Abstract
Purpose
Transient receptor potential vanilloid 1 (TRPV1) is a nonselective cation channel belonging to the transient receptor potential family, and it is expressed in different neoplastic tissues. Its activation is associated with regulation of cancer growth and progression. The aim of this research was to study the expression and pharmacological characteristics of TRPV1 in cells derived from human breast cancer MCF-7 cells.
Methods
TRPV1 presence was assessed by binding studies and Western blotting. Receptor binding characteristics were evaluated through competition assays, while 3-(4,5-dimethylthiazol-2-yl)-2,5,-dipheyltetrazolium bromide reduction assays were performed to confirm an early hypothesis regarding the modulation of cancer cell proliferation. The functionality of TRPV1 was evaluated by measuring Ca2+ uptake in the presence of increasing concentrations of TRPV1 agonists and antagonists.
Results
Binding studies identified a single class of TRPV1 (Bmax 1,492±192 fmol/mg protein), and Western blot showed a signal at 100 kDa corresponding to the molecular weight of human TRPV1. Among the different tested agonists and antagonists, anandamide (Ki: 2.8×10-11 M) and 5-iodoresiniferatoxin (5-I-RTX) (Ki: 5.6×10-11 M) showed the highest degrees of affinity for TRPV1, respectively. All tested TRPV1 agonists and antagonists caused a significant (p<0.05) decrease in cell growth rate in MCF-7 cells. For agonists and antagonists, the efficacy of tested compounds displayed the following rank order: resiniferatoxin>anandamide>capsaicin and 5-I-RTX=capsazepine, respectively.
Conclusion
These data indicate that both TRPV1 agonists and antagonists induce significant inhibition of MCF-7 cell growth. Even though the mechanisms involved in the antiproliferative effects of TRPV1 agonists and antagonists should be further investigated, it has been suggested that agonists cause desensitization of the receptor, leading to alteration in Ca2+-influx regulation. By contrast, antagonists cause a functional block of the receptor with consequent fatal dysregulation of cell homeostasis.
Keywords: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide; Competition assays; Functionality assays; MCF-7 cells; TRPV1 receptor
INTRODUCTION
Transient receptor vanilloid 1 (TRPV1) is a member of the transient receptor potential (TRP) family that is expressed in several neural and nonneuronal tissues of different animal species [1]. It is a Ca2+-permeable nonselective cation channel that can be activated by noxious heat (>43℃), low pH (<6), and various lipophilic compounds, including capsaicin and phopholipid derivatives [2]. TRPV1 is composed of six transmembrane spanning domains (S1-S6), intracellular N and C termini, and a pore-forming hydrophobic stretch between the fifth and the sixth domains [3].
Physiologically, TRPV1 is expressed in dorsal root, trigeminal, and nodose ganglion neurons that are associated with nociceptive afferents, enabling detection and integration of noxious stimuli [4] in different organs, such us the bladder [4], kidneys [5], brain [6], and lungs [7,8]. Moreover, there are strong indications that TRPV1 is involved in many pathological conditions, including abnormalities in pain sensation, epilepsy, osteoarthritis, pancreatitis, asthma, and non-insulin-dependent diabetes mellitus [9]. In the last decade, TRPV1 has also been identified in humans in neoplastic tissues such as squamous cell carcinoma of the tongue [10], prostate carcinoma [11], colon cancer [9], transitional urothelial cancer of the bladder [12], and breast cancer [9].
Based on this information, calcium is considered an important intracellular messenger that regulates physiological functions, including cellular differentiation, proliferation, and apoptosis. Additionally, it has been suggested that TRPV channels are involved in cancer growth and progression [13]. Santoni et al. [12] found, for example, that TRPV1 expression decreases during the development of transitional cell carcinoma of the human bladder, outlined its key role in the regulation of tumor cell proliferation, and even discussed the possibility of its use as a possible therapeutic target [7].
Breast cancer is a leading cause of death globally. Recent epidemiological data suggest that in 2008, almost 1.4 million women worldwide were diagnosed with breast cancer and approximately 459,000 deaths were recorded. The 5-year relative survival rate ranges from 12% to 90% depending on the diagnostic and therapeutic approaches used [14]. Traditional pharmacological treatments cause severe side effects; therefore, it is important to identify new targets to improve the clinical outcome of these patients [12].
Accordingly, the aim of this study was to identify and characterize TRPV1 in cells derived from human breast adenocarcinoma and study the modulation of cancer cell proliferation induced by different specific TRPV1 agonists and antagonists.
