Abstract
We evaluated the influence of socioeconomic factors on female cancer mortality using death data from the Cause of Death Statistics and the Korean Population and Housing Census databases collected in 2001, 2006, and 2011. We estimated Relative Index of Inequality (RII) of female cancer mortality using Poisson regression analysis. RII greater than 1 indicates increased mortality risk for women at the lowest educational level compared with women at the highest educational level. The RII for cervical cancer mortality was persistently greater than 1 for the entire study period, with a gradual increase over time. Subgroup analysis stratified by age (25-44 and 45-64 yr) revealed that younger women had increased RIIs of mortality due to cervical cancer and ovarian cancer during the entire study period. Older women had higher RII only for cervical cancer mortality, but the value was much lower than that for younger women. The RII for breast cancer mortality was greater than 1 for younger women since 2006. In conclusion, socioeconomic inequality in female cancer mortality has persisted for the last decade in Korea, which was most evident for cervical cancer, and for younger women.
Graphical Abstract
Keywords: Educational Status, Mortality, Neoplasms, Socioeconomic Factors, Women
INTRODUCTION
Korea has undergone rapid economic growth over the last several decades. During this time, Korea has instituted a National Cancer Screening Program (NCSP) to prevent avoidable cancer deaths (1). Thus, a decrease in mortality from preventable cancers and increase in life expectancy were expected to occur in Korea over this time period. However, cancer mortality in Korea has increased during the last several decades, with the rate of increment in cancer mortality being higher for women than for men (2). Given that the mortality rates from cancers affecting both sexes are usually lower in women than in men, it is necessary to examine mortality due to female specific cancers to better understand the reason for these differences in cancer mortality by sex. Mortality data for female-specific cancers such as breast, ovarian, cervical, and uterine cancer in Korea reveals that these cancers made up 4.8%, 2.4%, 3.1%, and 2.5% of total cancer mortality, respectively, in 2000. These values changed to 7.9%, 3.5%, 3.5%, and 1.3%, respectively in 2012 (3). This change in female cancer mortality warrants an investigation of relevant factors.
In addition to factors such as smoking, occupational and environmental carcinogens, diet, obesity, physical inactivity, and infection (4), socioeconomic status (SES) is associated with cancer incidence and mortality (5, 6). Although SES does not have a direct effect on the pathophysiology of disease, it is a modifiable risk factor that may affect the development and progression of cancer (7). A report that the Gini index, which reflects socioeconomic inequality, has increased during the same period in Korea (8) raises concern about increasing socioeconomic inequality in terms of health. In accordance with this concern, some studies of the Korean population have reported disparities in the rate of participation in cancer screening according to educational level (9, 10). However, the influence of SES on female cancer has not been clearly evaluated in Korea, or in other countries that have experienced rapid economic change.
Thus, in the present study we evaluated the influence of SES on female cancer mortality by examining time trends of socioeconomic inequality according to educational level over the last decade in Korea.
MATERIALS AND METHODS
Data and study subjects
We conducted this study using death certificate data from the Statistics Korea and Korean Population and Housing Census (KPHC) data. We extracted individual information for women 25-64 yr of age who died from four major female cancers (breast, cervical, ovarian, and uterine cancer) in 2001, 2006, and 2011 from the death certificate data, which included age, sex, educational status, cause of death, and date of death. Four major cancers were identified using International Classification of Disease-10 codes: C500-C509 (breast cancer); C530-539 (cervical cancer); C56 (ovarian cancer); and C540-C549, C55, and C570-574 (uterine cancer). Although breast cancer may occur in men, we included breast cancer in this study because it occurs mostly in women and is the second most common cancer among Korean women (3). Other gynecological cancers such as vaginal and vulvar cancer were excluded because of their extremely low incidences. Female cancer mortality of Korean women younger than 25 yr of age was not included because of the very low mortality rate in those young women. Female cancer mortality occurred among women 65 yr or older was also not included.
We extracted data on population size by age and educational level from KPHC in 2000, 2005, and 2010 in order to use the information as population denominators for calculating mortality rates. The KPHC has been conducted at 5-yr interval since 1926 to understand the size, distribution, and structure of the Korean population, as well as the housing of all Koreans and foreigners residing in Korea.
