Abstract
Echocardiographic parameters can predict cardiovascular events in several clinical settings. However, which echocardiographic parameter is most predictive of each cardiovascular or non-cardiovascular event in patients starting hemodialysis remains unresolved. Echocardiography was used in 189 patients at the time of starting hemodialysis. We established primary outcomes as follows: cardiovascular events (ischemic heart disease, cerebrovascular disease, peripheral artery disease, and acute heart failure), fatal non-cardiovascular events, all-cause mortality, and all combined events. The most predictable echocardiographic parameter was determined in the Cox hazard ratio model with a backward selection after the adjustment of multiple covariates. Among several echocardiographic parameters, the E/e' ratio and the left ventricular end-diastolic volume (LVEDV) were the strongest predictors of cardiovascular and non-cardiovascular events, respectively. After the adjustment of clinical and biochemical covariates, the predictability of E/e' remained consistent, but LVEDV did not. When clinical events were further analyzed, the significant echocardiographic parameters were as follows: s' for ischemic heart disease and peripheral artery disease, LVEDV and E/e' for acute heart failure, and E/e' for all-cause mortality and all combined events. However, no echocardiographic parameter independently predicted cerebrovascular disease or non-cardiovascular events. In conclusion, E/e', s', and LVEDV have independent predictive values for several cardiovascular and mortality events.
Keywords: Heart Failure, Diastolic; Echocardiography; Kidney Failure, Chronic; Renal Dialysis; Morbidity; Mortality
INTRODUCTION
End-stage renal disease (ESRD) significantly increases overall morbidity and mortality (1), and thus, its social and economic burden is globally large (2). Although patients with ESRD have received intensive attention for this reason, the prevalence rate of ESRD has not decreased but rather has continued to grow at approximately 3% per year (3). According to the Korean ESRD registry, the overall prevalence of ESRD in Korea is similar to the global trend (4). Because the risks of morbidity and mortality in Korean ESRD patients are still high, 3% of the national health care expenditure is used to provide care for ESRD patients even though they account for only 0.02%-0.03% of the total population (5). Accordingly, several efforts to identify modifiable factors associated with worse outcomes in Korean ESRD patients have been made (6), but more studies are needed.
Echocardiography is one of the modern methods used to estimate myocardial function quantitatively. In recent years, the assessment of myocardial systolic and diastolic functions using tissue Doppler imaging (TDI) has been established as a common approach to detect subclinical abnormalities because it can directly monitor mechanical wall function, which correlates with hydrodynamic responses. Previous studies have revealed that systolic velocity is a good marker of left ventricular (LV) function, which significantly correlates with LV ejection fraction or other estimates, such as LV contraction (ejection phase) and contractility (isovolumetric contraction), obtained by invasive methods (7). Diastolic velocity has also been shown to correlate with the time constant of LV relaxation and to correlate inversely with LV volumes and pressures (8). With this excellent accuracy, TDI parameters are proposed as reliable markers for prognosis (9).
Previous studies on ESRD patients have been conducted to predict morbidity and mortality outcomes using both echocardiography and TDI measurements (10, 11, 12, 13, 14). All of these results show significant correlations between certain echocardiographic parameters and outcomes. However, while these outcomes are all considered cardiovascular events or all-cause mortality, they have not analyzed the associations according to the cause or subtype of cardiovascular or non-cardiovascular events. Cardiovascular events are the most common cause for mortality in ESRD patients because they comprise almost 20%-40% of mortality cases (15). However, each cardiovascular event, such as ischemic heart disease, cerebrovascular disease, peripheral vascular disease, or acute heart failure, may be differentially associated with echocardiographic parameters, but this issue has not been studied. Non-cardiovascular death is also considered important because ESRD patients still have a high mortality risk attributable to this event. However, there is also little knowledge regarding the correlation between echocardiographic parameters and non-cardiovascular events. Herein, we have conducted the current study to address the correlations of echocardiographic parameters with several cardiovascular and non-cardiovascular events in patients starting hemodialysis.
MATERIALS AND METHODS
Patients and data collection
The present study follows the STROBE guideline reporting (Supplementary file). A total of 193 patients starting hemodialysis from January 2009 through December 2011 were enrolled. All patients were more than 20 yr old, did not have a history of kidney transplantation or congenital heart disease, and underwent a baseline echocardiography. Among the study subjects, 4 patients were excluded based on the unavailability of TDI data. Consequently, 189 patients were followed and reviewed from the day of baseline assessments until kidney transplantation or December 2013. The need for informed consent was waived because of non-interventional study design based on collected data.
Clinical parameters, such as age, sex, weight, height, diabetes mellitus, history of cardiovascular events, systolic/diastolic blood pressure, type of vascular access, and the use of medications including aspirin/other antiplatelet agents, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, calcium channel blockers, beta blockers, and diuretics, were recorded. Body mass index was calculated as (weight [kg]/height [m2]). Patients who were taking pills or insulin to reduce their blood glucose levels or who were diagnosed with diabetes mellitus by medical doctors were identified as having diabetes mellitus. Blood sample parameters, such as hemoglobin, calcium, phosphorus, uric acid, blood urea nitrogen, creatinine, cholesterol, and albumin, were measured at baseline.
Echocardiography
After 2 or 3 sessions of dialysis, all of the study patients underwent comprehensive echocardiographic examinations using commercially available ultrasound equipment (Vivid E9, GE Medical Systems, Horten, Norway) with subjects in a left lateral position. Standard M-mode echocardiographic examination included the following measurements: LV end-diastolic volume (LVEDV) (mL), LV end-systolic volume (mL), LV end-diastolic and end-systolic diameter (mm), diastolic and systolic interventricular septal wall thickness (mm), diastolic and systolic LV posterior wall thickness (mm), left atrial (LA) antero-posterior diameter (mm), aortic root diameter (mm), and regional wall motion abnormality (RWMA). LV mass index was determined using the method of Devereux et al. and indexed for body surface area (16). LA volume index was assessed using the biplane area-length method and indexed for body surface area (17). Regional wall thickness was measured as follows: 2×LV posterior wall thickness (mm) / LV end-diastolic diameter (mm). Fractional shortening was calculated with LV end-diastolic and end-systolic diameters (17). LV ejection fraction was determined by a modified biplane Simpson's method from the apical two-chamber and four-chamber views.
