Abstract
The first Korean Breast Cancer Treatment Consensus Conference Expert Panel reviewed and endorsed new evidence on aspects of local and regional therapies and diagnostic procedures that support the conservative application of results from recent clinical trials. This conference clarified the barriers that limit the application of recent clinical trial results, such as questions about level of evidence, differences between the setting of clinical trials and that of daily clinical practice, and medical necessities and environment. Detailed decisions recommended for the treatment and diagnosis, according to the from the consensus conference, are recorded including details of the votes. These recommendations differed in the degree of support for clinical consideration of disease extent and host factors, medical necessities, and environment.
Keywords: Breast neoplasms, Consensus, Practice guideline, Therapeutics
INTRODUCTION
Breast cancer has already become one of the principal female-specific cancers in South Korea, and its incidence has increased on annually [1]. The Korean Breast Cancer Society (KBCS) has issued guidelines for the appropriate examination and treatment of breast cancer since 1999. However, rapidly improving breast cancer treatments, including the development of new anticancer chemotherapy and targeted therapy, subtype-based selection of therapeutic methods, and expanded use of sentinel lymph node biopsy, have led to different interpretations and clinical applications among clinicians [2,3]. South Korea depends fully on the Health Insurance Review Agency (HIRA), a nonprofit, government-run health insurance body, to implement medical necessity doctrines. Only HIRA or individual patients pay medical expenses, without another statutory authorization for payment, because Korean law obliges all the medical institutions to become members of HIRA and to operate as nonprofit organizations. The medical and social environment in which patients receive treatment may also be affected by diverse stakeholders and may face specific challenges [2,3]. Thus, not only doctors, but also all persons involved in medical enterprises, are involved in decision-making in ambiguous situations.
KBCS held a consensus conference to provide medical staff new insights and to enable decision-making for the current best treatment practices. This consensus conference is, therefore neither, intended to define standard treatment, nor intended its contents to be immutable [4]. Detailed decision recommended for the treatment and diagnosis, according to the consensus conference, is recorded in Supplementary Table 2.
THE FIRST KOREAN BREAST CANCER TREATMENT CONSENSUS CONFERENCE: NEWS AND NEXT STEP FORWARD
The Korean Breast Cancer Treatment Consensus Conference was first held January 4, 2014, and was composed of three sections: local treatment, systemic treatment, and diagnostic evaluation of breast cancer. The local treatment section included the application of the results of several trials, which have been recently released, to clinical practice. In particular, since surgery may make semi-permanent or permanent changes through a one-off procedure, unlike medication, conservative surgeons are expected to be more cautious when making decisions in a clinic, even though the results of randomized prospective studies have been published or reflected in international treatment guidelines [5]. This consensus conference aimed to determine to the extent to which panelists apply the results of recent studies on the sentinel node-only procedure in clinical practice.
In the systemic treatment section, new insights into recently revealed inconsistencies between several clinical trials and the social environment, as well as gaps between HIRA criteria and these new insights, were described to identify the best treatment options available to clinicians.
In the diagnostic evaluation of breast cancer section, an attempt was made to educate and reach consensus on institutional changes made by HIRA that impact the diagnostic process. Panelists were asked the methods of changing the nonmedical environment, including selective coverage of health insurance for cancer patient follow-up and pathological tests, which would be accepted in clinical practice.
CONFERENCE HIGHLIGHTS: PANEL DELIBERATION
Local treatment
Surgery of the axilla
We asked about the results of the clinical trial, micrometastases and isolated tumor cells: relevant and robust or rubbish? (the MIRROR trial) [6] only in terms of micrometastatic disease in two categories, breast conserving surgery (BCS) and mastectomy. More than 90% of the BCS panel and nearly 80% of the mastectomy panel agreed that axillary lymph node dissection was unnecessary. However, no more than 62.5% were willing clinically apply the results from the clinical trial, locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group (ACOSOG) Z0011 randomized trial (ACOSOG Z0011 trial) [7]. Only 57.6% were willing to clinically apply the results of the trial, after mapping of the axilla: radiotherapy or surgery? (AMAROS trial) [8], regardless of the surgical method. Thus, the AMAROS Trial results were even less likely to be applied clinically than the ACOSOG Z0011 Trial. In all, 85.7% of the panelists who observed a clinical complete response and 54% of those who observed a clinically partial response in the axillary nodes after neoadjuvant chemotherapy were willing to use sentinel lymph node biopsy based on the results of the clinical trials, sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer (the ACOSOG Z1071 trial) [9] and sentinel lymph node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (the SENTINA trial) [10]. In addition, 71% of those observing complete response and 27% of those observing partial response answered they would omit axillary dissection if no nodal metastasis was found, given that sentinel lymph node biopsy had been used.
