B symptoms occurred as initial symptoms in 14 patients (50%). A serum LDH level >500 IU/L was noted in 17 patients (61%). All patients had a T-cell or null cell phenotype on immunohistochemistry. Eighteen patients (64%) had a T-cell phenotype. The ALK status was available for only 18 patients, and of them, 12 were positive.
Table 1.
Demographic and clinical characteristics of 28 pediatric ALCL patients.
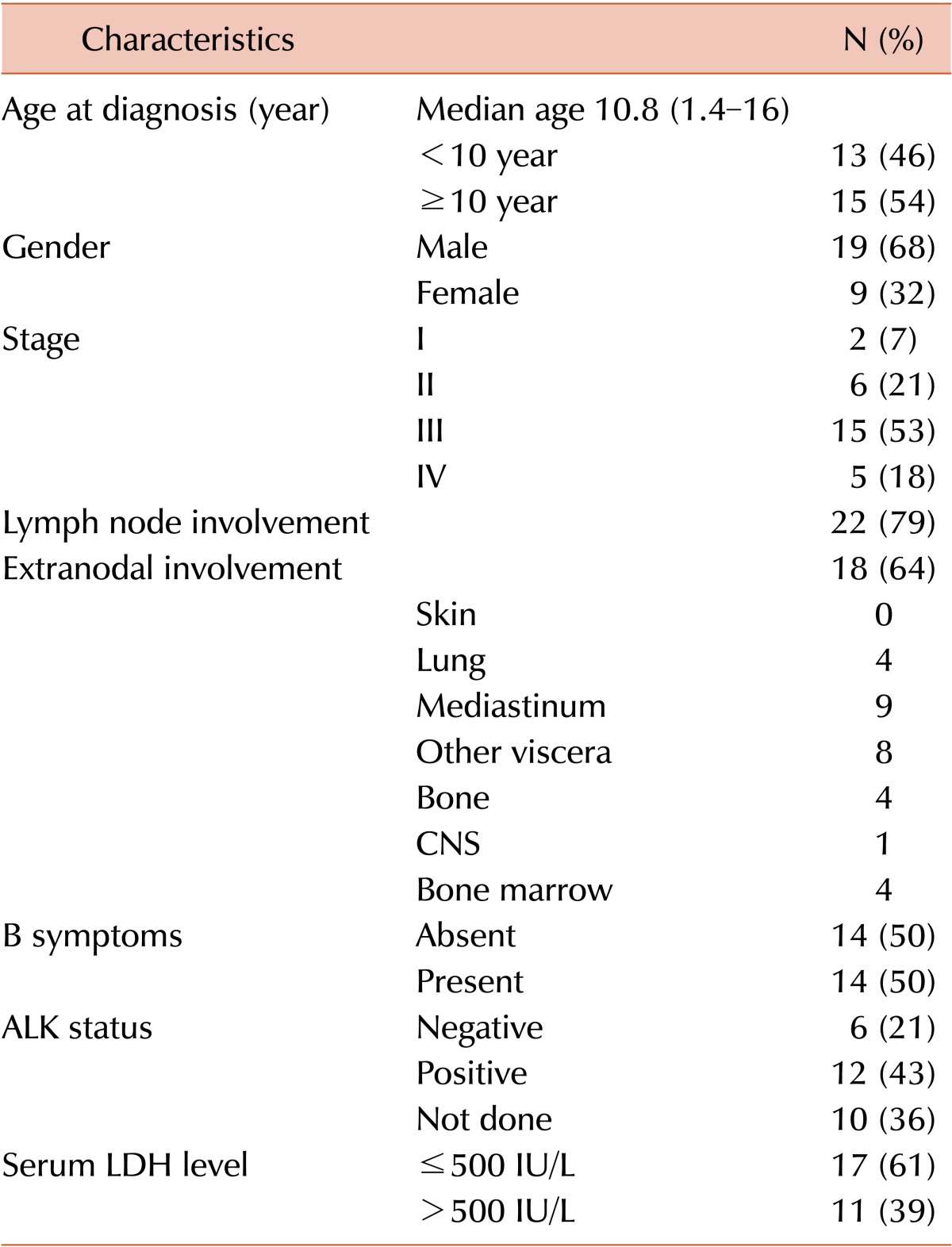
Abbreviations: CNS, central nervous system; ALK, anaplastic lymphoma kinase; LDH, lactate dehydrogenase.
