Abstract
This study analyzes the clinical characteristics of the brain metastasis (BM) of gynecologic cancer based on the type of cancer. In addition, the study examines the factors influencing the survival. Total 61 BM patients of gynecologic cancer were analyzed retrospectively from January 2000 to December 2012 in terms of clinical and radiological characteristics by using medical and radiological records from three university hospitals. There were 19 (31.1%) uterine cancers, 32 (52.5%) ovarian cancers, and 10 (16.4%) cervical cancers. The mean interval to BM was 25.4 months (21.6 months in ovarian cancer, 27.8 months in uterine cancer, and 33.1 months in cervical cancer). The mean survival from BM was 16.7 months (14.1 months in ovarian cancer, 23.3 months in uterine cancer, and 8.8 months in cervical cancer). According to a multivariate analysis of factors influencing survival, type of primary cancer, Karnofsky performance score, status of primary cancer, recursive partitioning analysis class, and treatment modality, particularly combined therapies, were significantly related to the overall survival. These results suggest that, in addition to traditional prognostic factors in BM, multiple treatment methods such as neurosurgery and combined chemoradiotherapy may play an important role in prolonging the survival for BM patients of gynecologic cancer.
Graphical Abstract

Keywords: Brain Metastasis; Ovarian Neoplasm; Uterine Cervical Neoplasm; Uterine Neoplasm, Outcome
INTRODUCTION
The American Cancer Society estimated about 1,284,900 new cases of cancer and more than 550,000 cancer-related deaths as of 2002 (1). Gynecologic malignancies accounted for approximately 13% of 647,400 cases of cancer and 10% of cancer death in women (1). The Korea Central Cancer Registry reported that 93,337 new cases of cancer in Korea in 2009 occurred in women (2). Gynecologic malignancies accounted for 7.7% of these cases (7,248), and 2,025 women died from their cancer, accounting for 7.8% of all cancer-related deaths in Korean women.
Brain metastasis (BM) occurs in 20% to 40% of all solid-tumor malignancies. However, gynecologic malignancies with BM occur much less frequently. Metastatic gynecologic diseases occur in 15% to 85% of all cases depending on the type of tumor (mainly by a direct extension, peritoneal seeding, or lymphatic dissemination). The most common metastasis sites include the liver, lung, bone, and lymph nodes (3). By contrast, central nervous system metastasis is presumed to occur through hematogenous seeding or a direct invasion from some previous bone metastasis, although the latter is rare. The development of metastatic foci is thought to be related to tumor cell behavior, host immune responses, and the number of tumor cells that embolize (4). Many patients have concurrent pulmonary metastasis and BM, and pulmonary disease may be a predisposing factor in the latter (5). Incidences of BM from ovarian, endometrial, and cervical cancer have been reported to be 0.3%-2.2%, 0.4%-1.2%, and 0.3%-0.9%, respectively (6). Despite these low rates, the occurrence of BM in gynecologic malignancies appears to have increased in recent years (6, 7, 8, 9). This increase may be due to increased survival times from improved treatment options and an early diagnosis of common diseases (10, 11). In particular, the Korean government supports regular physical checkups for common types of cancer through the National Health Insurance program. Regular screening programs for gynecologic cancer are also recommended every other year for women 40 yr and over. Unfortunately, no study in Korea has considered comprehensive data on BMs of gynecologic cancer because of their scarcity. This study considers retrospectively collected data on gynecologic cancer with BM over a 13-yr period from multiple institutes and analyzes the clinical characteristics of patients. In addition, the study determines the prognostic factors for overall patient survival.
MATERIALS AND METHODS
From January 2000 to December 2012, new BM cases were clinically and radiologically diagnosed in 61 patients with gynecologic cancer at Samsung Changwon Hospital, Gil Medical Center, and Dong-A Medical Center. Additional inclusion criteria included a pathological diagnosis of primary gynecologic malignancies and no prior therapy to the brain. Exclusion criteria included a history of treatment for malignancies other than gynecologic cancer, the presence of active double cancer, or the presence of a central nerve system disease and/or a neuromuscular disease unrelated to BM. Medical and radiological records of these patients were retrospectively analyzed.
Clinical characteristics of patients
A retrospective analysis of clinical variables was conducted for 61 BM patients with gynecologic cancer. These variables included patient age at BM diagnosis, the time interval to BM, the type of primary cancer, the stage of the primary tumor except BM, the presence of any extracranial metastasis at BM diagnosis, the status of primary cancer, the Karnofsky performance scale (KPS) score (12), and the recursive partitioning analysis (RPA) class. According to the classification method in Gaspar et al. (13), patients of RPA class 1 were characterized based on their age <65 yr, a KPS score ≥70, the absence of any extracranial metastasis at BM diagnosis, and good control of systemic diseases. RPA class 2 patients had a KPS score ≥70, were ≥65 yr, and had some uncontrolled systemic disease or systemic metastasis. Patients of RPA class 3 had a KPS score <70. BM diagnosed <60 days after the diagnosis of a primary lesion was considered as synchronous metastasis, whereas that diagnosed ≥60 days was considered metachronous metastasis.