METHODS
Cell culture
MCF-7 cells (cells derived from pleural effusion after pleural methastatisation of human breast adenocarcinoma) (ATCC Cell Culture-LGC Standards, Milan, Italy) were cultured in 75 mL flasks containing Dulbecco's Modified Eagles Medium (DMEM; Lonza, Basel, Switzerland), 20% fetal bovine serum (FBS; Sigma Aldrich, Milan, Italy), 2% L-glutamine (Lonza), and 2% penicillin-streptomicin-amphotericin B solution (Sigma Aldrich) at 37℃ with 5% CO2, 95% air, and complete humidity. When a confluence of 80% was reached, cells were detached using 0.1% trypsin/EDTA solution (Sigma Aldrich), centrifuged, and counted using Trypan Blue solution (Sigma Aldrich) with a Burker chamber. Cells were either resuspended at a concentration of 5×105 cells/mL or stored at -80℃ for further analysis.
Radioligand binding assays
Cell membrane suspensions obtained by fractioned ultracentrifugation were diluted to a final concentration of 1 mg protein/mL, and 100 µL aliquots were incubated for 1 hour at 37℃ in a shaking water bath with increasing concentrations (0.025-3.2 nM) of labeled resiniferatoxin (RTX) (3[H]RTX 43 Ci/mmol; PerkinElmer, Boston, USA) in the absence (total binding) and in the presence (nonspecific binding) of 1 µM unlabelled RTX. After stopping the incubation, the mixture was filtered under vacuum using presoaked glass fiber filters (Whatmann GF/B; Whatmann, Maidstone, UK). Filters were carefully washed with buffered saline solution (154 mM NaCl, 50 mM tris HCl, pH 7.4; Sigma Aldrich) and solubilized with 4 mL of scintillation fluid (Filter Count; Canberra Packard, Meridien, USA). The radioactivity retained on wet filters was measured for 2 minutes using a Tri-Carb 1600 TR Canberra Packard scintillator spectrometer (Canberra Packard) at an efficiency of 60%.
The equilibrium dissociation constant (Kd), expressed as nM, and the maximum number of binding sites (Bmax), expressed as fmol of specifically bound labeled ligand per milligram of membrane proteins, were determined (n=8) by Scatchard analysis [15] using GraphPad Prism Software (GraphPad Software, San Diego, USA).
Western blotting assay
To confirm the presence of TRPV1 in MCF-7, cell membrane suspensions were mixed with Laemmli buffer (5× solution: 3 mL 1 M Tris-HCl pH 6.8, 5 mL glicerol, 1.5 g sodium dodecyl sulfate [SDS], 50 mg bromophenol blue, and 320 µL β-mercaptoethanol [Sigma Aldrich]), diluted at 1× by adding phosphate buffered saline (PBS) and boiled for 5 minutes. A gel with 8% of acrilamide was used for SDS-PAGE procedure (Biosolve, Valkenswaard, The Netherlands). A lysate of urinary rat bladder was used as a positive control [4].
The samples were transferred to nitrocellulose membranes (Hybond ECL; Amersham, GE Healthcare, Milan, Italy) and dyed with Red Ponceau (Sigma Aldrich). After being washed three times, samples were probed with the primary anti-TRPV1 antibody (goat polyclonal antibody; Santa Cruz Biotechnology Inc., Heidelberg, Germany) (dilution 1:100) and incubated overnight at 5℃ on a shaking plate. At the end of the incubation period, samples were washed again and probed with secondary antibody (donkey antigoat IgG-HRP; Santa Cruz Biotechnology Inc.) (dilution 1:15,000) for 1 hour. The immunoreactive bands were visualized by enhanced chemiluminescence (ECL-Plus System; Amersham, GE Healthcare).
Functional studies
For the 45Ca2+ uptake assay, 250 µL of cell suspension (5×103/100 µL) was incubated for 20 minutes at 37℃ in a shaking bath in the presence of 1 µCi 45Ca2+/mL (45Ca 27.63 mCi/mL; PerkinElmer) in parallel sets of tubes with increasing concentrations (10-11-10-3 M) of selective agonists (RTX, capsaicin, and anandamide; Sigma Aldrich) and antagonists (5-iodoresiniferatoxin [5-I-RTX] and capsazepine; Sigma Aldrich). Immediately after incubation, 45Ca2+ was removed by washing with ice-cold PBS (140 mM NaCl, 27 mM KCl, 15 mM KH2 PO4, 90mM Na2HPO4, pH=7.4; Sigma Aldrich). Then, 3 mL of scintillation fluid (Ultima Gold; Canberra Packard) was added to the cell suspensions, and radioactivity (cpm/well) was measured by a scintillator spectrometer. The molar concentration of agonists producing 50% of the maximum possible response (EC50) and antagonist inhibitory concentration 50 (IC50) were then calculated [16].