Treatment results
All 28 patients received combination chemotherapy. Twenty-three (82%) patients achieved complete remission (CR). Of the five patients (18%) who did not achieve CR, three had progressive disease with treatment, and two had a partial response. Of the three patients with progressive disease, two patients had stage III disease and one had stage IV disease with bone marrow involvement. The two patients who achieved a partial response were treated with further chemotherapy.
Eight of the 28 patients (28%) relapsed (Table 2). The median time to relapse from diagnosis was 12.5 months with a range of 2-26 months. Of the eight relapsed patients, three had stage IV disease and five had stage III disease. All eight received second-line chemotherapy. Of the three (37%) of the patients who experienced a relapse died, one died from infection while in CR and two died from infection when their disease was refractory. The median time from relapse to death was four months, with a range of 3-16 months. Of the five patients who survived relapse, one was treated with salvage chemotherapy using the LSA2-L2 protocol [28], three were treated with high-dose chemotherapy followed by ASCT (HDCT-ASCT), and one was treated with brentuximab followed by HDCT-ASCT. A conditioning chemotherapy regimen included total body irradiation (total 6 Gy) and cyclophosphamide (50 mg/kg for 2 days) in two patients, and cytosine arabinoside (400 mg/m2 for 4 days), VP-16 (400 mg/m2 for 4 days), and melphalan (70 mg/m2) was administered as a conditioning chemotherapy in the other two patients. Two of the four patients treated with HDCT-ASCT after relapse experienced a second relapse. One of them was treated with allogeneic SCT; the other was treated with brentuximab followed by allogeneic SCT. Two relapsed patients were treated with brentuximab. One was treated with brentuximab followed by HDCT-ASCT after relapse, and the other one was treated with brentuximab followed by allogeneic SCT after a second relapse. Brentuximab was administered at 1.8 mg/kg four times per three weeks to the patient with stage III disease after relapse and at 1.8 mg/kg three times without a regular interval to the other patient with stage IV disease after a second relapse. The former showed a partial response, and she is now scheduled to undergo an allogeneic SCT. The latter is in CR. Brentuximab showed a successful outcome as a bridge to HSCT. No grade III-IV toxicities associated with brentuximab were observed. The five-year OS rate was 88%, and EFS rate was 69% (Fig. 1A). The five-year OS and EFS rates for the relapsed patients (N=8) were 56% and 33%, respectively (Fig. 1B).
Table 2.
Characteristics and outcomes of relapsed patients.
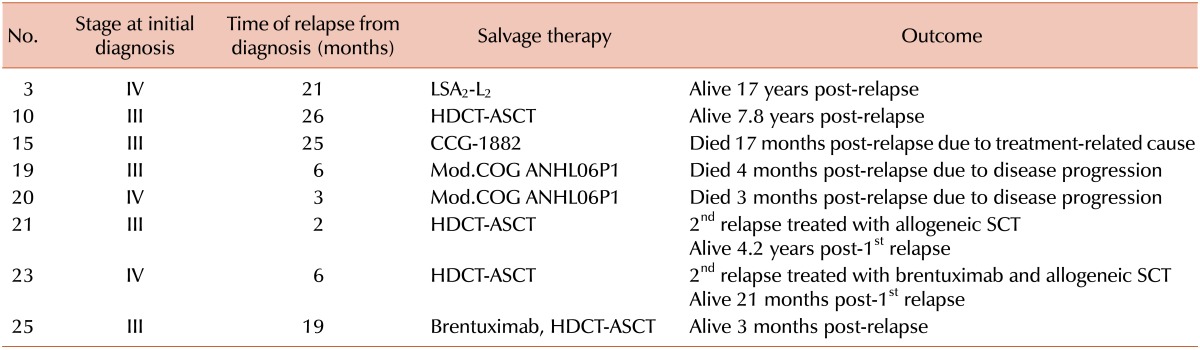
Abbreviations: HDCT-ASCT, high-dose chemotherapy-autologous stem cell transplantation; SCT, stem cell transplantation; CCG, Children's Cancer Group; COG, Children's Oncology Group.