Among the indicators of SES suggested on the basis of the Weverian framework, such as educational level, occupation, social class, income, wealth, marital status, race, and several social relationship indices (7), we selected educational level as an individual SES indicator for the present study because data on income, social class, or wealth was unavailable and less than 50% of Korean women were involved in economic activity.
Statistical methods
The subjects were categorized into four age groups: 25-34 yr, 35-44 yr, 45-54 yr, and 55-64 yr. We grouped subjects on the basis of educational status into four categories: elementary school or less (≤6 yr), middle school (7-9 yr), high school (10-12 yr), and college or higher (>12 yr).
Direct age-standardized cancer site specific mortality rates during 2001, 2006, and 2011 were calculated according to educational level using the respective numbers for the Korean population at the end of the previous year (i.e., 2000, 2005, and 2010). Then, time trends of the age-standardized mortality rates were assessed by the Spearman rank correlation test.
We calculated the relative index of inequality (RII) using Poisson regression to assess the degree of socioeconomic disparity in female cancer mortality according to educational level. RII is the rate ratio of the mortality rates of those at the lowest educational level compared with those at the highest educational level theoretically (11). An RII greater than 1 indicates that the mortality rate is increased in the group with lowest SES and thus, that inequality exists between different SES level. A larger RII indicates more severe inequality. If the RII is 2, then the mortality rate of the most disadvantaged is twice as high as that of the most advantaged.
Considering the rapid improvements in educational level of Korean women during the last several decades, we conducted a subgroup analysis by dividing the study sample into two birth year strata (born after or before 1955), which corresponds to two age strata (25-44 or 45-64 yr of age). As the distributions of educational level differ between younger and older women, we grouped subjects by educational level as follows: middle school or less, high school, and college or higher for the younger group (25-44 yr); elementary school or less, middle school, and high school and more for the older group (45-64 yr). All the analyses were conducted with SAS version 9.3 (SAS Institute, Inc., Cary, NC, USA).
Ethics Statement
The study was approved by the institutional review board of Samsung Medical Center (IRB No. 2011-10-070). The data used for this study do not include any identifiable personal information. As such, informed consent was waived by the board.
RESULTS
Table 1 shows mortality rate by educational level for cancers of the breast, cervix, ovary, and uterus. Through the whole study period, the mortality rate was highest for subjects with breast cancer, followed by cervical cancer, ovarian cancer, and uterine cancer. The total mortality rate from breast cancer and uterine cancer together gradually increased from 2001 to 2011, whereas total mortality from cervical cancer gradually decreased over the same period. There was no significant time trend in mortality from ovarian cancer. When we examined the time trend in mortality rates by educational level, we found that breast cancer mortality tended to progressively increase in women with lower educational level, while it has decreased in women with higher educational level. Cervical cancer mortality has gradually increased over time in women lower educational level, while no time trend was found for cervical cancer mortality in women with higher educational level. Uterine cancer mortality progressively increased over the study period for all educational levels. Ovarian cancer mortality has only slightly increased in women with the highest educational level.
Table 1.
Changes in age standardized mortality rates (per 100,000 persons) from female cancers from 2001 to 2011 according to educational level in Korean women 25-64 yr of age
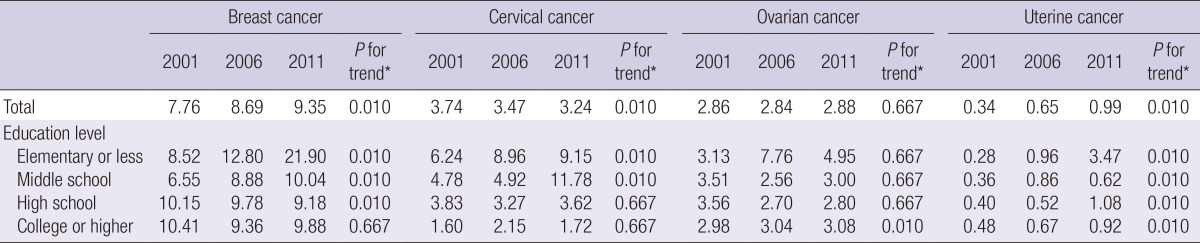
*Assessed by the Spearman rank correlation test.