Each case of gynecologic cancer was staged based on the International Federation of Obstetricians and Gynaecologists (FIGO) Staging System (14). All therapeutic modalities such as supportive medical treatment, neurosurgical resection, external beam radiotherapy for the brain, and systemic chemotherapy after the diagnosis of BM were analyzed.
Radiological features of brain metastasis
The parameters for the 61 patients included the BM number, size, and location and the associated hemorrhage of the brain lesion. BM size was defined as the maximum orthogonal diameter in T1-weighted and gadolinium-enhanced magnetic resonance (MR) images. The BM location was categorized as supratentorial or infratentorial. The involvement of cancer in the brain stem and the cerebrospinal fluid (CSF) was assessed using MR images. All patient-related data were extracted from a computerized database (PACS; m-view™, Marosis Corporation, Seoul, Korea).
Neurosurgical indications
Clinical indications included symptoms and signs of intracranial hypertension unresponsive to adequate medical therapy (e.g., corticosteroid and mannitol), intractable seizures, a reduced level of consciousness, progressive motor weakness, gait ataxia, and aphasia. Neuroimaging indications included lesion enlargement, associated hemorrhage, and a mass effect from edema unresponsive to maximum medical treatment. Basically, neurosurgical candidates were expected to survive more than 3 months.
Statistical analysis
Statistical analyses were conducted using SSPS 18.0 (SPSS Institute, Inc., Chicago, IL, USA). The overall survival time was estimated using the Kaplan-Meier method and analyzed based on the log-rank test. The Cox proportional hazard model was used to assess the effects of multiple covariates on the prognosis of BM from gynecologic cancers. The origin of primary cancer, age at BM diagnosis, the KPS score, the presence of extracranial metastasis, the status of primary cancer, the RPA class, the time interval between the diagnosis of primary cancer and BM, the BM number, and therapeutic modalities were regarded as candidate prognostic factors. Variables found to be significantly related to the survival time based on a univariate analysis (P<0.2) were subjected to a multivariate analysis. The multivariate analysis yielded the hazards ratio (HR) with 95% confidence intervals (CIs). The results were considered significant when the P value was less than 0.05.
Ethics statement
The study was approved by the institutional review boards of Samsung Changwon Hospital (2013-SCMC-094-00), Dong-A Medical Center (2013-12-011), and Gil Medical Center (GAIRB 2013-115). The informed consent was obtained from all the subjects if survived or one of family members if died or unconscious for collection of clinical data.
RESULTS
Clinical characteristics of patients
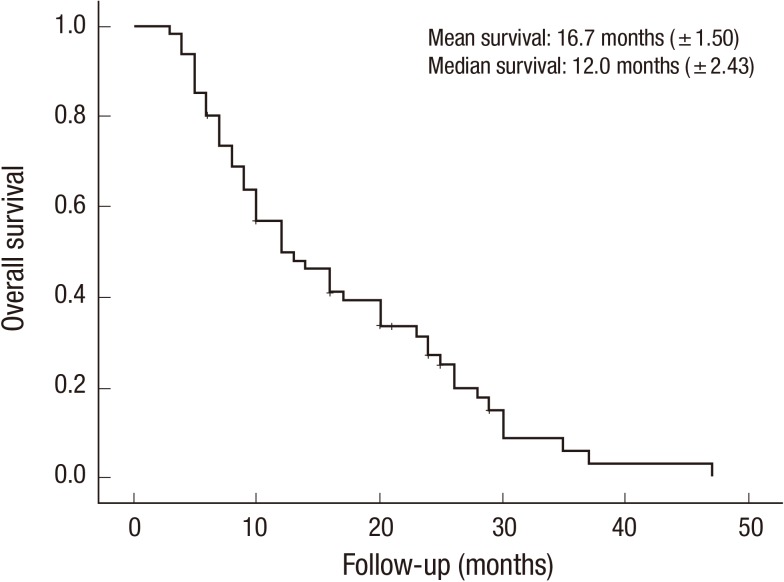
The mean survival time was 16.7 months (±1.50 months) (Fig. 1). The mean age of the patients at BM diagnosis was 54.7 yr and ranged from 22.6 to 79.4 yr. The mean interval to BM from the diagnosis of gynecologic cancer was 25.4 months and ranged from 0 to 84.3 months. All patients were followed up for at least 3 months, and the mean follow-up period was 19.8 months (range: 3.3-47.8 months). The primary origin of gynecologic cancer was the uterus in 19 patients (31.1%), the ovary in 32 patients (52.5%), and the cervix in 10 patients (16.4%). The histopathological diagnosis was endometrial cancer in 6 patients (9.8%), uterine adenocarcinoma in 11 patients (18.0%), uterine leiomyoma in 2 patients (3.3%), ovarian epithelial cancer in 23 patients (37.7%), ovarian germ cell cancer in 9 patients (14.8%), cervical squamous cell carcinoma in 6 patients (9.8%), and cervical adenocarcinoma in 4 patients (6.6%). Extracranial metastasis was found in 32 patients (52.5%; FIGO stage IV). Only 4 patients (6.6%) were categorized as RPA class I, and 29 patients (47.5%) were categorized as RPA class III for a KPS score below 70. As shown in Table 1, brain metastasis was diagnosed at 2 months after the diagnosis of primary gynecologic cancer in 54 patients (88.5%).