Fig. 1.
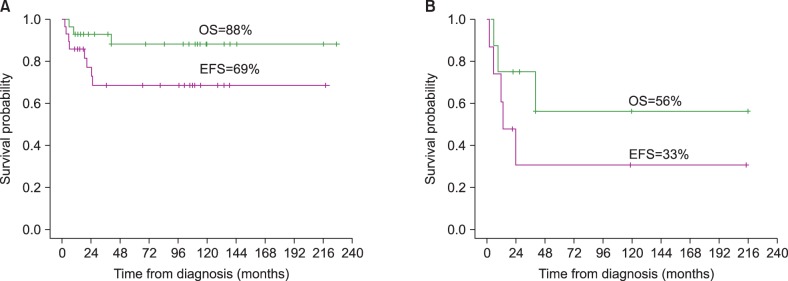
Overall survival (OS) and event-free survival (EFS) rates of 28 patients with anaplastic large cell lymphoma (A). OS and EFS rates of eight relapsed patients (B).
Prognostic factors
The log-rank test was performed to identify factors affecting survival (Table 3). Age at diagnosis, gender, stage, serum LDH level, the presence of B symptoms, ALK status, lymph node involvement, extranodal involvement, and the different types of treatment protocol were evaluated. There were no statistically significant factors affecting OS. Advanced stage (stages III and IV), the presence of B symptoms, lung involvement, and bone marrow involvement were identified as poor prognostic factors for EFS (P=0.02, 0.01, 0.01, and 0.02, respectively).
Table 3.
Prognostic factors for overall survival and event-free survival rates (log-rank test).
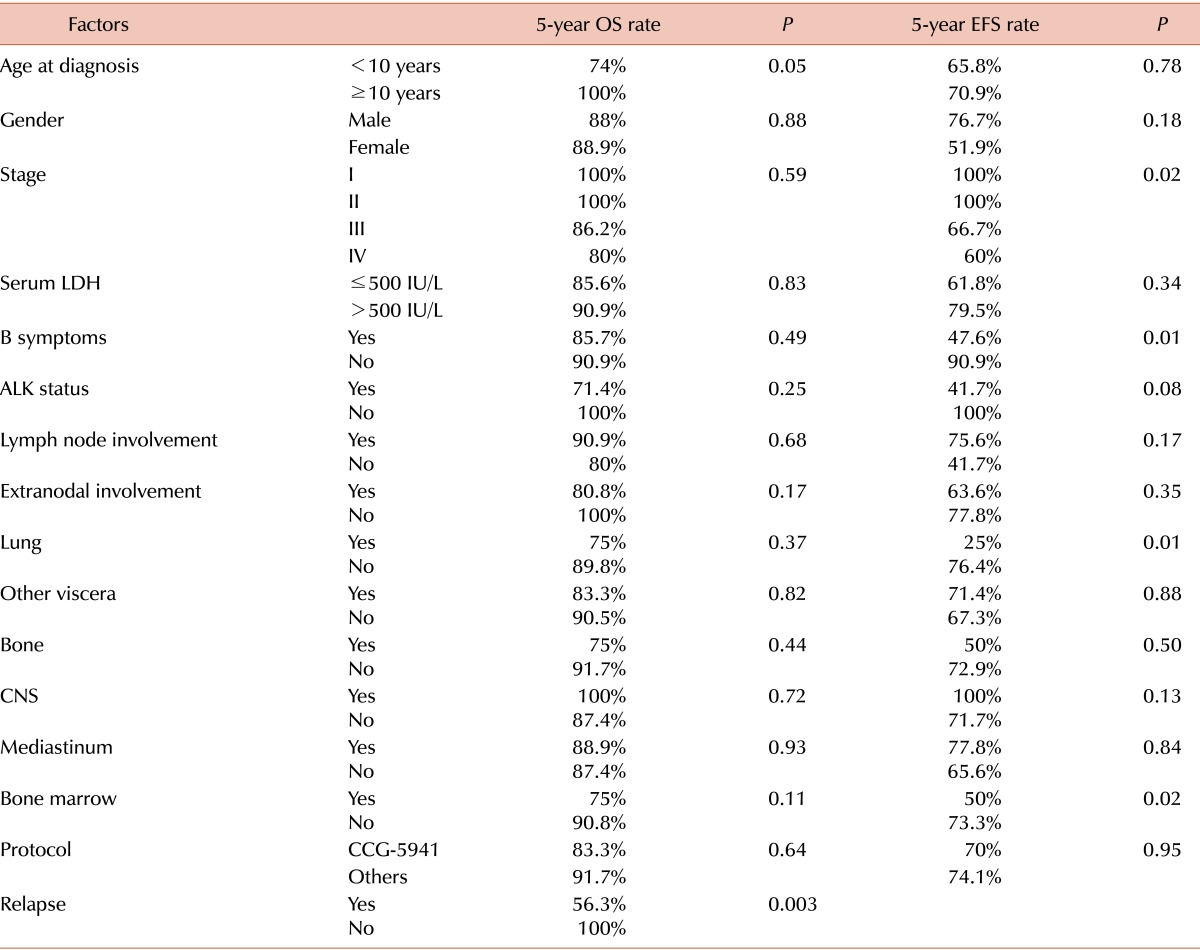
Abbreviations: OS, overall survival; EFS, event-free survival; LDH, lactate dehydrogenase; ALK, anaplastic lymphoma kinase; CNS, central nervous system.
DISCUSSION
The definition of ALCL has recently been reappraised, and options for its treatment are still under investigation. Our retrospective review of 28 patients diagnosed with ALCL in a single center contributes to the establishment of the clinical features, outcomes, prognostic factors, and treatments of refractory childhood ALCL in Korea. Among diverse factors, stage, the presence of B symptoms, and lung or bone marrow involvement were prognostic factors affecting EFS. Of the eight patients who relapsed, three died and five patients were alive after salvage therapy including HDCT-ASCT, brentuximab, and allogeneic SCT.
Various treatment strategies from short-pulse B-cell lineage NHL-type chemotherapy to prolonged lymphoblastic lymphoma/leukemia-type therapy have been the subject of many trials of several pediatric oncology groups; however, the optimal treatment for pediatric ALCL has not yet been established. In our study, the