Table 2 shows the RIIs of mortalities for four female cancers according to educational level. The RII of cervical cancer mortality was significantly greater than 1 over the entire study period, which indicates persistent socioeconomic inequality in cervical cancer mortality. The RII of cervical cancer mortality tended to increase over time. The RII of breast cancer mortality was significantly lower than 1 in 2001. However, this finding was reversed over time and the RII of breast cancer increased to 1.34 (95% confidence interval, 1.09-1.65) in 2011. The RII of ovarian cancer was also reversed through the period from 2001 to 2011 and increased to significantly greater than 1 in 2001. For cancers of the uterus, RII was not significant at any time (Table 2).
Table 2.
Change in relative index of inequality (95% confidence intervals) in mortality from female cancers from 2001 to 2011 according to educational level in Korean women 25-64 yr of age
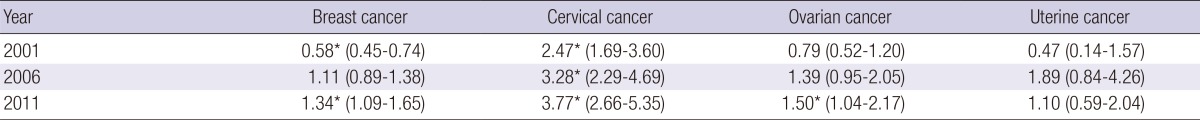
*Statistically significant (P value≤0.05), assessed by the Poisson regression analysis.
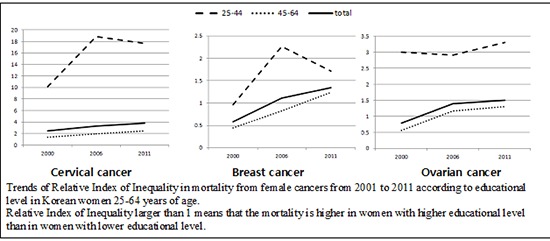
Table 3 shows the RIIs of mortalities for four female cancers according to educational level in two age strata. Socioeconomic inequality was more significant for women aged 25-44 yr than for older women. For younger women, the RII of breast cancer mortality did not differ from 1 in 2001, but increased to significantly higher than 1 since 2006. For older women, the RII was significantly lower than 1 in 2001, but not in 2006 and 2011. The RII of cervical cancer mortality was consistently greater than 1 over the entire study period for both younger and older women, but was much higher for younger women (>10) than for older women (<2). The RII of ovarian cancer for younger women was consistently greater than 1 over the entire study period, while it was lower than 1 or not different from 1 for older women over the entire study period. The RII of uterine cancer mortality did not differ significantly from 1 in both age strata during the entire study periods. There were no specific time trends of RII of cancer mortality for all four cancers across both age strata.
Table 3.
Relative index of inequality (95% confidence intervals) in age-stratified mortality (25-44 yr and 45-64 yr) from four female cancers from 2001 to 2011 according to educational level in Korean women
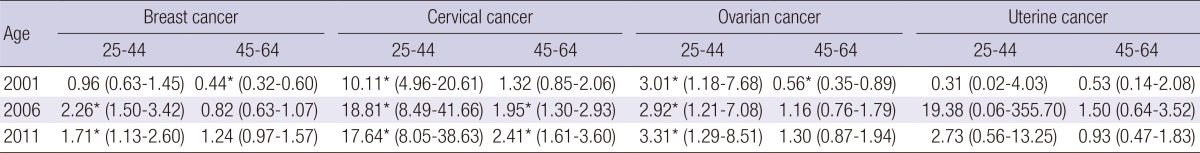
*Statistically significant (P value≤0.05), assessed by the Poisson regression analysis.