Fig. 1.
The overall survival curve of BM patients with gynecologic cancer based on Kaplan-Meier survival estimates.
Table 1.
Clinical characteristics of patients with brain metastasis of gynecological cancers (n = 61)

BM, brain metastasis; FIGO, the International Federation of Gynecology and Obstetrics; RPA, recursive partitioning analysis.
Five patients (8.2%) underwent only supportive treatment, and another 56 (91.8%) were treated with palliative methods such as neurosurgery, radiotherapy (including stereotactic radiosurgery), and/or chemotherapy. Monotherapy was performed in 23 patients (37.7%); dual therapy, in 26 (42.6%), and triple therapy, in 7 (11.5%). In terms of monotherapy, 2 patients (3.2%) underwent only neurosurgery; 19 (31.2%), only radiotherapy; and 2 (3.2%), only systemic chemotherapy. In terms of dual therapy, 10 (16.4%) were treated with a combination of neurosurgery and radiotherapy; 1 (1.6%), with a combination of neurosurgery and chemotherapy; and 15 (24.7%), with a combination of chemoradiotherapy. All therapeutic modalities, including neurosurgery and chemoradiotherapy, were applied to 7 patients (11.5%), as shown in Table 1.
Radiological features of brain metastasis
A single BM was found in 38 patients (62.3%), whereas multiple BMs were observed in 23 (37.7%). Among 85 BMs, 56 (65.9%) were located in the supratentorial area; 27 (31.8%), in the infratentorial area; and 2 (2.4%), in leptomeninges. Four brain stem involvements were found in 3 patients. There were 4 (6.6%) cases of some hemorrhagic complication of BM, and all originated from ovarian cancer (Table 2).
Table 2.
Radiological characteristics of brain metastasis of gynecological cancer (n = 61)

BM, brain metastasis.
A clinical comparison of brain metastasis by primary cancer
The mean ages at BM diagnosis were 56.3 yr (range: 34.2-75.7 yr) in patients with ovarian cancer; 58.2 (range: 38.5-79.4) in patients with uterine cancer; and 42.8 (range: 22.6-70.4) in patients with cervical cancer. RPA class III was categorized in 18 BM patients (56.3%) with ovarian cancer; 7 (36.8%) with uterine cancer; and 4 (40.0%) with cervical cancer. Extracranial metastasis (FIGO stage IV) was found in 18 patients (56.3%) with ovarian cancer; 8 (42.1%) with uterine cancer; and 6 (60.0%) with cervical cancer. Multiple BMs were found in 17 patients (53.1%) with ovarian cancer; 8 (42.1%) with uterine cancer; and 8 (80.0%) with cervical cancer (Table 3).
Table 3.
Clinical comparison of brain metastasis of gynecological cancer according to the primary origin (n=61)

BM, brain metastasis; FIGO, the International Federation of Gynecology and Obstetrics; RPA, recursive partitioning analysis.
No patient with cervical cancer was treated supportively, and all (100%) received radiotherapy for their BM. A relatively high proportion of BM patients of uterine cancer underwent neurosurgery (47.5%), but the 25.0% of BM patients of ovarian cancer and the 30.0% of BM patients of cervical cancer did neurosurgery. Despite the high proportion of patients treated with radiotherapy, the mean survival time for patients with cervical cancer was relatively short (8.4 months). By contrast, in uterine cancer patients, who received more neurosurgeries (47.4%), the mean survival time was relatively long (23.3 months). A relatively low proportion of BM patients with ovarian cancer received neurosurgical treatment (25.0%), whereas a relative high proportion received radiotherapy (84.8%). Their survival time was 14.1 months (Table 3). Actually, all the patients with BM of uterine cancer and cervical cancer who underwent neurosurgery received radiotherapy for brain lesions. However, 5 of 8 patients with BM of ovarian cancer who underwent neurosurgery received radiotherapy for brain.