A pulsed-wave Doppler for mitral inflow was further examined. The motion of the mitral annulus was recorded in the apical four-chamber view. Peak early (E) and late (A) diastolic velocities of the mitral inflow were measured using pulsed-wave Doppler with the sample volume at the tip of the mitral leaflets. Systolic (s') and early (e')/late (a') diastolic mitral annular velocities were also assessed on the septal side of the mitral annulus using pulsed-wave tissue Doppler imaging. The E/A and E/e' ratios for the LV filling index were calculated in accordance with the guideline (18). Tricuspid regurgitation velocity was recorded from a routine right ventricular inflow view using continuous-wave Doppler. Right ventricular systolic pressure was calculated using the modified Bernoulli equation (19).
Primary outcomes
When the patients were admitted to the hospital during the follow-up period, the cause of hospitalization was determined by a review of medical records or telephone interviews. Non-fatal and fatal cardiovascular events included ischemic heart disease; cerebrovascular disease, such as ischemic and hemorrhagic stroke; peripheral vascular disease; and acute heart failure. The mortality data were obtained from medical records and the national database of Statistics Korea. The causes of mortality were reviewed using the code data of the International Classification of Diseases, 10th revision (ICD-10), which was provided by the National Statistical Office of Korea, or telephone interviews. These causes were classified as cardiovascular (e.g., myocardial infarction or aggravation of heart failure) and non-cardiovascular events (e.g., infection or bleeding). Accordingly, we established the primary outcomes as follows: cardiovascular events (ischemic heart disease, cerebrovascular disease, peripheral artery disease, and acute heart failure), fatal non-cardiovascular events, all-cause mortality, and all combined events (all above events).
Statistical analysis
All of the analyses and calculations were performed using SPSS (SPSS version 21.0, IBM, Armonk, NY, USA) and STATA (STATA version 12.0, StataCorp LP, College Station, TX, USA). The data are presented as means±standard deviation (SD) for continuous variables and as proportions for categorical variables. The hazard ratios (HRs) and 95% confidence intervals (CIs) for morbidity and mortality rates were calculated using the Cox proportional hazard models for several clinical outcomes, including cardiovascular events (ischemic heart disease, cerebrovascular disease, peripheral artery disease, and acute heart failure), fatal non-cardiovascular events, all-cause mortality, and all combined events. To prevent co-linearity among significant echocardiographic parameters, the backward stepwise selection method was used. Additionally, to find the independent echocardiographic parameters, clinical and biochemical covariates with P<0.1 in the univariate analysis were adjusted. Important covariates such as age, sex, and diabetes mellitus were adjusted in all models to have adequate confounder control. To account for possible nonlinear relationships between echocardiographic parameters and outcomes, we applied the fractional polynomials method and showed the relationship as a fitted curve. A P value of less than 0.05 was considered significant.
Ethics statement
The study protocol complied with the Declaration of Helsinki and achieved full approval from the institutional review board at the Seoul National University Bundang Hospital (no. B-1304/200-108). Informed consent was waived by the board.
RESULTS
Baseline characteristics of the study subjects
Table 1 shows the baseline characteristics of the study subjects. The mean age was 63.7 yr. All of the subjects were of Asian descent. More than half of the patients were diagnosed with diabetes mellitus. The study subjects were followed for a mean duration of 33.3±15.3 months (maximum of 5.1 yr). Mean echocardiographic measurements are shown in Table 2. The mean value of the LV mass index was 133.4 g/m2: male, 135.6±36.98 g/m2; female, 130.4±40.68 g/m2. When LV hypertrophy was defined with parameters of over 115.0 g/m2 in male and over 98.0 g/m2 in female subjects, 66.7% and 76.5% of the male and female subjects had LV hypertrophy, respectively. The mean velocities of E, s', and e' were 73.1 cm/s, 7.3 cm/s, and 5.6 cm/s, respectively. With these results, the mean of the E/e' ratio was 15.1.
Table 1.
Baseline characteristics of study subjects
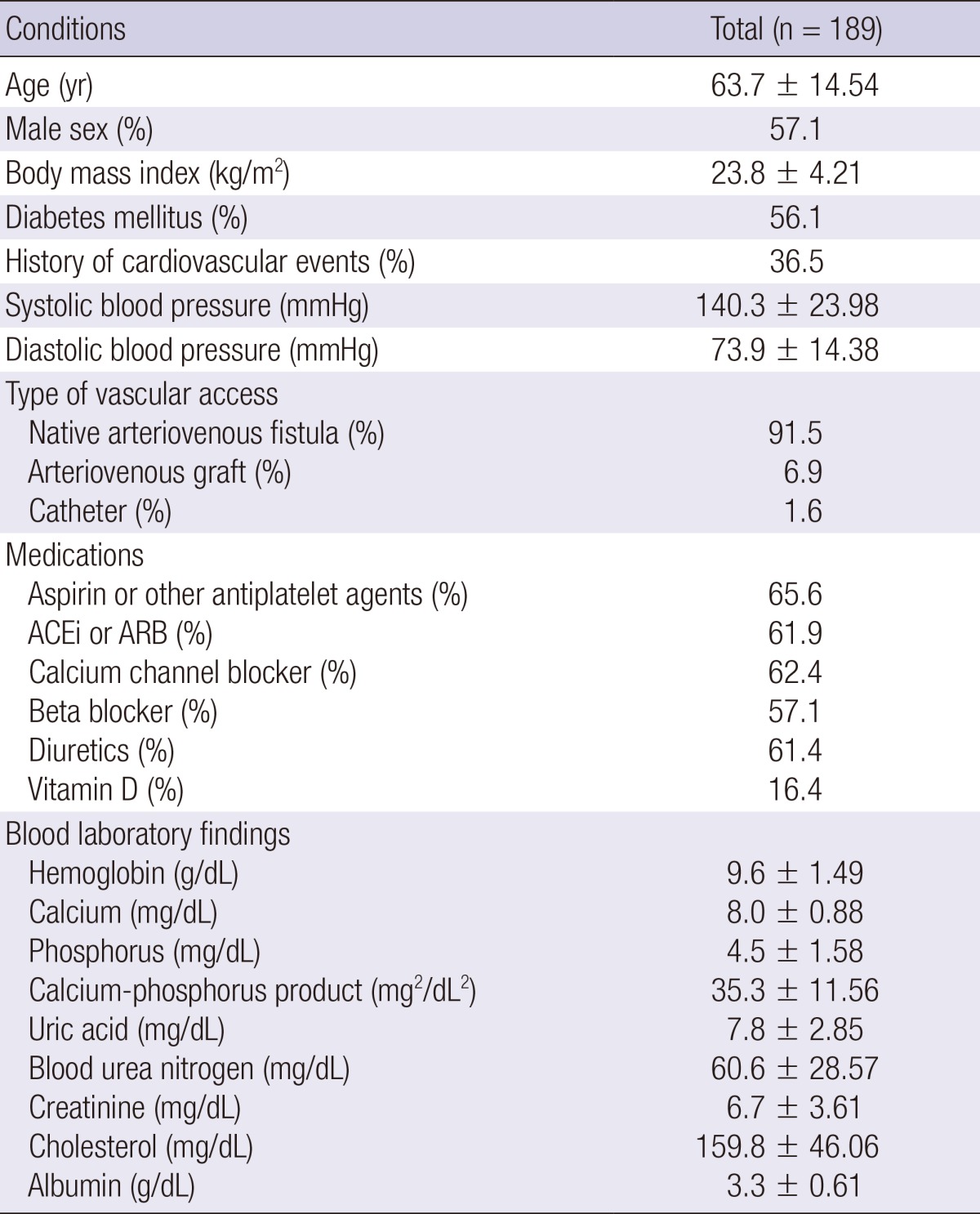
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker.