Radiation therapy
For ductal carcinoma in situ, 91% of the panelists believed that radiotherapy could be omitted for some patients. However, about three-fourths of the panelists supported criteria in the fifth KBCS guidelines and the National Comprehensive Cancer network (NCCN) guidelines® but suggested the criteria be supplemented. Among those patients with invasive ductal carcinoma, 55.9% of panelists indicated that there was a low risk group requiring no radiotherapy. However, only 67.7% supported the criteria in clinical trials such as lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer initiated by the Cancer and Leukemia Group B (CALGB) 9343 [11] and postoperative radiotherapy in minimum-risk elderly (PRIME) II [12].
Systemic treatment
Human epidermal growth factor receptor 2-targeted therapy
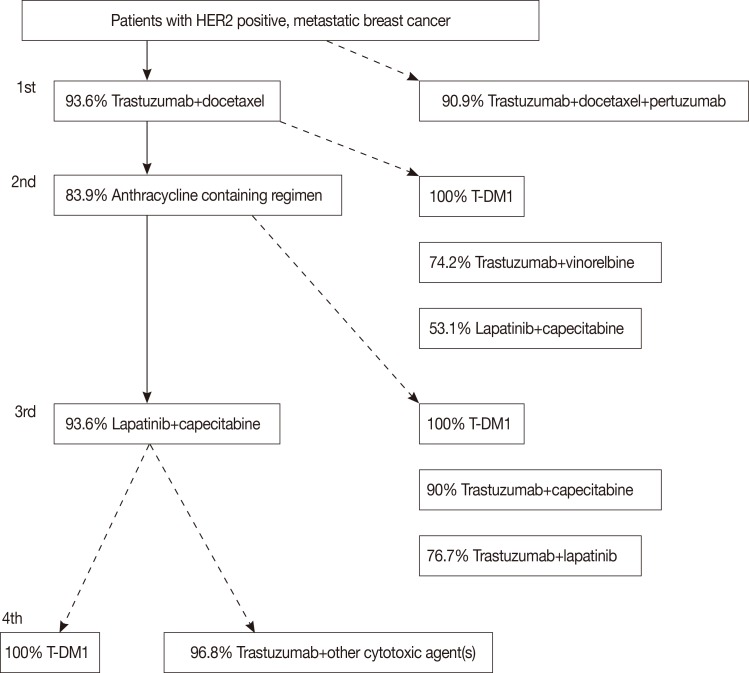
When this survey was conducted, HIRA's legal regimen, whether covered by HIRA or not, for patients with human epidermal growth factor receptor 2 (HER2)-positive and hormone receptor-negative metastatic breast cancer was trastuzumab with taxane, which was inconsistent with NCCN guidelines®. Clinicians were asked what treatment they would select when medical necessity was inconsistent with medical environment (Figure 1). Panelists were first asked about the NeoSphere trial, a phase III evaluation of pertuzumab and trastuzumab for HER2-positive metastatic breast cancer (the CLEOPATRA trial) [13], an open-label study of trastuzumab emtansine (T-DM1) versus capecitabine+lapatinib in patients with HER2-positive locally advanced or metastatic breast cancer" (the EMILIA trial) [14], capecitabine with or without lapatinib for women with refractory advanced or metastatic breast cancer (EGF100151) [15], and trastuzumab in combination with vinorelbine or taxane-based chemotherapy in patients with metastatic breast cancer (the TRAVIOTA trial) [16] for HER2-positive metastatic breast cancer. The responses are summarized in Figure 1.