Factors influencing overall survival in brain metastasis patients with gynecologic cancer
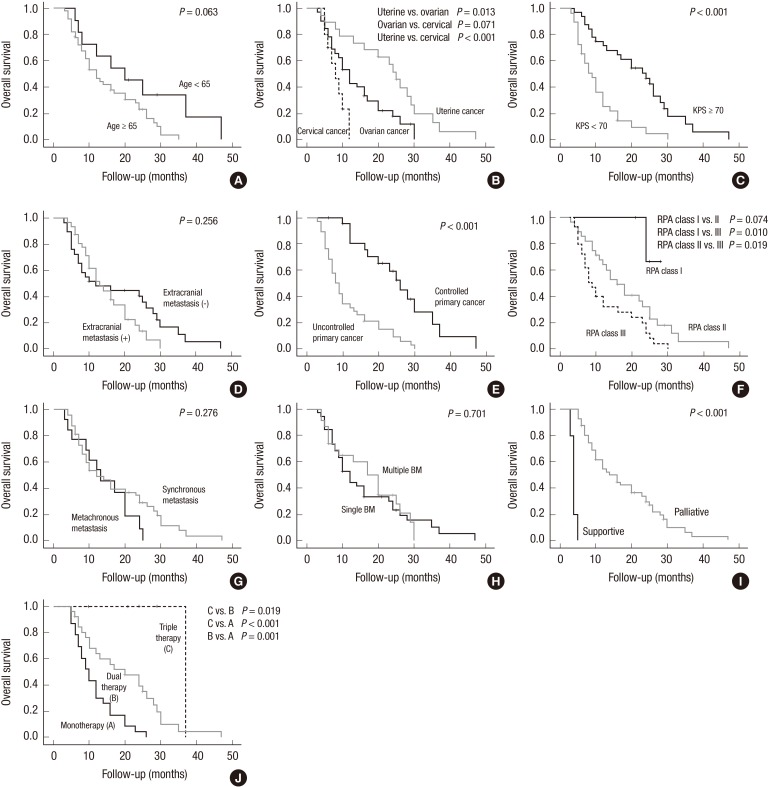
According to the univariate analysis, age (<65 yr), BM from uterine cancer, KPS ≥70, a lack of extracranial metastasis, the lower RPA class, synchronous BMs, single BMs, and multiple treatment modalities increased the overall survival time (Table 4).
Table 4.
Factors affecting overall survival in univariate and multivariate survival analysis using Cox proportional hazard model
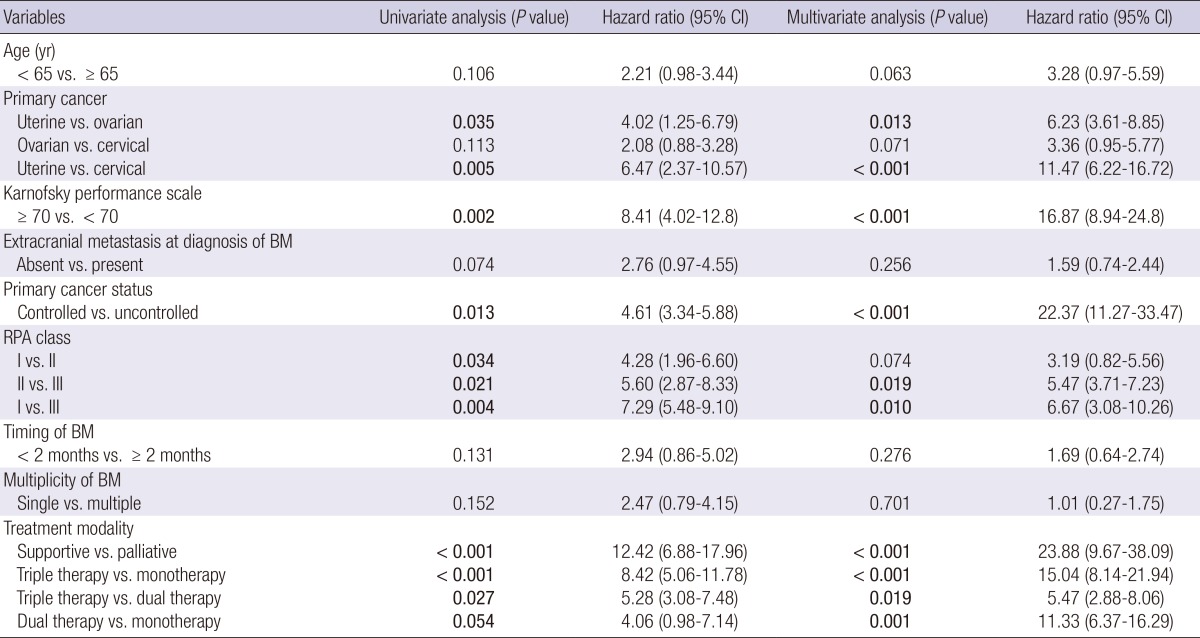
Dual therapy: neurosurgery plus chemotherapy, or neurosurgery plus radiotherapy, or chemotherapy plus radiotherapy; Triple therapy: combination treatment of neurosurgery and chemoradiotherapy. BM, brain metastasis; CI, confidence interval; RPA, recursive partitioning analysis.