DISCUSSION
With the purpose of reducing cancer mortality in the Korean population, the Korean government launched the NCSP targeting cervical, breast, and gastric cancers in 1999. Initially, the target population was limited to medical aid beneficiary, who were the socioeconomically most deprived population. Since then, the program has gradually been expanded to include beneficiaries of the National Health Insurance Corporation (NHIC) in an effort to cover more deprived individuals first and more affluent persons later. Coverage was extended to those within the lower 20% of NHIC premiums in 2002, to those within the lower 50% of NHIC premiums in 2005, and to all beneficiaries in 2010. Thus, since 2010, these programs have provided most Korean women with free screening against cervical cancer (if they are aged 30 yr or older) and breast cancer (if they are aged 40 yr or older) if they are within the lower 50% of NHIC premiums, or for 10% of out-of-pocket expenses if they are within the upper 50% of NHIC premiums. (12) However, there are no specific recommended screening tests for cancers of the ovary or uterus. Thus, we hypothesized that socioeconomic disparities in mortality from cervical and breast cancer would decline over time since the introduction of the NCSP, while we expected no significant changes in mortality from ovary and uterine cancer due to socioeconomic disparity.
However, in this study, we found that socioeconomic inequality in mortality from cervical cancer persisted throughout the decade after the introduction of the NCSP. In addition, subgroup analysis by age strata showed that such socioeconomic disparity was more evident in younger women than in older women for mortality due to cancers of the cervix, breast, and ovary.
In the present study the RII, which reflects socioeconomic disparity, was greatest for cervical cancer among the four major female cancers. The inverse association between SES and cervical cancer mortality observed in the present study is consistent with the findings of previous studies (5, 13). Lower participation rates in cervical cancer screening by women of low SES may explain the higher cervical cancer mortality in women with lower educational levels in Korea (9, 10). Thus, strategies to encourage women of lower SES to more actively participate in cancer screening programs are essential. A previous study found that changes in the time trends of cervical cancer mortality might be related to changes in the patterns of marriage and sexual behavior over time (14). Multiple sexual partners, earlier age at first sexual intercourse, and less frequent use of barrier protection have been suggested as risk factors for cervical cancer (15). However, little is known regarding the roles of these putative risk factors in explaining the higher cervical cancer mortality in Korean women of low SES observed in the present study, which warrants further analyses. In addition, the provision of free immunization with human papilloma virus vaccine for girls of low SES has been shown to be critical to reduce the socioeconomic gap in cervical cancer mortality (16).
Breast cancer was once portrayed as a "disease of affluence" because the incidence (17) and mortality (5, 6, 18, 19) of breast cancer were higher in more industrialized countries and among more affluent people. This finding may occur because women of higher SES are more likely to use estrogen replacements, to give birth at later ages, and to have lower fertility levels compared with women of lower SES (20). On the other hand, some studies have suggested that the relationship between breast cancer and educational level may differ according to race (19), nationality (21), and age (18) of the studied population. An inverse association between SES and breast cancer has also been reported (22, 23), especially with respect to mortality. Several explanations were suggested as possible reasons for the higher mortality among women of lower SES, such as more advanced stage at diagnosis of breast cancer, greater number of co-morbid conditions, and higher levels of obesity compared with women of higher SES (24).
Up to now, few studies have addressed the effects of socioeconomic disparity on breast cancer mortality among Korean women (25, 26). In those studies, the mortality rate from breast cancer was higher among more educated women than among less educated women before 2004. However, that previous study did not examine data regarding breast cancer mortality after 2004. The findings of our study are comparable to the findings of the previous study as comparisons are made up to 2006 for older women. However, interestingly, the socioeconomic disparity in breast cancer mortality according to educational level observed in our study turned in the opposite direction in 2006, especially for younger women. Similar changing patterns of socioeconomic inequality in breast cancer mortality over time have also been observed in the United States (22). Several explanations for the patterns of breast cancer mortality according to SES changing from positive to inverse directions are possible. First, the gap in reproductive patterns between women of lower SES and those of higher SES is decreasing due to an overall trend toward low birth rates worldwide. Second, socioeconomic differentials in survival by SES may have been aggravated because of limited accessibility to earlier detection and timely, high-quality treatment among women of lower SES (22).