Competition assays
Receptor binding characteristics were studied by displacement assays using TRPV1 selective agonists (capsaicin, anandamide, and RTX; Sigma Aldrich) and antagonists (capsazepine, 5-I-RTX, and SB-366791; Sigma Aldrich). Aliquots of 100 µL of membrane suspension (1 mg protein/mL) were incubated with increasing concentrations (10-10-10-3 M) of the TRPV1 unlabelled agonists and antagonists in presence of 1.2 nM 3[H]RTX. The concentration of unlabelled ligand that it took to inhibit 50% (IC50) of 3[H]RTX specific binding was determined from the competition curves obtained by nonlinear regression analysis of the data (GraphPad Prism; GraphPad Software). The fit of the one-site model was tested by R2 by nonlinear regression and by the runs test. The affinity constant (Ki) for the competitors were calculated using the Cheng-Prussoff equation [17].
Proliferation assay
The MTT (Sigma Aldrich) reduction assay, a routine method for quantitating cell viability, was used [18]. Cells (5×103 cells/100 µL) were transferred into 96-well plates in order to perform the MTT assay at 24, 48, and 72 hours after seeding. After an overnight incubation at 37℃ and 5% CO2, the medium was changed (DMEM, FBS 5%, antibiotic and antimycotic solution 2%, L-glutamine 2%), and increasing concentrations of agonists (capsaicin [10-10-10-4 M] and RTX [10-10-10-6 M]) and antagonists (capsazepine [10-10-10-4 M] and 5-I-RTX [10-10-10-6 M]) were added.
After adding MTT (Sigma Aldrich) solution, the plates were incubated at 37℃ and 5% CO2 for 4 hours. At the end of incubation, the medium was removed, and 100 µL of dimethyl sulfoxide (DMSO; Sigma Aldrich) was added in order to lysate the formazan crystals. The plates were further incubated for 10 minutes at room temperature, and the optical density of the wells was determined using a plate reader (Poverwave x; Bio-Tek Instruments Inc., Winooski, USA) at a wavelength of 570 nm [19]. The results, expressed as mean±SEM, were analyzed using GraphPad Prism Software as well as applying the Kruskal-Wallis test and a Dunn post-test. Significant values were considered p<0.05.
RESULTS
Binding assay
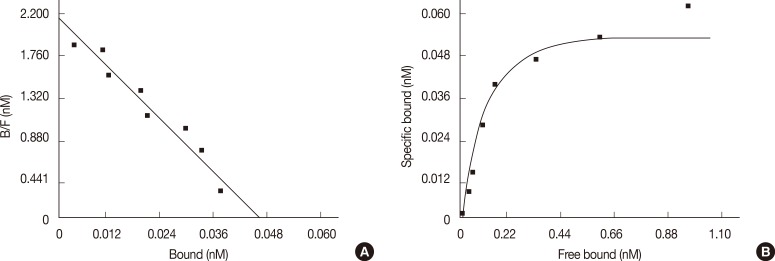
Scatchard analysis identified a single class of TRPV1 characterized by high affinity for 3[H]RTX (Figure 1). The Bmax value was 1,492±192 fmol/mg of protein (mean±SEM) and the d value was 0.03±0.004 nM (mean±SEM, r>0.9).
Figure 1.
Scatchard plot (A) and saturation curve of MCF-7 binding membranes (B). One hundred microliters of cell membrane suspension were incubated with increasing concentration (0.025-3.2 nM) of 3[H]RTX and in presence or in absence of 1 µM unlabelled RTX. The specific bound labeled ligand was determined by Scatchard analysis using GraphPad Prism Software (GraphPad Software) (n=8).
RTX=resiniferatoxin.
Western blotting assays
Figure 2 shows a signal corresponding to a molecular weight of about 100 kDa, which is consistent with that reported for human TRPV1 [20]. The same signal was observed in rat urinary bladder lysate, which was used as a positive control.
Figure 2.