Figure 1.
Flow sheet of questions on patients with human epidermal growth factor receptor 2 (HER2)-positive, metastatic breast cancers; panels were asked the 1st line treatment to 4th line treatment in sequence with (line) or without (dotted line) consideration of practical issues, showing T-DM 1, if practical, commanded 100% agreement at each sequence.
Second, panelists were asked about regimens used in preoperative chemotherapy for HER2-positive breast cancer. They were also asked about clinical trials such as neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomized controlled superiority trial with a parallel HER2-negative cohort [17] and taxol epirubicin cyclophosphamide herceptin neoadjuvant study (the TECHNO trial) [18]. Only 9.7% of the panelists chose the regimen suggested by the NOAH trial, whereas most of the panelists chose anthracycline with cyclophosphamide, followed by taxane with trastuzumab combination, 96.9% when paclitaxel is used and 100% when docetaxel is used.
Systemic therapy in luminal A-like breast cancer: chemoendocrine versus endocrine
First, biomarkers that might affect the decision of whether or not anticancer therapy would be provided to postmenopausal women with T1c (1.8 cm), N0, estrogen receptor (ER)-positive breast cancer were addressed. Of the panelists, 60.6% were willing to use a multigene assay and half, not including abstentions, were willing to provide anticancer therapy even when progesterone receptor (PR), KI-67, and the histologic grade were all favorable (2-Q6-2). Furthermore, 73.5% to 93.9% suggested the need for anticancer chemotherapy cases where PR, KI-67, and the histologic grade were unfavorable. Of note, the panelists unanimously agreed that anticancer chemotherapy was needed in cases with positive nodes.
Application of sonography in breast cancer surveillance
We assessed whether insurance coverage for ultrasonography in cancer patients affected radiological follow-up since it began in 2013. Of the panelists, 11.0% used mammography alone for the follow-up of breast cancer patients, while 77.8% and 13.5% used ultrasonography and magnetic resonance imaging (MRI), respectively, before the coverage. Furthermore, 55.9% indicated that insurance coverage for ultrasonography would affect radiological follow-up. Finally, 93.1% of panelists indicated that the results of an in situ hybridization (ISH) test would affect the selection of adjuvant therapy if immunohistochemistry (IHC)-based HER2 was found to be 2+. However, if IHC-based HER2 was found to be 1+, no more than 25.0% and 42.9% were willing to carry out an ISH test in ER-positive and ER-negative patients, respectively.
DISCUSSION
Level of evidence: is it only the study design that matters?
Not more than 62.5% panelists were willing to clinically apply the ACOSOG Z0011 trial [7] results, and only 57.6% were willing to clinically apply the results of the AMAROS trial [8], regardless of the surgical method. Thus, the AMAROS Trial was even less likely to be applied clinically than the ACOSOG Z0011 trial.
This seems to reflect assessment results, which have provided evidence for panelists in the medical community, thus providing weightage to evidence-based medicine. In evidence-based medicine, the assessment of data is very important, and clinicians are trained to evaluate the quality of evidence. In particular, the quality of evidence should be evaluated in terms of the quality of supportive literature and the quantity, consistency, and immediacy of the evidence, as well as the study design. The ACOSOG Z0011 trial has been criticized for its design, accrual, and data collection methods [19]. Some researchers indicated that ACOSOG Z0011 had an insufficient follow-up period, and others suggested that its results should be applied selectively. On the basis of these reports, it can be presumed that clinicians may resist the application of ACOSOG Z0011 results. A survey concerning the effects of the ACOSOG Z0011 results on medical practice in France found that it was not widely accepted in the clinical field, with 61.9% willing to use axillary node dissection in case of macrometastases that met the ACOSOG Z0011 criteria [20].
Even fewer panelists (57.6%) were willing to clinically use the results of the AMAROS study. Despite intervention with radiotherapy, the lower rate for the AMAROS study compared to the ACOSOG trial is probably owing to the inclusion of modified radical mastectomy patients in the questionnaire (see Q4).