Although more evidence and longer study periods are needed, the findings of the present study raise concerns regarding the possible aggravation of socioeconomic inequality in breast cancer mortality in the future, especially among younger women. A previous study reported that socioeconomic disparities in receiving breast cancer screening tests are not found in countries with population-based screening programs, while significant socioeconomic disparities are observed in countries with opportunistic screening programs (27). Korea provides population-based screening against breast cancer and is therefore expected to have low levels of socioeconomic disparity in breast cancer mortality. Therefore, the increasing socioeconomic disparity observed in our study suggests that the Korean screening program may not be effective for young women of lower SES. Considering that death from breast cancer typically occurs several years after diagnosis and that the peak age of breast cancer death is 50 to 59 yr (28), it may be necessary to increase the screening participation rates of younger women, including those in their 40s. The NCSP participation rates tend to be higher among Korean women in their 50s, while abnormal results are more frequently found among women in their 40s (29). Thus, making efforts to further encourage younger women of lower SES to participate in screening programs may help reduce the socioeconomic inequalities in breast cancer mortality observed in this study. The NCSP against breast cancer should be more focused on people of lower SES in order to eliminate or diminish these socioeconomic disparities.
Without including age as a variable, we detected no association between ovarian cancer mortality and educational level in our study; but, age-stratified analysis revealed socioeconomic disparity in ovarian cancer mortality among younger women. Previous studies in Caucasian and Mexican populations have reported positive (5), inverse (30), and null associations (17) between SES and ovarian cancer incidence and mortality. Differences in the age distributions of populations between studies may explain such diverse findings. Up to now, no specific screening guidelines for the early detection of ovarian cancer have been recommended. Thus, further studies examining the relationship between SES and ovarian cancer, as well as more effective ways of preventing death from ovarian cancer, are needed.
Interestingly, the inequality of female cancer mortality according to educational level was more evident among women aged 25-44 yr than among women aged 45 yr or older for mortalities from cervical cancer, ovarian cancer, and breast cancer in the present study. Given that the educational levels of Korean women have increased remarkably during the decades since the Korean War (9), lower educational levels among younger women probably reflect more disadvantaged conditions than the same low educational levels among older women. Thus, this difference may have resulted in the greater socioeconomic disparity in female cancer mortality according to educational level that we observed among younger women than among older women.
This study has some limitations. First, we calculated age-standardized mortality rates and RIIs in five-year intervals rather than annually. This was necessary because the KPHC, the only survey providing population statistics by educational level, is conducted at five-year intervals. Second, we observed time trends for only 10 yr, which may be too short a study period to provide sufficient data. To properly evaluate the effects of the NCSP in preventing cancer mortality and reducing inequalities in cancer mortality, a follow-up study conducted 10 to 20 yr after the program's inception is necessary. Third, educational level may not be the best SES indicator for Koreans, given that Koreans tend to be more enthusiastic about education than other populations. Thus, by using education level as a SES indicator our study might have underestimated socioeconomic inequality in female cancer mortality. Fourth, the present study did not include women 65 yr or older. Thus, it is hard to generalize the findings of this study to women older than 64 yr.
In conclusion, socioeconomic inequality in female cancer mortality has persisted during the last decade in Korea, especially for cervical cancer mortality, even after the introduction of the NCSP. Age-stratified analysis revealed greater socioeconomic disparity in mortality from cancers of the cervix, ovary and breast among younger women than among older women. These findings suggest that diverse efforts to eliminate or reduce socioeconomic gaps in cancer mortality are necessary, especially for younger women in Korea.
Footnotes
This research work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and future Planning (2011-0013545 and 2014R1A2A2A01002705).
All authors have no potential conflicts of interest.
Conceived and designed the study: MH Kim, YM Song, K Jung-Choi, H Kim. Data collection: MH Kim, H Kim. Analyzed the data: MH Kim, YM Song, K Jung-Choi. Wrote the first draft of the manuscript: MH Kim, YM Song, K Jung-Choi, H Kim. Wrote the paper: MH Kim, YM Song, K Jung-Choi, H Kim. ICMJE criteria for authorship read and met: MH Kim, YM Song, K Jung-Choi, H Kim. Agree with manuscript results and conclusions: MH Kim, YM Song, K Jung-Choi, H Kim.