Table 2.
Echocardiographic parameters and associations with cardiovascular events
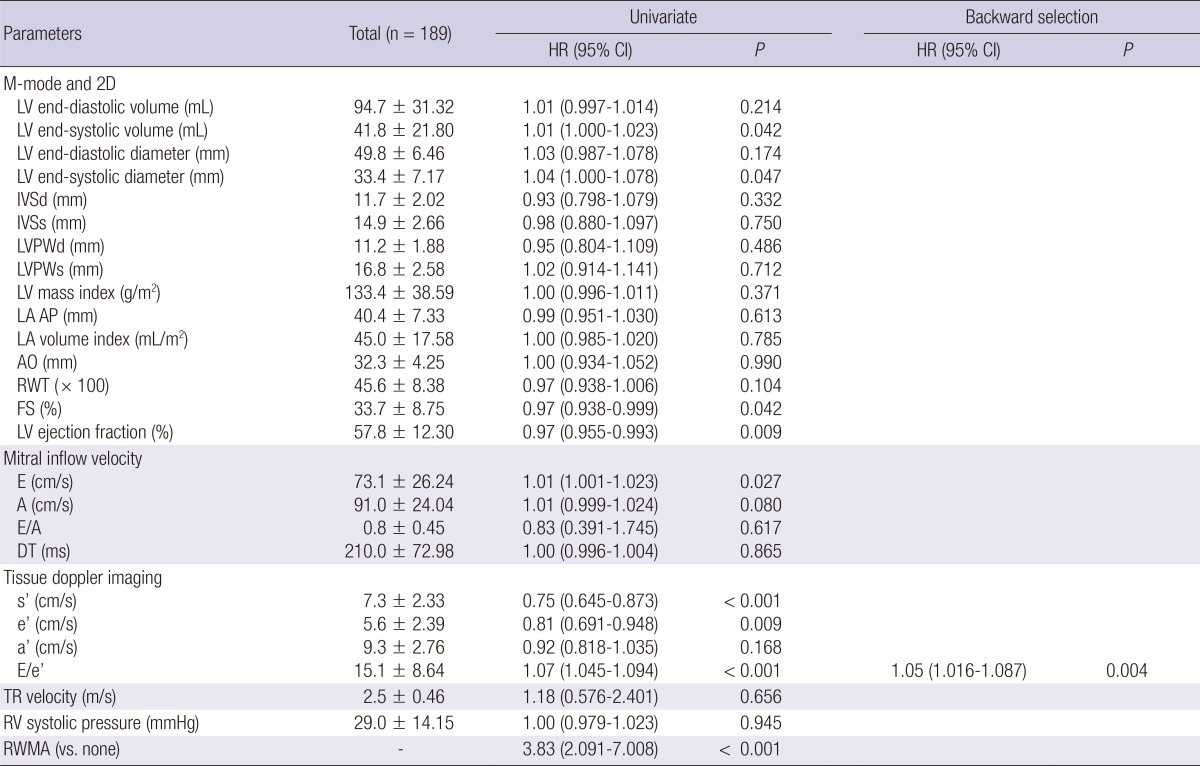
HR, hazard ratio; CI, confidence interval; LV, left ventricle; IVSd, diastolic interventricular septal wall thickness, IVSs, systolic interventricular septal wall thickness; PWd, diastolic left ventricular posterior wall thickness; PWs, systolic left ventricular posterior wall thickness; LA AP, left atrial antero-posterior diameter; AO, aortic root diameter; RWT, relative wall thickness; FS, fractional shortening; E, early diastolic transmitral inflow velocity; A, late diastolic transmitral flow velocity; DT, deceleration time; s', mitral annular systolic velocity; e', early diastolic mitral annular velocity; a', late diastolic mitral annular velocity; RV, right ventricle; TR, tricuspid regurgitation; RWMA, regional wall motion abnormality.
Echocardiographic parameters associated with cardiovascular and non-cardiovascular events
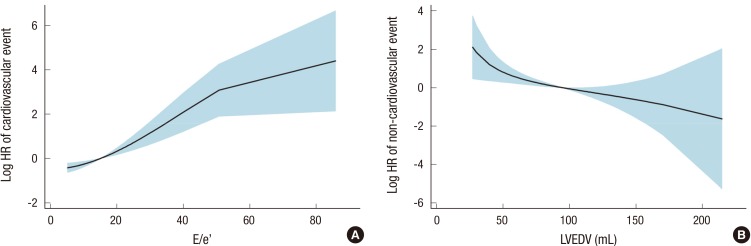
Among the study subjects, 47 patients (24.9%) underwent 49 fatal and non-fatal cardiovascular events after hemodialysis. The subtypes of cardiovascular events were as follows: ischemic heart disease, 17 cases (34.7%); cerebrovascular disease, 9 cases (18.4%); peripheral artery disease, 4 cases (8.2%); and acute heart failure, 19 cases (38.8%). Throughout the following period, 58 patients (30.7%) died; 9 of the deaths had cardiovascular causes, and the rest had non-cardiovascular causes. Among the non-cardiovascular causes, infectious death was most common (n=18). Other non-cardiovascular causes included complications of hemodialysis (n=5), cancer (n=3), and non-cardiovascular hemorrhage (n=2). Each echocardiographic parameter was included in the univariate hazard model as a continuous variable, whereas the RWMA was included as a categorical variable (Table 2). Accordingly, low systolic (s') and early diastolic (e') mitral annular velocities increased the risk of cardiovascular events. Additionally, a high E/e' ratio was associated with the high risk of cardiovascular events. After applying the backward selection model, the E/e' ratio was only a significant predictor of cardiovascular events. The fitted curve between the HR of cardiovascular events and E/e' is shown in Fig. 1A. Additional analysis was conducted for non-cardiovascular events (Table 3). In the univariate analysis, only LVEDV showed a significant correlation with non-cardiovascular events. Systolic/diastolic mitral annular velocities or E/e' were not correlated with non-cardiovascular events. The fitted curve between the HR of non-cardiovascular events and LVEDV is shown in Fig. 1B.