Available medicine versus practical medicine
The panelists had a preference for dual blocker use in patients with HER2-positive, metastatic breast cancer (93.6% agreement for trastuzumab+docetaxel+pertuzumab), a high preference for TDM-1 (100% selection as a second-, third-, and fourth-line drug), and a low preference for lapatinib (53.1%) as the second-line drug. Recommendations from this conference do not imply that each panel member was in full agreement. Among more than 50 questions, only six questions achieved 100% agreement, all of which were about systemic treatment (Table 1). The fact that 100% of panelists were in favor of T-DM1, which is not available in Korea, suggests that clinicians are clearly open to using available treatments, not only practicable treatments, even if HIRA's regulations strongly influence actual clinical practice.
Table 1.
Questions commanded 100% agreement from the Expert Panel

All of the questions were issues regarding systemic treatment.
ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2; AC=doxorubicin cyclophosphamide.
Chemotherapy in the molecular era
Not more than 60.6% of the panelists suggested the need for multigene assays in postmenopausal women with T1cN0, ER-positive breast cancer. Panelists were found to rely heavily on adjuvant cytotoxic chemotherapy, with half willing to use these assays even when PR, KI-67, and the histologic grade were all favorable, and all panelists were willing to use these assays in patients with lymph node-positive breast cancer.
The St. Gallen consensus conference in 2013 suggested that a major unresolved question is the threshold for use of adjuvant cytotoxic chemotherapy in patients with luminal A or B disease [3]. Unlike the 2013 St. Gallen consensus conference in which active discussions were used to decide cases for which chemotherapy could be added to hormone therapy, the first Korean Breast Cancer Treatment Consensus Conference aimed to identify cases where anticancer chemotherapy could be left out and to determine a different therapeutic base, with 50% willing to use anticancer treatment even when all the conditions were favorable. In terms of lymph node-positive disease, most panelists in the St. Gallen consensus conference incited that cancer being nodal-positive per se was not an indication for chemotherapy [3], whereas all the panelists in this conference were willing to use anticancer treatment for lymph node-positive disease and they tended to support the application of anticancer therapy based on TMN stage rather than on the breast cancer subtype. This tendency is probably due to the different epidemiologic features of breast cancer in Korean women compared to that in Western women.
Even if the incidence of breast cancer, the second most common cancer in Korean women, recently has been increasing, its incidence per 100,000 persons remains at 39.8, which is lower than that in the Western society. However, the median age of occurrence in Korea was reported as 50 years in 2011, which is lower by approximately 10 years than that reported in the United States, which was 61 years between 2006 and 2010 [21]. The recent increase in breast cancer cases among Korean women cannot be explained based on biologic factors alone [22] and is presumed to be affected by lifestyle changes associated with Westernization [23,24,25,26,27]. The results of epidemiologic studies implying ethnic differences do not contain enough evidence supporting biological differences in breast cancer between Western and Korean women. It is therefore necessary to consider whether Western and Korean breast cancer patients with the same biomarkers currently in clinical use, such as TNM stage, ER, PR, HER2, and Ki-67, can be treated differently and, if so, it is imperative to make clinical decisions on the basis for these different treatments.
Limitations and prospective meeting
The conclusions of this consensus conference have several limitations, which might be overcome by additional experience. First, we did not have sufficient time to develop the questions in advance. Since we had no more than 8 months after the committee was formed, we lacked time and manpower to develop key questions and were restricted to reviewing entire evidence. In addition, the lack of resources made it difficult to develop key questions, which were key elements for this event. Panelists also were given insufficient time to provide answers. Additional resources should be provided to support thorough deliberation for the next consensus conference. Second, the absence of a conference manual may have introduced some errors. It is necessary to develop a system of standard operating procedures that will allow the conference to proceed systematically. Third, we failed to examine conflicts of interest (COI) in advance. Even if a wide range of questions are given and a large number of experts from diverse fields are appointed to the panel, it is necessary to manage COIs more proactively given its importance. The sixth guideline committee of the KBSC decided to overcome limitations in holding the event, taking into account the fact that this was the first consensus conference. We appreciate the participants' devotion, which ensured the high quality of this consensus conference.