five-year OS and EFS rates for the total cohort of 28 pediatric ALCL patients were 88% and 69%, respectively, and more than a half of the patients were treated with CCG-5941, a chemotherapy protocol designed for T-cell lineage lymphoblastic leukemia. This result was comparable to those of previous studies. In the BFM group, ALCL was treated according to the short-pulse chemotherapy strategy proven to be efficacious for mature B-cell NHL. In an analysis of the BFM group trial NHL-BFM 90, Seidemann et al. [10] reported a five-year EFS rate of 76% for 89 patients with newly diagnosed ALCL. Thirty-four patients treated with the AIEOP LNH 92 protocol, which is based on a modified LSA2-L2 acute leukemia protocol, showed a 10-year EFS of 65%. The treatment consisted of an induction of a remission phase, followed by consolidation and maintenance for a total duration of 24 months [12]. The CCG-5941 protocol was a compressed aggressive multi-agent T-cell lineage chemotherapy regimen consisting of three weeks of induction therapy, followed by a three-week consolidation period followed by six courses of maintenance chemotherapy every seven weeks; for 86 patients with systemic ALCL treated with CCG-5941 the five-year EFS rate was 68% [14]. Although many studies, including our own, have found that leukemia-type chemotherapy regimens result in successful treatment outcomes, there is still no consensus on the optimal treatment for pediatric ALCL.
In our study, we found no significant prognostic factors for OS. Four factors, including advanced stage, B symptoms, lung involvement, and bone marrow involvement, were significant prognostic factors for poor EFS. In a large European intergroup study of 225 patients, B symptoms, skin lesions, mediastinal involvement, visceral involvement (lung, liver, or spleen), and elevated LDH levels were identified as risk factors of disease progression and relapse in ALCL [29]. Although our study population was small, we found that similar factors affected EFS.