Fig. 1.
Fitted curves between the strongest predictive echocardiographic parameters and the hazard ratios of cardiovascular (A) and non-cardiovascular (B) events. HR, hazard ratio; E, early diastolic transmitral inflow velocity; e', early diastolic mitral annular velocity; LVEDV, left ventricular end-diastolic volume.
Table 3.
Echocardiographic parameters and associations with the non-cardiovascular events
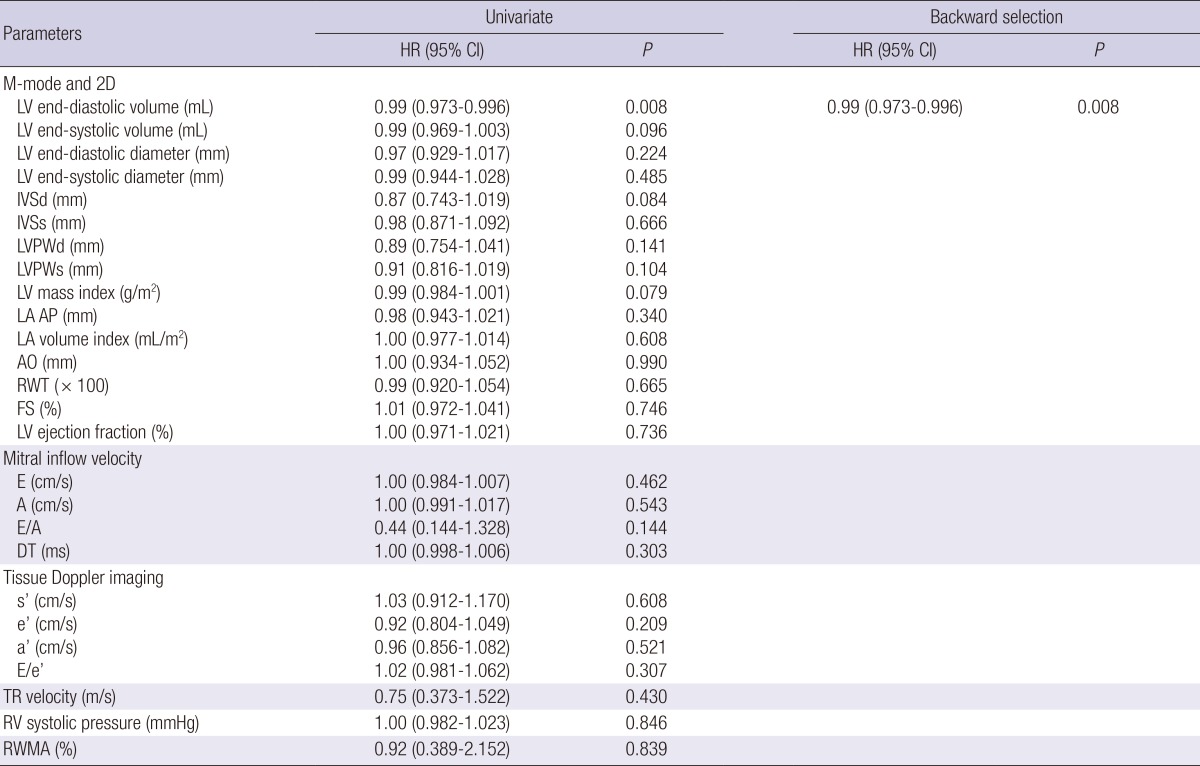
HR, hazard ratio; CI, confidence interval; LV, left ventricle; IVSd, interventricular septal thickness at end-diastole; IVSs, interventricular septal thickness at end-systole; PWd, posterior wall thickness at end-diastole; PWs, posterior wall thickness at end-systole; LA AP, left atrial antero-posterior diameter; AO, aortic root diameter; RWT, relative wall thickness; FS, fractional shortening; E, early diastolic transmitral inflow velocity; A, late diastolic transmitral flow velocity; DT, deceleration time; s', mitral annular systolic velocity; e', early diastolic mitral annular velocity; a', late diastolic mitral annular velocity; RV, right ventricle; TR, tricuspid regurgitation; RWMA, regional wall motion abnormality.
Several clinical and biochemical parameters, such as age, history of cardiovascular events, diastolic blood pressure, type of vascular access, use of antiplatelet agents and angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker, hemoglobin, calcium, creatinine, and cholesterol had a P value <0.10 in the univariate analysis for several clinical events (Table 4). Therefore, these parameters plus sex and diabetes mellitus were adjusted to the multivariate Cox model throughout the study. Although multiple clinical and biochemical covariates were adjusted to the cardiovascular Cox model, the E/e' ratio also remained significant (Table 5). However, LVEDV was not included in the final non-cardiovascular model with the adjustment of multiple covariates. Although E/e' was included instead of LVEDV, E/e' was not associated (P=0.908) with the risk of non-cardiovascular events; HR, 1.00 (0.959-1.049).
Table 4.