We found that this panel has different opinions from conventional treatment guidelines, including those of the NCCN [28], and the 2013 St. Gallen International Expert consensus on the primary therapy for early breast cancer [3], both of which support less extensive treatment. Not more than 62.5% of the panelists said they were willing to clinically apply the results of the ACOSOG Z0011 trial [7]. The panel was equally divided (46.9% vs. 46.9% with 6.3% abstentions) on whether they are willing to provide anticancer therapy to postmenopausal breast cancer patients in which all of the prognostic factors are favorable, such as being positive for ER and PR, with low Ki-67 and histologic grade. One of the two major factors contributing to the difference in opinion is that the panel is heavily influenced by HIRA, which does not cover multigene assays despite their superior accuracy and reproducibility. However, more importantly, these results are a reflection of clinicians' anxiety. We believe that substantial progress in evidence relevant to various clinical questions could help provide more tailored treatment based on biological markers or clinicopathologic surrogate definitions of cancer subtype in this era of molecular biology.
CONCLUSIONS
The first Korean Breast Cancer Treatment Consensus Conference is the only and the first multidisciplinary joint conference based on the voluntary participation of expert panelists from various clinical backgrounds. This conference served as a good opportunity to present current Korean practices for treating breast cancer. We believe these recommendations can be applied until the next consensus conference, where further discussion will be held. We hope that the ideas and findings generated at this conference will lead to more appropriate breast cancer treatment.
Footnotes
The authors declare that they have no competing interests.
Supplementary Materials
Professional composition of the panels at first Korean Breast Cancer Treatment Consensus Conference
Records of votes
References
- 1.Ko BS, Noh WC, Kang SS, Park BW, Kang EY, Paik NS, et al. Changing patterns in the clinical characteristics of Korean breast cancer from 1996-2010 using an online nationwide breast cancer database. J Breast Cancer. 2012;15:393–400. doi: 10.4048/jbc.2012.15.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldhirsch A, Wood WC, Senn HJ, Glick JH, Gelber RD International Consensus Panel on the Treatment of Primary Breast Cancer. Fifth International Conference on Adjuvant Therapy of Breast Cancer, St. Gallen, March 1995. Eur J Cancer. 1995;31:1754–1759. doi: 10.1016/0959-8049(95)00479-3. [DOI] [PubMed] [Google Scholar]
- 3.Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glick JH. Meeting highlights: adjuvant therapy for breast cancer. J Natl Cancer Inst. 1988;80:471–475. doi: 10.1093/jnci/80.7.471. [DOI] [PubMed] [Google Scholar]
- 5.McCulloch P, Taylor I, Sasako M, Lovett B, Griffin D. Randomised trials in surgery: problems and possible solutions. BMJ. 2002;324:1448–1451. doi: 10.1136/bmj.324.7351.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Boer M, van Deurzen CH, van Dijck JA, Borm GF, van Diest PJ, Adang EM, et al. Micrometastases or isolated tumor cells and the outcome of breast cancer. N Engl J Med. 2009;361:653–663. doi: 10.1056/NEJMoa0904832. [DOI] [PubMed] [Google Scholar]
- 7.Giuliano AE, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252:426–432. doi: 10.1097/SLA.0b013e3181f08f32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galimberti V, Cole BF, Zurrida S, Viale G, Luini A, Veronesi P, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol. 2013;14:297–305. doi: 10.1016/S1470-2045(13)70035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310:1455–1461. doi: 10.1001/jama.2013.278932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14:609–618. doi: 10.1016/S1470-2045(13)70166-9. [DOI] [PubMed] [Google Scholar]