ALCL in children and adolescents has successful treatment outcomes with first-line chemotherapy, but it still has a relapse rate of 25-35% after first-line chemotherapy [18]. In our study, 8 of 28 patients (28.5%) relapsed. Various treatment options for patients with relapsed disease have emerged because of clinical trials. Vinblastine mono-therapy, HDCT-ASCT, allogeneic SCT, and new, targeted therapies have been suggested, but their efficacies and toxicities are still under investigation. Of our eight patients who relapsed, four received HDCT-ASCT. Allogeneic SCT was added as another option at second relapse after HDCT-ASCT. Although the EFS of the relapsed patients in our cohort was poor, their OS rate was still favorable. These results suggest that relapsed patients with ALCL still have a chance of salvage following a second relapse. In a previous retrospective analysis, 39 patients with relapsed or refractory ALCL who received HDCT-ASCT had a five-year EFS of 59%, and the survival rate was associated with the time to relapse and CD3 expression [18]. In another study, Woessmann et al. [30] reported a three-year EFS of 75% in 20 patients with high-risk relapsed or refractory ALCL who underwent allogeneic SCT. ALCL is distinguished by molecular and immunohistochemical characteristics, including the expression of the surface antigen CD30 and the ALK protein in more than a half of all cases. These characteristics have provided targets for the development of new therapies [21]. For example, brentuximab vedotin is an anti-CD30 antibody-cytotoxic drug conjugate, and crizotinib is an inhibitor of ALK tyrosine kinase; both are currently in trials for the treatment of pediatric ALCL [21, 22, 23]. In our study, two relapsed patients were treated with brentuximab plus HDCT-ASCT or allogeneic SCT. In our experience, brentuximab was a particularly successful therapy for second relapse after HDCT-ASCT as a bridge to allogeneic SCT. Although we could not evaluate the efficacy and treatment outcomes because of the small number of patients, our study suggests that brentuximab is a feasible option for children with relapsed, refractory ALCL.
In our study, treatment regimens were left to the physician's discretion; therefore, the treatment regimens or criteria were not uniform. Therefore, we have a limited ability to evaluate whether the treatment regimen has an effect on outcomes.
In conclusion, despite being limited by the small number of patients, our study has shown that treatment outcomes with multi-agent chemotherapy in Korean children with ALCL are similar to those of previous Western reports, and that relapsed patients can be salvaged with HDCT-ASCT or allogeneic SCT. Further investigation of the role of new, targeted therapies is warranted for relapsed pediatric patients.
Footnotes
No potential conflicts of interest relevant to this article were reported.
References
- 1.Pillon M, Gregucci F, Lombardi A, et al. Results of AIEOP LNH-97 protocol for the treatment of anaplastic large cell lymphoma of childhood. Pediatr Blood Cancer. 2012;59:828–833. doi: 10.1002/pbc.24125. [DOI] [PubMed] [Google Scholar]
- 2.Wright D, McKeever P, Carter R. Childhood non-Hodgkin lymphomas in the United Kingdom: findings from the UK Children's Cancer Study Group. J Clin Pathol. 1997;50:128–134. doi: 10.1136/jcp.50.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delsol G, Al Saati T, Gatter KC, et al. Coexpression of epithelial membrane antigen (EMA), Ki-1, and interleukin-2 receptor by anaplastic large cell lymphomas. Diagnostic value in so-called malignant histiocytosis. Am J Pathol. 1988;130:59–70. [PMC free article] [PubMed] [Google Scholar]