Baseline clinical and biochemical characteristics associated with cardiovascular, non-cardiovascular, and all combined events
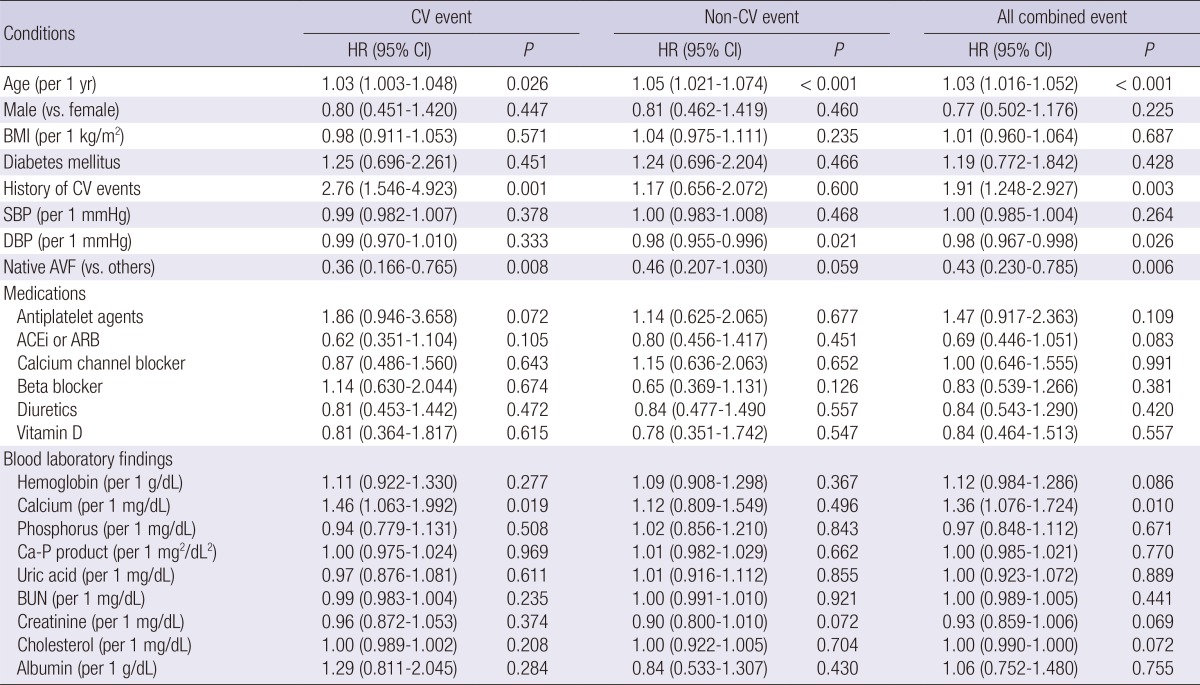
CV, cardiovascular; HR, hazard ratio; CI, confidence interval; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; AVF, arteriovenous fistula; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; Ca-P, calcium-phosphorus.
Table 5.
Multivariate Cox proportional hazard models for cardiovascular and non-cardiovascular events
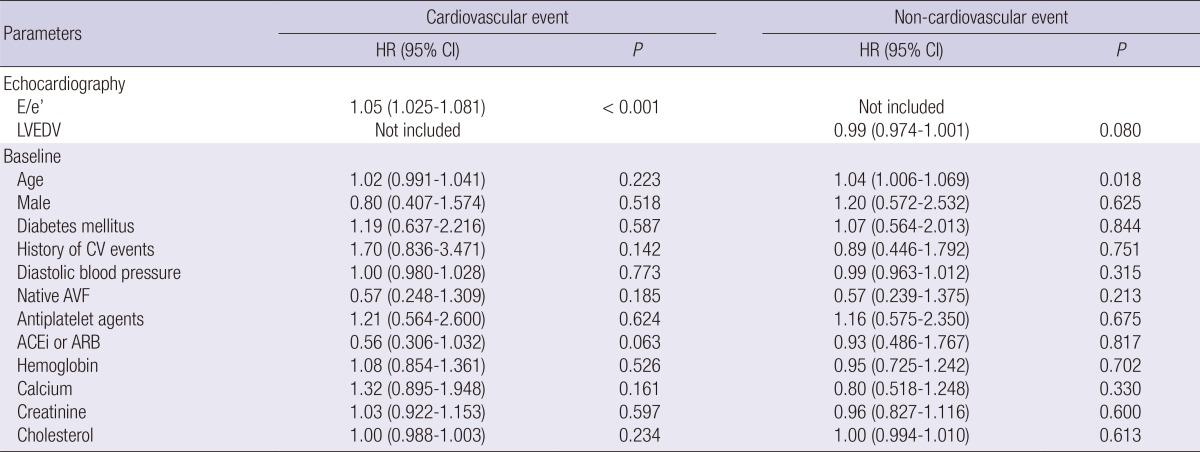
HR, hazard ratio; CI, confidence interval; E, early diastolic transmitral inflow velocity; e', early diastolic mitral annular velocity; LVEDV, left ventricular end-diastolic volume; CV, cardiovascular; AVF, arteriovenous fistula; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker.
Echocardiographic parameters associated with each clinical event
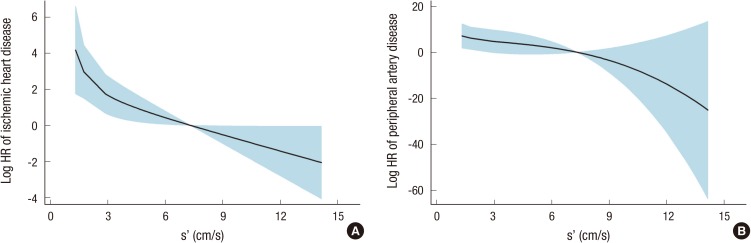
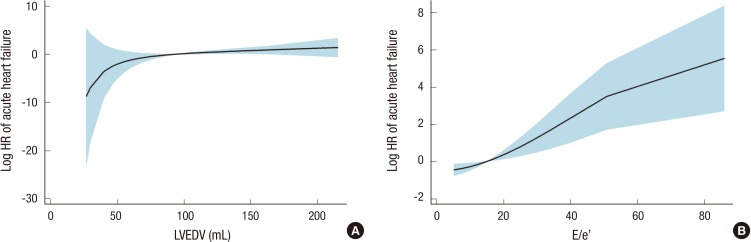
We further analyzed the association of echocardiographic parameters according to each outcome, including ischemic heart disease, cerebrovascular disease, peripheral artery disease, acute heart failure, fatal infectious disease, all-cause mortality, and all combined events (Table 6). Among echocardiographic parameters, s' was selected as the strongest predictor in the Cox models for ischemic heart disease and peripheral artery disease. Fig. 2A and B show the fitted curves between s' and the HRs of ischemic heart disease and peripheral artery disease, respectively. After the adjustment of multiple covariates, the significance of s' remained consistent for ischemic heart disease and remained partially significant for peripheral artery disease. Both LVEDV and the E/e' ratio were significantly correlated with acute heart failure risk. The fitted curves of acute heart failure risk with LVEDV or E/e' are shown in Fig. 3. These correlations remained significant after the adjustment of multiple covariates. However, neither cerebrovascular disease nor fatal infectious disease had an association with any echocardiographic parameter. All-cause mortality attributable to fatal cardiovascular and non-cardiovascular events could be predicted by LVEDV, diastolic interventricular septal wall thickness, and E/e'. However, E/e' remained significant after the adjustment of clinical and biochemical covariates. In the multivariate Cox model for all combined events, only E/e' was significant.
Table 6.