- 11.Hughes KS, Schnaper LA, Bellon JR, Cirrincione CT, Berry DA, McCormick B, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31:2382–2387. doi: 10.1200/JCO.2012.45.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunkler I. PRIME II breast cancer trial. Clin Oncol (R Coll Radiol) 2004;16:447–448. doi: 10.1016/j.clon.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Swain SM, Kim SB, Cortés J, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14:461–471. doi: 10.1016/S1470-2045(13)70130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cameron D, Casey M, Oliva C, Newstat B, Imwalle B, Geyer CE. Lapatinib plus capecitabine in women with HER-2-positive advanced breast cancer: final survival analysis of a phase III randomized trial. Oncologist. 2010;15:924–934. doi: 10.1634/theoncologist.2009-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burstein HJ, Keshaviah A, Baron AD, Hart RD, Lambert-Falls R, Marcom PK, et al. Trastuzumab plus vinorelbine or taxane chemotherapy for HER2-overexpressing metastatic breast cancer: the trastuzumab and vinorelbine or taxane study. Cancer. 2007;110:965–972. doi: 10.1002/cncr.22885. [DOI] [PubMed] [Google Scholar]
- 17.Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375:377–384. doi: 10.1016/S0140-6736(09)61964-4. [DOI] [PubMed] [Google Scholar]
- 18.Untch M, Fasching PA, Konecny GE, Hasmüller S, Lebeau A, Kreienberg R, et al. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol. 2011;29:3351–3357. doi: 10.1200/JCO.2010.31.4930. [DOI] [PubMed] [Google Scholar]
- 19.Sabel MS. The need for axillary lymph node dissection in T1/T2 breast cancer surgery: counterpoint. Cancer Res. 2013;73:7156–7160. doi: 10.1158/0008-5472.CAN-13-2094. [DOI] [PubMed] [Google Scholar]
- 20.Barranger E, Houvenaeghel G, Classe JM. Axillary support in breast cancer: survey practice in France. Gynecol Obstet Fertil. 2013;41:433–436. doi: 10.1016/j.gyobfe.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Kim Z, Min SY, Yoon CS, Lee HJ, Lee JS, Youn HJ, et al. The basic facts of Korean breast cancer in 2011: results of a nationwide survey and breast cancer registry database. J Breast Cancer. 2014;17:99–106. doi: 10.4048/jbc.2014.17.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colditz GA, Bohlke K, Berkey CS. Breast cancer risk accumulation starts early: prevention must also. Breast Cancer Res Treat. 2014;145:567–579. doi: 10.1007/s10549-014-2993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shin HR, Joubert C, Boniol M, Hery C, Ahn SH, Won YJ, et al. Recent trends and patterns in breast cancer incidence among Eastern and Southeastern Asian women. Cancer Causes Control. 2010;21:1777–1785. doi: 10.1007/s10552-010-9604-8. [DOI] [PubMed] [Google Scholar]
- 24.Wu AH, Wan P, Hankin J, Tseng CC, Yu MC, Pike MC. Adolescent and adult soy intake and risk of breast cancer in Asian-Americans. Carcinogenesis. 2002;23:1491–1496. doi: 10.1093/carcin/23.9.1491. [DOI] [PubMed] [Google Scholar]
- 25.Wu AH, Yu MC, Tseng CC, Stanczyk FZ, Pike MC. Dietary patterns and breast cancer risk in Asian American women. Am J Clin Nutr. 2009;89:1145–1154. doi: 10.3945/ajcn.2008.26915. [DOI] [PubMed] [Google Scholar]
- 26.Ziegler RG, Hoover RN, Nomura AM, West DW, Wu AH, Pike MC, et al. Relative weight, weight change, height, and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1996;88:650–660. doi: 10.1093/jnci/88.10.650. [DOI] [PubMed] [Google Scholar]
- 27.Ziegler RG, Hoover RN, Pike MC, Hildesheim A, Nomura AM, West DW, et al. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1993;85:1819–1827. doi: 10.1093/jnci/85.22.1819. [DOI] [PubMed] [Google Scholar]
- 28.Clinical practice guidelines in oncology v.3. National Comprehensive Cancer Network; 2014. [Accessed August 25th, 2014]. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Professional composition of the panels at first Korean Breast Cancer Treatment Consensus Conference
Records of votes