The samples used for Western blot analysis were at different concentrations: 25, 50, and 100 µg of protein. The image shows a signal corresponding to the molecular weight around 100 kDa that is reported to correspond to transient receptor potential vanilloid 1 (TRPV1) receptor. The signal was also present in the positive control (first column, K+), composed by proteic lysate of rat urinary bladder.
Functional studies
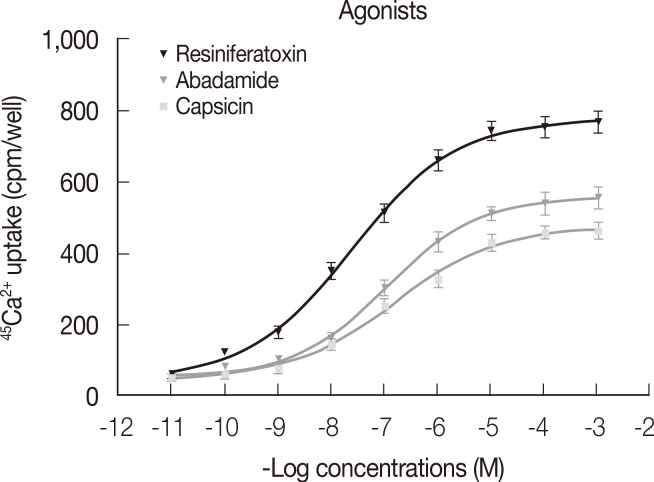
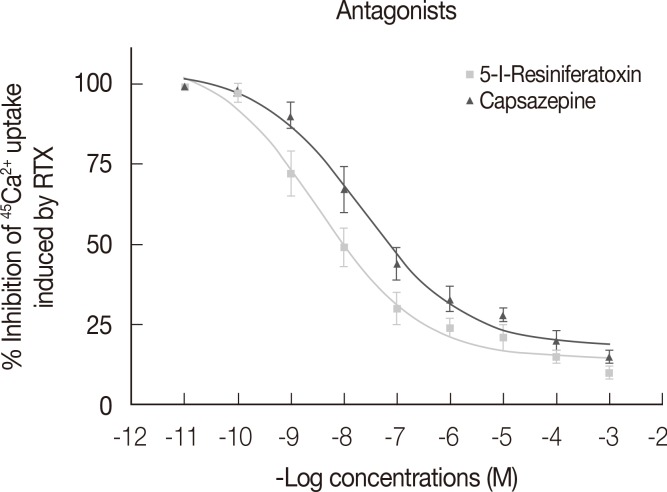
Figure 3 shows dose-response curves describing Ca2+ uptake induced by increasing concentrations of RTX, capsaicin, and anandamide. The efficacy of tested compounds displayed the following rank order: RTX (EC50=2.4×10-8 M, R2>0.9, E max=777.0 cpm)>anandamide (EC50=1.10×10-7 M, R2>0.9, E max=557.0 cpm)>capsaicin (EC50=1.52×10-7 M, R2>0.9, E max=478.8 cpm). Figure 4 shows the percentage of Ca2+ influx inhibition induced by increasing concentrations of the two tested antagonists 5-I-RTX and capsazepine. The IC50 for 5-I-RTX and capsazepine were 5.6×10-11 M, R2>0.9, E max=108.3% and 7.9×10-11 M, R2>0.9, E max=104.0%, respectively. All significant differences were observed between antagonists.
Figure 3.
During functional studies, the stimulation of 45Ca2+ uptake in MCF-7 membranes was induced by adding increasing concentrations (10-11-10-3) of selective agonists: anandamide, resiniferatoxin (RTX), and capsaicin. For each data point in each experiment, four wells were assayed (n=4). The error bars indicate SEM (GraphPad Prism Software; GraphPad Software).
Figure 4.
Inhibition of resiniferatoxin (RTX) induced 45Ca2+ uptake by two selective transient receptor potential vanilloid 1 antagonists (5-iodoresiniferatoxin [5-I-RTX] and capsazepine) in MCF-7 cell membranes. For each data point in each experiment, four wells were assayed (n=4). The error bars indicate SEM (GraphPad Prism Software; GraphPad Software). The antagonists have been tested in the presence of RTX 2 nM for the measurement of the percentage of inhibition of 45Ca2+ uptake (IC50) (GraphPad Prism Software).