Significant echocardiographic parameters related to each clinical event
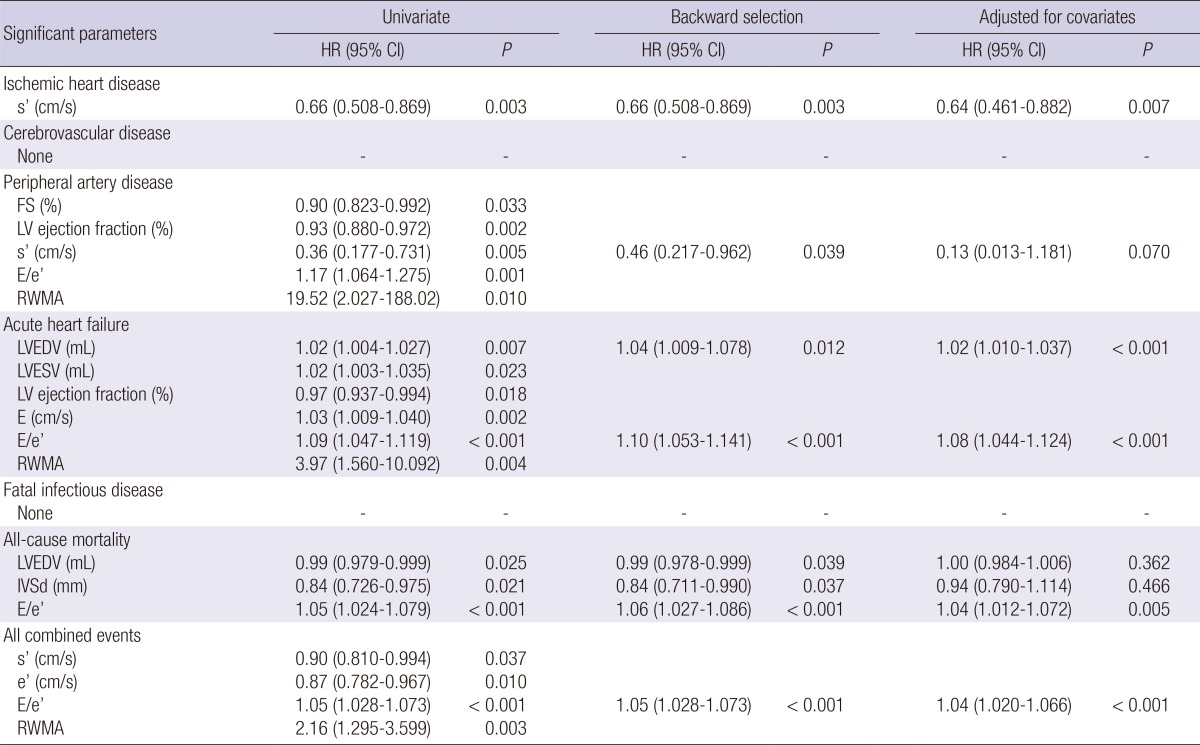
HR, hazard ratio; CI, confidence interval; s', mitral annular systolic velocity; FS, fractional shortening; LV, left ventricular; E, early diastolic transmitral inflow velocity; e', early diastolic mitral annular velocity; RWMA, regional wall motion abnormality; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; IVSd, interventricular septal thickness at end-diastole.
Fig. 2.
Fitted curves between the mitral annular systolic velocity (s') and the hazard ratios of ischemic heart disease (A) and peripheral artery disease (B). HR, hazard ratio; s', mitral annular systolic velocity.
Fig. 3.
Fitted curves of the hazard ratios for acute heart failure with left ventricular end-diastolic volume (A) or E/e' (B). HR, hazard ratio; LVEDV, left ventricular end-diastolic volume; E, early diastolic transmitral inflow velocity; e', early diastolic mitral annular velocity.
DISCUSSION
All previous studies have focused on the correlations between echocardiographic parameters and cardiovascular events or all-cause mortality. Additionally, the present study first determined whether the associations of echocardiographic parameters were different according to each cardiovascular or non-cardiovascular event. The E/e' ratio, representative of LV diastolic function, was the strongest predictor of all cardiovascular events. We further conducted analyses after stratification by the subtype of cardiovascular events. As a result, s', representative of LV systolic function, was associated with the risk of ischemic heart disease. For peripheral artery disease, there was a marginal significance with s' after the adjustment of multiple covariates. Both high LVEDV and E/e' were significantly associated with acute heart failure risk. When considering the outcomes of all-cause mortality or all combined events, E/e' had only a significant association. However, there was no independent predictor of cerebrovascular disease or non-cardiovascular events among the echocardiographic parameters.
LV diastolic dysfunction has been extensively documented to be associated with high morbidity and mortality (20). Thus far, the E/A ratio or deceleration time using the mitral inflow pattern in conventional Doppler echocardiography has been used to evaluate LV diastolic dysfunction (21). However, these markers do not accurately reflect LV diastolic function because of the strong dependency on volume (22) and may exhibit pseudonormalization of the LV filling pattern where the mitral flow is apparently normal despite the presence of chronic diastolic dysfunction (23). This phenomenon is particularly challenging in patients undergoing hemodialysis, for whom the relatively high preload before a dialysis session can mask a restrictive pattern (24). Alternative echocardiographic parameters can be successfully used to overcome these limitations of conventional parameters. One alternative is the E/e' ratio, which is measured using TDI. E/e' is known to be the most reliable parameter of LV diastolic function in hemodialysis patients (10).
Previous studies have demonstrated that the E/e' ratio is a strong predictor of mortality and is superior in this regard to other clinical or echocardiographic parameters in several clinical settings, such as hypertension, acute myocardial infarction, and cardiac arrhythmia. The superiority of E/e' has also been documented in previous studies on patients with ESRD, where the echocardiography was conducted during maintenance hemodialysis or peritoneal dialysis (10, 14, 25) but not at the time of starting dialysis. There are no previous studies on the correlation between E/e' at the start of dialysis and cardiovascular or other events. The present study primarily confirms that E/e' assessed at the start of dialysis is most predictive for cardiovascular risk (especially acute heart failure) as well as other risks, such as all-cause mortality and all combined events. A high E/e' ratio (LV diastolic dysfunction) may be prevalent in patients with ESRD due to the conditions related to myocardial fibrosis (26). These conditions include old age, hypertension, uremia, and bone mineral disorders (27). The present study adjusted multiple clinical and biochemical parameters as representatives of these conditions in the multivariate analyses, but E/e' remained significant as the predictor of cardiovascular events. Therefore, we suggest that the conditions aggravating E/e' should be identified and monitored, although not much is known regarding when and how to monitor these conditions.