- 4.Stein H, Foss HD, Durkop H, et al. CD30(+) anaplastic large cell lymphoma: a review of its histopathologic, genetic, and clinical features. Blood. 2000;96:3681–3695. [PubMed] [Google Scholar]
- 5.Stansfeld AG, Diebold J, Noel H, et al. Updated Kiel classification for lymphomas. Lancet. 1988;1:292–293. doi: 10.1016/s0140-6736(88)90367-4. [DOI] [PubMed] [Google Scholar]
- 6.Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J. Lymphoma classification-from controversy to consensus: the R.E.A.L. and WHO Classification of lymphoid neoplasms. Ann Oncol. 2000;11(Suppl 1):3–10. [PubMed] [Google Scholar]
- 7.Jaffe ES. The 2008 WHO classification of lymphomas: implications for clinical practice and translational research. Hematology Am Soc Hematol Educ Program. 2009:523–531. doi: 10.1182/asheducation-2009.1.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wrobel G, Mauguen A, Rosolen A, et al. Safety assessment of intensive induction therapy in childhood anaplastic large cell lymphoma: report of the ALCL99 randomised trial. Pediatr Blood Cancer. 2011;56:1071–1077. doi: 10.1002/pbc.22940. [DOI] [PubMed] [Google Scholar]
- 9.Reiter A, Schrappe M, Tiemann M, et al. Successful treatment strategy for Ki-1 anaplastic large-cell lymphoma of childhood: a prospective analysis of 62 patients enrolled in three consecutive Berlin-Frankfurt-Munster group studies. J Clin Oncol. 1994;12:899–908. doi: 10.1200/JCO.1994.12.5.899. [DOI] [PubMed] [Google Scholar]
- 10.Seidemann K, Tiemann M, Schrappe M, et al. Short-pulse B-non-Hodgkin lymphoma-type chemotherapy is efficacious treatment for pediatric anaplastic large cell lymphoma: a report of the Berlin-Frankfurt-Münster Group Trial NHL-BFM 90. Blood. 2001;97:3699–3706. doi: 10.1182/blood.v97.12.3699. [DOI] [PubMed] [Google Scholar]
- 11.Banavali SD, Goyal L, Bhagwat RV, et al. Vinblastine based short-pulse B-non-Hodgkin’s lymphoma type chemotherapy (CT) with maintenance therapy is highly efficacious treatment for anaplastic large cell lymphoma (ALCL) Blood. 2006;108(ASH Annual Meeting):abst 4673. [Google Scholar]
- 12.Rosolen A, Pillon M, Garaventa A, et al. Anaplastic large cell lymphoma treated with a leukemia-like therapy: report of the Italian Association of Pediatric Hematology and Oncology (AIEOP) LNH-92 protocol. Cancer. 2005;104:2133–2140. doi: 10.1002/cncr.21438. [DOI] [PubMed] [Google Scholar]
- 13.Laver JH, Kraveka JM, Hutchison RE, et al. Advanced-stage large-cell lymphoma in children and adolescents: results of a randomized trial incorporating intermediate-dose methotrexate and high-dose cytarabine in the maintenance phase of the APO regimen: a Pediatric Oncology Group phase III trial. J Clin Oncol. 2005;23:541–547. doi: 10.1200/JCO.2005.11.075. [DOI] [PubMed] [Google Scholar]
- 14.Lowe EJ, Sposto R, Perkins SL, et al. Intensive chemotherapy for systemic anaplastic large cell lymphoma in children and adolescents: final results of Childrens Cancer Group Study 5941. Pediatr Blood Cancer. 2009;52:335–339. doi: 10.1002/pbc.21817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dokmanovic L, Krstovski N, Vukanic D, et al. Pediatric non-Hodgkin lymphoma: a retrospective 14-year experience with Berlin-Frankfurt-Münster (BFM) protocols from a tertiary care hospital in Serbia. Pediatr Hematol Oncol. 2012;29:109–118. doi: 10.3109/08880018.2011.652342. [DOI] [PubMed] [Google Scholar]
- 16.Garner R, Li Y, Gray B, et al. Long-term disease control of refractory anaplastic large cell lymphoma with vinblastine. J Pediatr Hematol Oncol. 2009;31:145–147. doi: 10.1097/MPH.0b013e31819146f8. [DOI] [PubMed] [Google Scholar]