Competition assays
The results obtained by competition assays are shown in Tables 1 and 2. The Ki values demonstrate that tested agonists displaced the radioligand from its specific binding sites in the following order of potency: anandamide>capsaicin>RTX. For the antagonists, the order of potency was 5-I-RTX>capsazepine>SB-366791.
Table 1.
Inhibition of 3[H]RTX binding to transient receptor potential vanilloid 1 by selected agonists
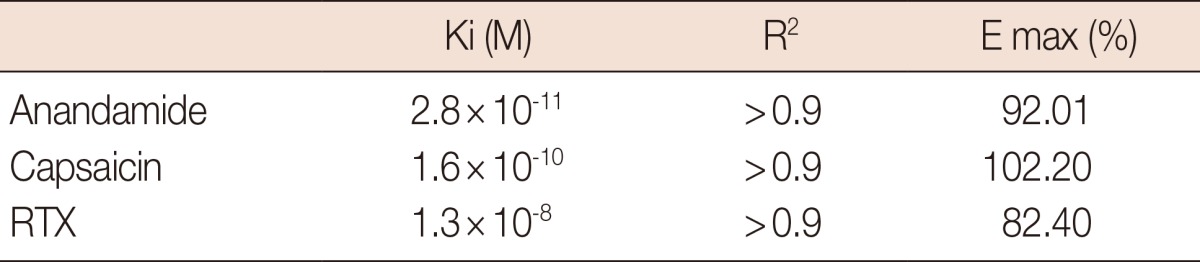
Competition assays using antagonists compounds.
Ki=affinity constant; E max=efficacy; RTX=resiniferatoxin.
Table 2.
The affinities (Ki) for the different selective transient receptor potential vanilloid 1 antagonists
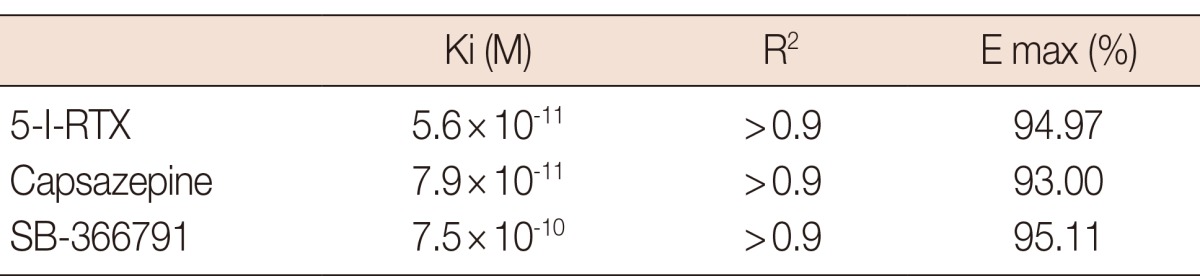
Competition assays using antagonists compounds.
Ki=affinity constant; E max=efficacy; 5-I-RTX=5-iodoresiniferatoxin.