The LV systolic dysfunction is evident in almost half of patients undergoing hemodialysis and can be induced by hemodialysis itself (28). Furthermore, this pathological condition has an association with cardiovascular morbidity and mortality in patients with ESRD (29). To assess LV systolic function, ejection fraction or fractional shortening can be measured by conventional echocardiography. However, these parameters have some limitations, such as high inter- and intra-observer variability or dependency on LV loading. Instead, the mitral annular systolic velocity (s') is more useful for assessing LV systolic function (7). The present study also found associations of s' with ischemic heart disease, or partially, with peripheral artery disease in patients undergoing hemodialysis, although there was no previous knowledge of this association before the present study. The result is plausible, because inadequate cardiac output by systolic dysfunction increases sympathetic neurohormonal pathways and eventually changes either the structure or the functioning of heart and peripheral vessels (29, 30).
We could not determine the independent relationship of cerebrovascular disease with several echocardiographic parameters including systolic and diastolic dysfunctions. Previous studies have shown that heart failure increases the risk of cerebrovascular disease (31). However, all previous studies defined heart failure by using one echocardiographic parameter or by putting echocardiographic and clinical parameters together. Therefore, the objectives of these studies were not identical to ours. We aimed to find the most predictive parameter for the outcome, and thus put them into the competition in the analyses. Accordingly, any echocardiographic parameter could not hold a dominant position over other parameters. If several parameters were considered together, the model might successfully predict cerebrovascular disease. This issue will be investigated in the future studies.
All previous echocardiographic studies have reviewed either cardiovascular events or all-cause mortality but not non-cardiovascular events. The present study primarily addressed this issue, and thus, LVEDV was identified as the strongest predictor of fatal non-cardiovascular events. However, the impact of this variable was not significant after the adjustment of multiple clinical and biochemical parameters. It is well known that LVEDV changes with age (32), and elderly ESRD patients have a greater risk of non-cardiovascular risk (e.g., infection, frailty, and complications of ESRD) than younger patients (33). However, the independent correlation between echocardiographic parameters and non-cardiovascular risk may appear in a larger population.
Although the present results are informative, this study has certain limitations. First, the observational study design limits the full applicability of our conclusions to other clinical settings, despite the details of the outcome data. Second, we did not measure echocardiographic parameters repeatedly following hemodialysis. However, there is no consensus regarding how to monitor heart function in patients with ESRD. Based on these limitations, whether reducing the E/e' ratio or other echocardiographic parameters will decrease the risks of morbidity and mortality cannot be determined by our study alone. Third, we did not collect some valuable information, such as vascular calcification, parathyroid status, and vitamin D levels. These factors could participate in the predictive models of outcomes, although we gathered the use of vitamin D.
In summary, the present study has thoroughly evaluated the prognostic value of echocardiographic parameters for several cardiovascular and non-cardiovascular events. As a result, the E/e' ratio was the strongest predictor of acute heart failure, all cardiovascular events, all-cause mortality, and all combined events. Furthermore, s' had a significant relationship with ischemic heart disease, or partially, with peripheral artery disease. However, no echocardiographic parameter independently predicted the risk of cerebrovascular disease or non-cardiovascular events. In conclusion, certain echocardiographic parameters, such as E/e', s', and LVEDV can be successfully used to predict several clinical events in patients starting hemodialysis. Accordingly, the present study may be helpful to guide further surveillance in patients with abnormal echocardiographic findings. Further studies are needed to address the schedule or the implication of further surveillance in those patients.
Footnotes
The authors have no potential conflicts of interest to declare in relation to this article.
Conception and coordination of the study: KY Na. Design of ethical issues: SS Han. Acquisition of data: YS Park, SH Baek, SY Ahn. Data review: S Kim, HJ Chin, DW Chae. Statistical analysis: SS Han. Manuscript preparation: SS Han, GY Cho, KY Na. Manuscript approval: all authors.
References
- 1.Locatelli F, Del Vecchio L, Manzoni C. Morbidity and mortality on maintenance haemodialysis. Nephron. 1998;80:380–400. doi: 10.1159/000045210. [DOI] [PubMed] [Google Scholar]
- 2.Schieppati A, Remuzzi G. Chronic renal diseases as a public health problem: epidemiology, social, and economic implications. Kidney Int Suppl. 2005:S7–S10. doi: 10.1111/j.1523-1755.2005.09801.x. [DOI] [PubMed] [Google Scholar]
- 3.Collins AJ, Foley RN, Gilbertson DT, Chen SC. The state of chronic kidney disease, ESRD, and morbidity and mortality in the first year of dialysis. Clin J Am Soc Nephrol. 2009;4:S5–S11. doi: 10.2215/CJN.05980809. [DOI] [PubMed] [Google Scholar]
- 4.Jin DC. Current status of dialysis therapy in Korea. Korean J Intern Med. 2011;26:123–131. doi: 10.3904/kjim.2011.26.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt KU, Nahas ME, Jaber BL, Jadoul M, Levin A, et al. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72:247–259. doi: 10.1038/sj.ki.5002343. [DOI] [PubMed] [Google Scholar]
- 6.Han SS, Ahn JM, Chin HJ, Chae DW, Oh KH, Joo KW, Kim YS, Ahn C, Han JS, Kim S, et al. Impact of C-reactive protein and pulse pressure evaluated at the start of peritoneal dialysis on cardiovascular events in the course of treatment with peritoneal dialysis. Perit Dial Int. 2010;30:300–310. doi: 10.3747/pdi.2009.00064. [DOI] [PubMed] [Google Scholar]