However, after a multi-factor adjustment through a multivariate analysis, BM from uterine cancer (uterine vs. ovarian, HR of 6.23, P, 0.013; uterine vs. cervical, HR of 11.47, P<0.001), KPS ≥70 (HR of 16.87, P<0.001), BM with well-controlled primary cancer (HR of 22.37, P<0.001), the RPA class (II vs. III, HR of 5.47, P, 0.019; I vs. III, HR of 6.67, P, 0.010), palliative treatment (vs. supportive treatment, HR of 23.88, P<0.001), triple therapy (vs. monotherapy, HR of 15.04, P<0.001; vs. dual therapy, HR of 5.47, P, 0.019), and dual therapy (vs. monotherapy, HR of 11.33, P, 0.001) were independently associated with a longer overall survival time (Table 4 and Fig. 2).
Fig. 2.
The overall survival curve of BM patients with gynecologic cancer based on factors influencing the survival time: (A) age ( ≥ 65 yr vs. < 65 yr), (B) the type of gynecologic cancer, (C) the Karnofsky performance score ( ≥ 70 vs. < 70), (D) extracranial metastasis (presence vs. absence), (E) the primary cancer status (well controlled vs. poorly controlled), (F) the recursive partitioning analysis class, (G) the timing of brain metastasis (synchronous vs. metachronous), (H) the BM number (single vs. multiple), (I) the purpose of treatment (supportive vs. palliative), and (J) therapeutic modalities.
DISCUSSION
As expected, the results indicate a poor prognosis of BM from gynecologic cancer. The mean survival time for BM patients with gynecologic cancer was 16.7 months. This, however, is better than that reported in previous studies. According to the previous reports, more specifically, the median survival time after BM diagnosis from ovarian cancer was 6 to 7 months (15, 16, 17); that from endometrial cancer was 1 to 2 months (18); and that from cervical cancer was 9.9 months (3). This increase in the survival time for BM patients with gynecologic cancer may be due to improved surgical and chemotherapy techniques because chemotherapy has been cited as the major factor increasing the incidence of BM from these malignancies (19). Noteworthy is that 39% of BM patients were heavily treated with three or more sessions of chemotherapy (20). Increased diagnostic sensitivity resulting from improved cerebral imaging technologies has made it possible to detect small intracranial lesions earlier during the course of disease recurrence (21). In fact, diagnosing BM has become easier with modern diagnostic modalities. However, 92% of the patients in present study were symptomatic at BM diagnosis (22). This may be because gynecologic oncologists have difficulty observing BM as a result of its low incidence and because a cranial radiographic evaluation is typically not conducted with no evident symptoms. In this regard, efforts to find asymptomatic, small, and solitary BMs should produce a better prognosis after treatment. Many patients had solitary BMs (44.5%), small BMs (<2 cm; 21.2%), no pulmonary disease (56.2%), no extracranial disease (24.1%), and good performance. These results are consistent with the findings of previous studies (15, 20, 23, 24, 25). Gynecologic cancer patients with such favorable clinical factors are typically considered good candidates for aggressive BM treatment.
In this study, a majority of BM patients (91.8%) with gynecologic cancer received palliative treatment because the primary disease was often advanced and the patients' general condition was generally poor. Management approaches included highly supportive care, surgical resection, and radiotherapy (26). Treatment algorithms for a heterogeneous population of BM patients are typically based on their prognosis and whether the aim is symptom palliation, an increase in the survival time, or both (27). Because patient survival depends on disease control, both intracranially and extracranially, patients with an active extracranial disease may be candidates for systemic treatment (28). In general, treatment complexity and resource use have increased in recent decades. Treatment options for BMs of gynecologic cancer depend on many factors. The Radiation Therapy Oncology Group recommended that the treatment choice for BM from extracranial primary cancer be based on the KPS score, the primary tumor status, the presence of extracranial metastasis, and age (28). In addition, the treatment choice for BM from gynecologic cancer should be made on an individual basis by carefully considering the ultimate treatment purpose. Patients with poor performance and extensive metastasis may not benefit from definitive treatment, but symptomatic care to minimize the effect of brain edema may be helpful. Patients who cannot undergo surgery may benefit from whole-brain radiotherapy (WBRT), which can ameliorate neurologic symptoms and improve the quality of life.