References
- 1.Cho B, Lee CM. Current situation of national health screening systems in Korea. J Korean Med Assoc. 2011;54:666–669. [Google Scholar]
- 2.Statistics Korea. Causes of death statistics in 2011. [accessed on 31 March 2014]. Available at http://kostat.go.kr/portal/english/news/1/1/15/index.board?bmode=read&aSeq=271601&pageNo=&rowNum=10&amSeq=&sTarget=&sTxt=
- 3.Statistics Korea. Korean statistial information service. [accessed on 6 August 2014]. Available at http://kosis.kr/statisticsList/statisticsList_01List.jsp?vwcd=MT_ZTITLE&parmTabId=M_01_01#SubCont.
- 4.Goldman L, Cecil RL, Schafer AI. Goldman's Cecil medicine. 24th ed. Philadelphia: Elsevier/Saunders; 2012. [Google Scholar]
- 5.Faggiano F, Lemma P, Costa G, Gnavi R, Pagnanelli F. Cancer mortality by educational level in Italy. Cancer Causes Control. 1995;6:311–320. doi: 10.1007/BF00051406. [DOI] [PubMed] [Google Scholar]
- 6.Heck KE, Wagener DK, Schatzkin A, Devesa SS, Breen N. Socioeconomic status and breast cancer mortality, 1989 through 1993: an analysis of education data from death certificates. Am J Public Health. 1997;87:1218–1222. doi: 10.2105/ajph.87.7.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berkman LF, Kawachi I. Social epidemiology. New York: Oxford University Press; 2000. [Google Scholar]
- 8.Cheon BY, Chang JH, Shin GS, Kang JW, Lee SW, Kim BH, Joo H. Growing inequality and its impact in Korea: country report for Korea. [accessed on 15 March 2014]. Available at http://gini-research.org/system/uploads/439/original/Korea.pdf?1370077269.
- 9.Jang SN, Cho SI, Hwang SS, Jung-Choi K, Im SY, Lee JA, Kim MK. Trend of socioeconomic inequality in participation in cervical cancer screening among Korean women. J Prev Med Public Health. 2007;40:505–511. doi: 10.3961/jpmph.2007.40.6.505. [DOI] [PubMed] [Google Scholar]
- 10.Park SJ, Park WS. Identifying barriers to Papanicolaou smear screening in Korean women: Korean National Health and Nutrition Examination Survey 2005. J Gynecol Oncol. 2010;21:81–86. doi: 10.3802/jgo.2010.21.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackenbach JP, Kunst AE. Measuring the magnitude of socio-economic inequalities in health: an overview of available measures illustrated with two examples from Europe. Soc Sci Med. 1997;44:757–771. doi: 10.1016/s0277-9536(96)00073-1. [DOI] [PubMed] [Google Scholar]
- 12.Kim Y, Jun JK, Choi KS, Lee HY, Park EC. Overview of the National Cancer screening programme and the cancer screening status in Korea. Asian Pac J Cancer Prev. 2011;12:725–730. [PubMed] [Google Scholar]
- 13.Simard EP, Fedewa S, Ma J, Siegel R, Jemal A. Widening socioeconomic disparities in cervical cancer mortality among women in 26 states, 1993-2007. Cancer. 2012;118:5110–5116. doi: 10.1002/cncr.27606. [DOI] [PubMed] [Google Scholar]
- 14.Murphy MF, Mant DC, Goldblatt PO. Social class, marital status, and cancer of the uterine cervix in England and Wales, 1950-1983. J Epidemiol Community Health. 1992;46:378–381. doi: 10.1136/jech.46.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Sanjosé S, Bosch FX, Muñoz N, Shah K. Social differences in sexual behaviour and cervical cancer. IARC Sci Publ. 1997:309–317. [PubMed] [Google Scholar]
- 16.Chan JK, Berek JS. Impact of the human papilloma vaccine on cervical cancer. J Clin Oncol. 2007;25:2975–2982. doi: 10.1200/JCO.2007.10.8662. [DOI] [PubMed] [Google Scholar]