- 7.Edvardsen T, Urheim S, Skulstad H, Steine K, Ihlen H, Smiseth OA. Quantification of left ventricular systolic function by tissue Doppler echocardiography: added value of measuring pre- and postejection velocities in ischemic myocardium. Circulation. 2002;105:2071–2077. doi: 10.1161/01.cir.0000014614.63980.ba. [DOI] [PubMed] [Google Scholar]
- 8.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 9.Dandel M, Lehmkuhl H, Knosalla C, Hetzer R. Tissue Doppler imaging: diagnostic and prognostic value. J Am Coll Cardiol. 2007;50:1614. doi: 10.1016/j.jacc.2007.06.044. author reply 5. [DOI] [PubMed] [Google Scholar]
- 10.Sharma R, Pellerin D, Gaze DC, Mehta RL, Gregson H, Streather CP, Collinson PO, Brecker SJ. Mitral peak Doppler E-wave to peak mitral annulus velocity ratio is an accurate estimate of left ventricular filling pressure and predicts mortality in end-stage renal disease. J Am Soc Echocardiogr. 2006;19:266–273. doi: 10.1016/j.echo.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Tripepi G, Benedetto FA, Mallamaci F, Tripepi R, Malatino L, Zoccali C. Left atrial volume monitoring and cardiovascular risk in patients with end-stage renal disease: a prospective cohort study. J Am Soc Nephrol. 2007;18:1316–1322. doi: 10.1681/ASN.2006080881. [DOI] [PubMed] [Google Scholar]
- 12.Yigla M, Fruchter O, Aharonson D, Yanay N, Reisner SA, Lewin M, Nakhoul F. Pulmonary hypertension is an independent predictor of mortality in hemodialysis patients. Kidney Int. 2009;75:969–975. doi: 10.1038/ki.2009.10. [DOI] [PubMed] [Google Scholar]
- 13.Patel RK, Jardine AG, Mark PB, Cunningham AF, Steedman T, Powell JR, McQuarrie EP, Stevens KK, Dargie HJ, Jardine AG. Association of left atrial volume with mortality among ESRD patients with left ventricular hypertrophy referred for kidney transplantation. Am J Kidney Dis. 2010;55:1088–1096. doi: 10.1053/j.ajkd.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwabuchi Y, Ogawa T, Inoue T, Otsuka K, Nitta K. Elevated E/E' predicts cardiovascular events in hemodialysis patients with preserved systolic function. Intern Med. 2012;51:155–160. doi: 10.2169/internalmedicine.51.6250. [DOI] [PubMed] [Google Scholar]
- 15.de Jager DJ, Grootendorst DC, Jager KJ, van Dijk PC, Tomas LM, Ansell D, Collart F, Finne P, Heaf JG, De Meester J, et al. Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA. 2009;302:1782–1789. doi: 10.1001/jama.2009.1488. [DOI] [PubMed] [Google Scholar]
- 16.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 17.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, et al. Chamber Quantification Writing Group; American Society of Echocardiographys Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiographys Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 19.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. quiz 86-8. [DOI] [PubMed] [Google Scholar]
- 20.Mandinov L, Eberli FR, Seiler C, Hess OM. Diastolic heart failure. Cardiovasc Res. 2000;45:813–825. doi: 10.1016/s0008-6363(99)00399-5. [DOI] [PubMed] [Google Scholar]
- 21.Giannuzzi P, Imparato A, Temporelli PL, de Vito F, Silva PL, Scapellato F, Giordano A. Doppler-derived mitral deceleration time of early filling as a strong predictor of pulmonary capillary wedge pressure in postinfarction patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 1994;23:1630–1637. doi: 10.1016/0735-1097(94)90667-x. [DOI] [PubMed] [Google Scholar]
- 22.Barberato SH, Mantilla DE, Misocami MA, Gonçalves SM, Bignelli AT, Riella MC, Pecoits-Filho R. Effect of preload reduction by hemodialysis on left atrial volume and echocardiographic Doppler parameters in patients with end-stage renal disease. Am J Cardiol. 2004;94:1208–1210. doi: 10.1016/j.amjcard.2004.07.100. [DOI] [PubMed] [Google Scholar]
- 23.Barberato SH, Pecoits Filho R. Prognostic value of left atrial volume index in hemodialysis patients. Arq Bras Cardiol. 2007;88:643–650. doi: 10.1590/s0066-782x2007000600004. [DOI] [PubMed] [Google Scholar]
- 24.Pecoits-Filho R, Bucharles S, Barberato SH. Diastolic heart failure in dialysis patients: mechanisms, diagnostic approach, and treatment. Semin Dial. 2012;25:35–41. doi: 10.1111/j.1525-139X.2011.01011.x. [DOI] [PubMed] [Google Scholar]
- 25.Rakhit DJ, Zhang XH, Leano R, Armstrong KA, Isbel NM, Marwick TH. Prognostic role of subclinical left ventricular abnormalities and impact of transplantation in chronic kidney disease. Am Heart J. 2007;153:656–664. doi: 10.1016/j.ahj.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 26.Burlew BS, Weber KT. Cardiac fibrosis as a cause of diastolic dysfunction. Herz. 2002;27:92–98. doi: 10.1007/s00059-002-2354-y. [DOI] [PubMed] [Google Scholar]
- 27.Losi MA, Memoli B, Contaldi C, Barbati G, Del Prete M, Betocchi S, Cavallaro M, Carpinella G, Fundaliotis A, Parrella LS, et al. Myocardial fibrosis and diastolic dysfunction in patients on chronic haemodialysis. Nephrol Dial Transplant. 2010;25:1950–1954. doi: 10.1093/ndt/gfp747. [DOI] [PubMed] [Google Scholar]
- 28.Wang AY, Wang M, Lam CW, Chan IH, Lui SF, Sanderson JE. Heart failure with preserved or reduced ejection fraction in patients treated with peritoneal dialysis. Am J Kidney Dis. 2013;61:975–983. doi: 10.1053/j.ajkd.2012.12.030. [DOI] [PubMed] [Google Scholar]
- 29.Kishi T. Heart failure as an autonomic nervous system dysfunction. J Cardiol. 2012;59:117–122. doi: 10.1016/j.jjcc.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Delis KT, Lennox AF, Nicolaides AN, Wolfe JH. Sympathetic autoregulation in peripheral vascular disease. Br J Surg. 2001;88:523–528. doi: 10.1046/j.1365-2168.2001.01735.x. [DOI] [PubMed] [Google Scholar]
- 31.Alberts VP, Bos MJ, Koudstaal P, Hofman A, Witteman JC, Stricker B, Breteler M. Heart failure and the risk of stroke: the Rotterdam study. Eur J Epidemiol. 2010;25:807–812. doi: 10.1007/s10654-010-9520-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin FY, Devereux RB, Roman MJ, Meng J, Jow VM, Jacobs A, Weinsaft JW, Shaw LJ, Berman DS, Callister TQ, et al. Cardiac chamber volumes, function, and mass as determined by 64-multidetector row computed tomography: mean values among healthy adults free of hypertension and obesity. JACC Cardiovasc Imaging. 2008;1:782–786. doi: 10.1016/j.jcmg.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 33.Munshi SK, Vijayakumar N, Taub NA, Bhullar H, Lo TC, Warwick G. Outcome of renal replacement therapy in the very elderly. Nephrol Dial Transplant. 2001;16:128–133. doi: 10.1093/ndt/16.1.128. [DOI] [PubMed] [Google Scholar]