To date, surgical resection in combination with WBRT, particularly for solitary BMs, is known as the best option for controlling BM from gynecologic cancer. In addition, this therapeutic modality is more likely to increase patient survival than WBRT-only treatment (28, 29). According to previous research, the median survival time for patients who underwent surgery in conjunction with WBRT or chemotherapy was 16 months (range: 4-41 months), whereas it was only 4 months (range: 1-24 months) for patients without surgery (29). According to the another study, the mean survival time was 28 months for four ovarian cancer patients with solitary BMs who underwent surgical resection, WBRT, and chemotherapy (30). All deaths in that study were from some recurrent systemic disease with no clinical evidence of brain relapse. Most studies have found that BM patients with surgical resection with or without radiotherapy or chemotherapy are more likely to survive than those without surgery (17, 23, 31, 32). Considering only surgically treated patients, Cormio et al. (29) focused on prognostic factors and found the disease-free interval between the diagnosis of ovarian cancer and the appearance of BM and the presence of extracranial disease at BM diagnosis to be the only factors influencing patient survival. Solitary BMs are usually considered more suitable for surgical resection than are multiple BMs (29, 33). According to a review, 46% of single-BM patients underwent surgical resection, whereas only 5% of those with multiple lesions did (23).
In this study, 25 patients received systemic chemotherapy, which may play a role in some diseases, particularly in systemic or incompletely resected intracranial diseases. In BM patients, the blood-brain barrier is breached, resulting in a higher drug concentration from systemic chemotherapy and potentially a more favorable response. McMeekin et al. (23) concluded that chemotherapy, independent of surgery, is an independent predictor of patient survival and that its increases the survival time by controlling both intracranial and extracranial diseases.
In the present study, multimodal therapy increased the survival time, which is consistent with the findings of previous studies. Cormio et al. (34) reported that 2 out of 10 patients treated with surgery in conjunction with WBRT survived 28 and 83 months after their BM diagnosis. Cormio et al. (35) reported different outcomes for BM patients with ovarian cancer. Those patients receiving only radiotherapy between 1982 and 1994 survived only 5 months, whereas those with surgical resection followed by radiotherapy and/or chemotherapy between 1995 and 2010 survived 18 months. Mahmoud-Ahmed et al. (36) showed similar findings. The median survival time for patients treated with radiation in conjunction with surgery was 15 months, whereas that for patients receiving only radiation was 2.4 months.
This study's results are based on data from multiple institutes but have some limitations. First, this retrospective analysis was conducted using data from three institutes and thus was not conducted at the national level. In this regard, the results may not accurately represent the national pattern. Second, patients with an extremely severe condition were less likely to have a complete evaluation, potentially introducing some study bias. Third, it is difficult to independently conclude multiple therapeutic modalities as the most effective treatment modality for BMs patients with gynecologic cancer because of a lack of non-randomization and some selection bias introduced by the choice of the treatment modality. Finally, because of the rarity of BMs of gynecologic cancer, not all types of gynecologic cancer could be included. To address these limitations, future research should provide a more comprehensive and comparative analysis (e.g., a randomized prospective study) at the national level. Knowledge of prognostic factors for BMs patients with gynecologic cancer should improve patient outcomes and the management of individual patients.
In conclusion, although BM rarely occurs in patients with gynecologic cancer, BMs of gynecologic cancer were examined and analyzed retrospectively for a relatively long period of time in this multi-institutes-based study. Improvements in therapeutic modalities and the early detection of brain metastasis may explain the long survival time found in this study. Consistent with the findings of previous research, the results highlight traditional factors influencing the prognosis of BM of solid cancer, including the performance status of patients, the status of primary cancer, and the RPA class. As expected, aggressive and multimodality treatment methods such as neurosurgery and combination chemoradiotherapy increased the survival time for BMs patients with gynecologic cancer.
ACKNOWLEDGMENTS
Dr. Jae Hyun Kwon moved to Samsung Changwon Hospital from Dong-A University Medical Center at May 1, 2012. At present, current address for Dr. Kwon is Sungkyunkwan University School of Medicine, Samsung Changwon Hospital, Changwon, Korea.
Footnotes
The authors disclose no conflict of interest in the materials, methods, and findings of this manuscript.
Conception and coordination of the study: Soyi Lim, YZ Kim. Design of ethical issues: Soyi Lim, JH Kwon, YZ Kim. Acquisition of data: Soyi Lim, JH Kwon, YZ Kim. Data review: YZ Kim. Statistical analysis: YZ Kim. Manuscript preparation: YZ Kim. Manuscript approval: Soyi Lim, JH Kwon, YZ Kim.