- 17.Yared J, Kimball A. The role of high dose chemotherapy and autologous stem-cell transplantation in peripheral T-cell lymphoma: a review of the literature and new perspectives. Cancer Treat Rev. 2013;39:51–59. doi: 10.1016/j.ctrv.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Woessmann W, Zimmermann M, Lenhard M, et al. Relapsed or refractory anaplastic large-cell lymphoma in children and adolescents after Berlin-Frankfurt-Muenster (BFM)-type firstline therapy: a BFM-group study. J Clin Oncol. 2011;29:3065–3071. doi: 10.1200/JCO.2011.34.8417. [DOI] [PubMed] [Google Scholar]
- 19.Gross TG, Hale GA, He W, et al. Hematopoietic stem cell transplantation for refractory or recurrent non-Hodgkin lymphoma in children and adolescents. Biol Blood Marrow Transplant. 2010;16:223–230. doi: 10.1016/j.bbmt.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sureda A, Canals C, Arranz R, et al. Allogeneic stem cell transplantation after reduced intensity conditioning in patients with relapsed or refractory Hodgkins lymphoma. Results of the HDR-ALLO study - a prospective clinical trial by the Grupo Español de Linfomas/Trasplante de Médula Osea (GEL/TAMO) and the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2012;97:310–317. doi: 10.3324/haematol.2011.045757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foyil KV, Bartlett NL. Brentuximab vedotin and crizotinib in anaplastic large-cell lymphoma. Cancer J. 2012;18:450–456. doi: 10.1097/PPO.0b013e31826aef4a. [DOI] [PubMed] [Google Scholar]
- 22.Gibb A, Jones C, Bloor A, et al. Brentuximab vedotin in refractory CD30+ lymphomas: a bridge to allogeneic transplantation in approximately one quarter of patients treated on a Named Patient Programme at a single UK center. Haematologica. 2013;98:611–614. doi: 10.3324/haematol.2012.069393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mosse YP, Lim MS, Voss SD, et al. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: a Children's Oncology Group phase 1 consortium study. Lancet Oncol. 2013;14:472–480. doi: 10.1016/S1470-2045(13)70095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park KD, Shin HY, Ahn HS. Childhood Ki-1 lymphoma: clinical features and treatment outcome. Korean J Hematol. 1995;30:455–462. [Google Scholar]
- 25.Murphy SB. Classification, staging and end results of treatment of childhood non-Hodgkin's lymphomas: dissimilarities from lymphomas in adults. Semin Oncol. 1980;7:332–339. [PubMed] [Google Scholar]
- 26.Uckun FM, Nachman JB, Sather HN, et al. Poor treatment outcome of Philadelphia chromosome-positive pediatric acute lymphoblastic leukemia despite intensive chemotherapy. Leuk Lymphoma. 1999;33:101–106. doi: 10.3109/10428199909093730. [DOI] [PubMed] [Google Scholar]
- 27.Heath JA, Steinherz PG, Altman A, et al. Human granulocyte colony-stimulating factor in children with high-risk acute lymphoblastic leukemia: a Children's Cancer Group Study. J Clin Oncol. 2003;21:1612–1617. doi: 10.1200/JCO.2003.07.129. [DOI] [PubMed] [Google Scholar]
- 28.Mora J, Filippa DA, Qin J, Wollner N. Lymphoblastic lymphoma of childhood and the LSA2-L2 protocol: the 30-year experience at Memorial-Sloan-Kettering Cancer Center. Cancer. 2003;98:1283–1291. doi: 10.1002/cncr.11615. [DOI] [PubMed] [Google Scholar]
- 29.Le Deley MC, Reiter A, Williams D, et al. Prognostic factors in childhood anaplastic large cell lymphoma: results of a large European intergroup study. Blood. 2008;111:1560–1566. doi: 10.1182/blood-2007-07-100958. [DOI] [PubMed] [Google Scholar]
- 30.Woessmann W, Peters C, Lenhard M, et al. Allogeneic haematopoietic stem cell transplantation in relapsed or refractory anaplastic large cell lymphoma of children and adolescents-a Berlin-Frankfurt-Münster group report. Br J Haematol. 2006;133:176–182. doi: 10.1111/j.1365-2141.2006.06004.x. [DOI] [PubMed] [Google Scholar]