- 17.La Vecchia C, Negri E, Franceschi S. Education and cancer risk. Cancer. 1992;70:2935–2941. doi: 10.1002/1097-0142(19921215)70:12<2935::aid-cncr2820701234>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 18.Khang YH, Lynch JW, Kaplan GA. Health inequalities in Korea: age- and sex-specific educational differences in the 10 leading causes of death. Int J Epidemiol. 2004;33:299–308. doi: 10.1093/ije/dyg244. [DOI] [PubMed] [Google Scholar]
- 19.Kim C, Eby E, Piette JD. Is education associated with mortality for breast cancer and cardiovascular disease among black and white women? Gend Med. 2005;2:13–18. doi: 10.1016/s1550-8579(05)80005-1. [DOI] [PubMed] [Google Scholar]
- 20.Strand BH, Tverdal A, Claussen B, Zahl PH. Is birth history the key to highly educated women's higher breast cancer mortality? A follow-up study of 500,000 women aged 35-54. Int J Cancer. 2005;117:1002–1006. doi: 10.1002/ijc.21239. [DOI] [PubMed] [Google Scholar]
- 21.Strand BH, Kunst A, Huisman M, Menvielle G, Glickman M, Bopp M, Borell C, Borgan JK, Costa G, Deboosere P, et al. EU Working Group on Socioeconomic Inequalities in Health. The reversed social gradient: higher breast cancer mortality in the higher educated compared to lower educated. A comparison of 11 European populations during the 1990s. Eur J Cancer. 2007;43:1200–1207. doi: 10.1016/j.ejca.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 22.Albano JD, Ward E, Jemal A, Anderson R, Cokkinides VE, Murray T, Henley J, Liff J, Thun MJ. Cancer mortality in the United States by education level and race. J Natl Cancer Inst. 2007;99:1384–1394. doi: 10.1093/jnci/djm127. [DOI] [PubMed] [Google Scholar]
- 23.Sprague BL, Trentham-Dietz A, Gangnon RE, Ramchandani R, Hampton JM, Robert SA, Remington PL, Newcomb PA. Socioeconomic status and survival after an invasive breast cancer diagnosis. Cancer. 2011;117:1542–1551. doi: 10.1002/cncr.25589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woods LM, Rachet B, Coleman MP. Origins of socio-economic inequalities in cancer survival: a review. Ann Oncol. 2006;17:5–19. doi: 10.1093/annonc/mdj007. [DOI] [PubMed] [Google Scholar]
- 25.Jung-Choi K, Khang YH, Cho HJ. Changes in contribution of causes of death to socioeconomic mortality inequalities in Korean adults. J Prev Med Public Health. 2011;44:249–259. doi: 10.3961/jpmph.2011.44.6.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung-Choi K, Khang YH, Cho HJ. Socioeconomic differentials in cause-specific mortality among 1.4 million South Korean public servants and their dependents. J Epidemiol Community Health. 2011;65:632–638. doi: 10.1136/jech.2009.100651. [DOI] [PubMed] [Google Scholar]
- 27.Palència L, Espelt A, Rodríguez-Sanz M, Puigpinós R, Pons-Vigués M, Pasarín MI, Spadea T, Kunst AE, Borrell C. Socio-economic inequalities in breast and cervical cancer screening practices in Europe: influence of the type of screening program. Int J Epidemiol. 2010;39:757–765. doi: 10.1093/ije/dyq003. [DOI] [PubMed] [Google Scholar]
- 28.Osborne C, Ostir GV, Du X, Peek MK, Goodwin JS. The influence of marital status on the stage at diagnosis, treatment, and survival of older women with breast cancer. Breast Cancer Res Treat. 2005;93:41–47. doi: 10.1007/s10549-005-3702-4. [DOI] [PubMed] [Google Scholar]
- 29.Sung NY, Park EC, Shin HR, Choi KS. Participation rate and related socio-demographic factors in the national cancer screening program. J Prev Med Public Health. 2005;38:93–100. [PubMed] [Google Scholar]
- 30.Bernal A, Méndez-Moran L, Fajardo-Gutiérrez A, González-Lira G, Escudero P, Ortiz H. Univariate and multivariate analysis of risk factors for ovarian cancer: case-control study, Mexico City. Arch Med Res. 1995;26:245–249. [PubMed] [Google Scholar]