References
- 1.Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA Cancer J Clin. 2002;52:23–47. doi: 10.3322/canjclin.52.1.23. [DOI] [PubMed] [Google Scholar]
- 2.Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2009. Cancer Res Treat. 2012;44:11–24. doi: 10.4143/crt.2012.44.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tangjitgamol S, Levenback CF, Beller U, Kavanagh JJ. Role of surgical resection for lung, liver, and central nervous system metastases in patients with gynecological cancer: a literature review. Int J Gynecol Cancer. 2004;14:399–422. doi: 10.1111/j.1048-891x.2004.14326.x. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen SL, Posner JB. Brain metastasis localized to an area of infarction. J Neurooncol. 1983;1:191–195. doi: 10.1007/BF00165602. [DOI] [PubMed] [Google Scholar]
- 5.Wroński M, de Palma P, Arbit E. Leiomyosarcoma of the uterus metastatic to brain: a case report and a review of the literature. Gynecol Oncol. 1994;54:237–241. doi: 10.1006/gyno.1994.1201. [DOI] [PubMed] [Google Scholar]
- 6.Ogawa K, Yoshii Y, Aoki Y, Nagai Y, Tsuchida Y, Toita T, Kakinohana Y, Tamaki W, Iraha S, Adachi G, et al. Treatment and prognosis of brain metastases from gynecological cancers. Neurol Med Chir (Tokyo) 2008;48:57–62. doi: 10.2176/nmc.48.57. discussion -3. [DOI] [PubMed] [Google Scholar]
- 7.Mayer RJ, Berkowitz RS, Griffiths CT. Central nervous system involvement by ovarian carcinoma: a complication of prolonged survivial with metastatic disease. Cancer. 1978;41:776–783. doi: 10.1002/1097-0142(197802)41:2<776::aid-cncr2820410253>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 8.Deutsch M, Beck D, Manor D, Brandes J. Metastatic brain tumor following negative second-look operation for ovarian carcinoma. Gynecol Oncol. 1987;27:116–120. doi: 10.1016/0090-8258(87)90238-1. [DOI] [PubMed] [Google Scholar]
- 9.Hardy JR, Harvey VJ. Cerebral metastases in patients with ovarian cancer treated with chemotherapy. Gynecol Oncol. 1989;33:296–300. doi: 10.1016/0090-8258(89)90515-5. [DOI] [PubMed] [Google Scholar]
- 10.Bindal RK, Sawaya R, Leavens ME, Lee JJ. Surgical treatment of multiple brain metastases. J Neurosurg. 1993;79:210–216. doi: 10.3171/jns.1993.79.2.0210. [DOI] [PubMed] [Google Scholar]
- 11.Patchell RA. Brain metastases. Neurol Clin. 1991;9:817–824. [PubMed] [Google Scholar]
- 12.Karnofsky DA, Abelmann WH, Craver LF, Burchenal JH. The use of the nitrogen mustards in the palliative treatment of carcinoma: with particular reference to bronchogenic carcinoma. Cancer. 1948;1:634–656. [Google Scholar]
- 13.Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, McKenna WG, Byhardt R. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 14.Benedet JL, Bender H, Jones H, 3rd, Ngan HY, Pecorelli S. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 2000;70:209–262. [PubMed] [Google Scholar]
- 15.Gadducci A, Tana R, Teti G, Fanucchi A, Pasqualetti F, Cionini L, Genazzani AR. Brain recurrences in patients with ovarian cancer: report of 12 cases and review of the literature. Anticancer Res. 2007;27:4403–4409. [PubMed] [Google Scholar]
- 16.Cohen ZR, Suki D, Weinberg JS, Marmor E, Lang FF, Gershenson DM, Sawaya R. Brain metastases in patients with ovarian carcinoma: prognostic factors and outcome. J Neurooncol. 2004;66:313–325. doi: 10.1023/b:neon.0000014516.04943.38. [DOI] [PubMed] [Google Scholar]
- 17.Anupol N, Ghamande S, Odunsi K, Driscoll D, Lele S. Evaluation of prognostic factors and treatment modalities in ovarian cancer patients with brain metastases. Gynecol Oncol. 2002;85:487–492. doi: 10.1006/gyno.2002.6653. [DOI] [PubMed] [Google Scholar]
- 18.Monaco E, 3rd, Kondziolka D, Mongia S, Niranjan A, Flickinger JC, Lunsford LD. Management of brain metastases from ovarian and endometrial carcinoma with stereotactic radiosurgery. Cancer. 2008;113:2610–2614. doi: 10.1002/cncr.23868. [DOI] [PubMed] [Google Scholar]
- 19.Muggia FM, Blessing JA, Sorosky J, Reid GC. Phase II trial of the pegylated liposomal doxorubicin in previously treated metastatic endometrial cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2002;20:2360–2364. doi: 10.1200/JCO.2002.08.171. [DOI] [PubMed] [Google Scholar]
- 20.Kolomainen DF, Larkin JM, Badran M, A'Hern RP, King DM, Fisher C, Bridges JE, Blake PR, Barton DP, Shepherd JH, et al. Epithelial ovarian cancer metastasizing to the brain: a late manifestation of the disease with an increasing incidence. J Clin Oncol. 2002;20:982–986. doi: 10.1200/JCO.2002.20.4.982. [DOI] [PubMed] [Google Scholar]
- 21.Kim TJ, Song S, Kim CK, Kim WY, Choi CH, Lee JH, Lee JW, Bae DS, Kim BG. Prognostic factors associated with brain metastases from epithelial ovarian carcinoma. Int J Gynecol Cancer. 2007;17:1252–1257. doi: 10.1111/j.1525-1438.2007.00941.x. [DOI] [PubMed] [Google Scholar]
- 22.Chura JC, Marushin R, Boyd A, Ghebre R, Geller MA, Argenta PA. Multimodal therapy improves survival in patients with CNS metastasis from uterine cancer: a retrospective analysis and literature review. Gynecol Oncol. 2007;107:79–85. doi: 10.1016/j.ygyno.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 23.McMeekin DS, Kamelle SA, Vasilev SA, Tillmanns TD, Gould NS, Scribner DR, Gold MA, Guruswamy S, Mannel RS. Ovarian cancer metastatic to the brain: what is the optimal management? J Surg Oncol. 2001;78:194–200. doi: 10.1002/jso.1149. discussion -1. [DOI] [PubMed] [Google Scholar]
- 24.Pectasides D, Pectasides M, Economopoulos T. Brain metastases from epithelial ovarian cancer: a review of the literature. Oncologist. 2006;11:252–260. doi: 10.1634/theoncologist.11-3-252. [DOI] [PubMed] [Google Scholar]
- 25.Lee YK, Park NH, Kim JW, Song YS, Kang SB, Lee HP. Gamma-knife radiosurgery as an optimal treatment modality for brain metastases from epithelial ovarian cancer. Gynecol Oncol. 2008;108:505–509. doi: 10.1016/j.ygyno.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 26.Richards GM, Khuntia D, Mehta MP. Therapeutic management of metastatic brain tumors. Crit Rev Oncol Hematol. 2007;61:70–78. doi: 10.1016/j.critrevonc.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Khuntia D, Brown P, Li J, Mehta MP. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol. 2006;24:1295–1304. doi: 10.1200/JCO.2005.04.6185. [DOI] [PubMed] [Google Scholar]
- 28.Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, Markesbery WR, Macdonald JS, Young B. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 29.Cormio G, Maneo A, Colamaria A, Loverro G, Lissoni A, Selvaggi L. Surgical resection of solitary brain metastasis from ovarian carcinoma: an analysis of 22 cases. Gynecol Oncol. 2003;89:116–119. doi: 10.1016/s0090-8258(03)00060-x. [DOI] [PubMed] [Google Scholar]
- 30.Salvati M, Cervoni L. Solitary cerebral metastasis from ovarian carcinoma: report of 4 cases. J Neurooncol. 1994;19:75–77. doi: 10.1007/BF01051051. [DOI] [PubMed] [Google Scholar]
- 31.Geisler JP, Geisler HE. Brain metastases in epithelial ovarian carcinoma. Gynecol Oncol. 1995;57:246–249. doi: 10.1006/gyno.1995.1134. [DOI] [PubMed] [Google Scholar]
- 32.Kaminsky-Forrett MC, Weber B, Conroy T, Spaëth D. Brain metastases from epithelial ovarian carcinoma. Int J Gynecol Cancer. 2000;10:366–371. doi: 10.1046/j.1525-1438.2000.010005366.x. [DOI] [PubMed] [Google Scholar]
- 33.Cormio G, Maneo A, Parma G, Pittelli MR, Miceli MD, Bonazzi C. Central nervous system metastases in patients with ovarian carcinoma. A report of 23 cases and a literature review. Ann Oncol. 1995;6:571–574. doi: 10.1093/oxfordjournals.annonc.a059246. [DOI] [PubMed] [Google Scholar]
- 34.Cormio G, Lissoni A, Losa G, Zanetta G, Pellegrino A, Mangioni C. Brain metastases from endometrial carcinoma. Gynecol Oncol. 1996;61:40–43. doi: 10.1006/gyno.1996.0093. [DOI] [PubMed] [Google Scholar]
- 35.Cormio G, Loizzi V, Falagario M, Lissoni AA, Resta L, Selvaggi LE. Changes in the management and outcome of central nervous system involvement from ovarian cancer since 1994. Int J Gynaecol Obstet. 2011;114:133–136. doi: 10.1016/j.ijgo.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 36.Mahmoud-Ahmed AS, Suh JH, Barnett GH, Webster KD, Belinson JL, Kennedy AW. The effect of radiation therapy on brain metastases from endometrial carcinoma: a retrospective study. Gynecol Oncol. 2001;83:305–309. doi: 10.1006/gyno.2001.6384. [DOI] [PubMed] [Google Scholar]