Proliferation assay
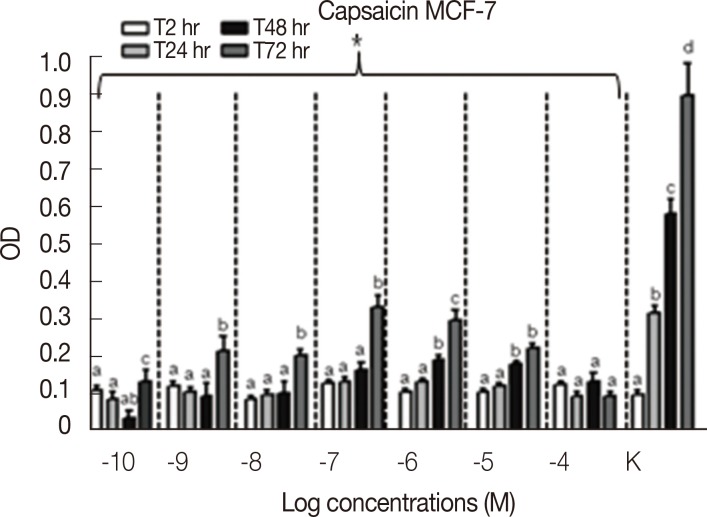
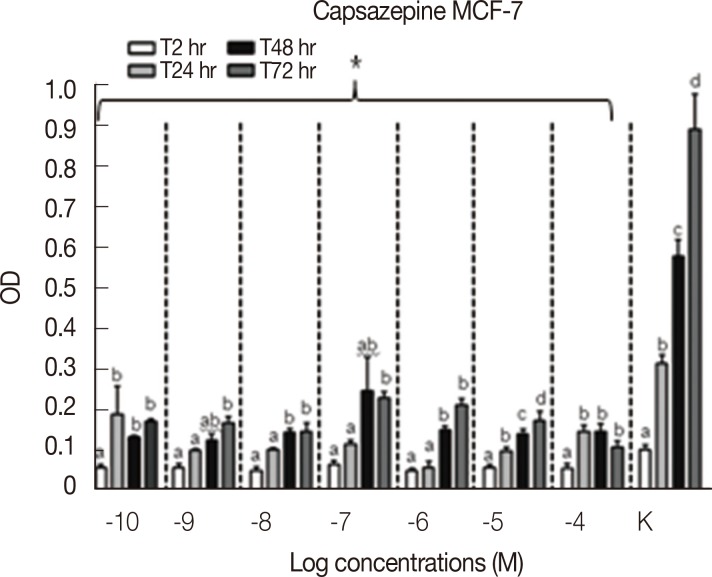
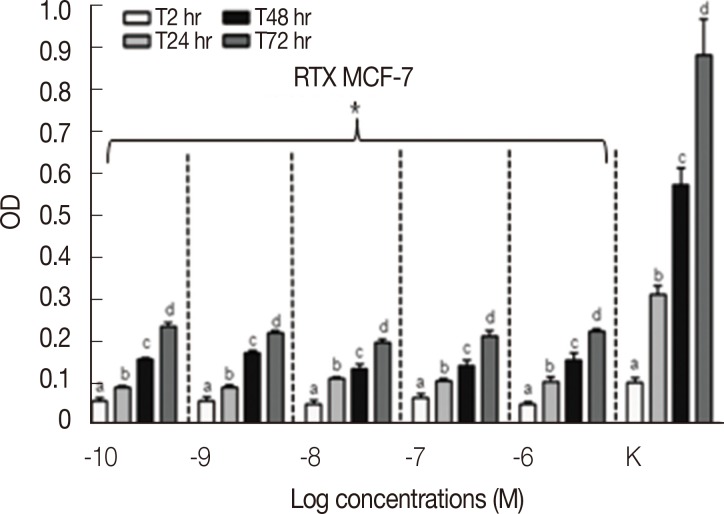
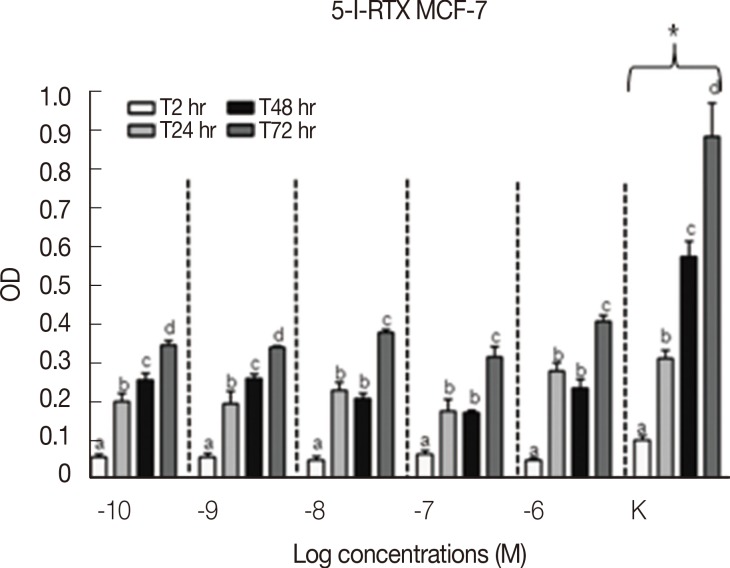
Results concerning the effects induced by different concentrations of capsaicin, capsazepine, RTX, and 5-I-RTX on MCF-7 viability are represented in Figures 5,6,7,8. All agonists and antagonists caused a significant decrease (p<0.05) in treated cell viability compared with controls. Moreover, RTX (Figure 7) was the only compound to show time-dependent behavior.
Figure 5.
MTT assay (n=6) to evaluate the modulation of MCF-7 cells growth after the treatment with increasing concentration (10-10-10-4 M) of capsaicin at different experimental time points (2, 24, 48, and 72 hours). All the samples treated with increasing concentrations of capsaicin demonstrated a significant decrease (p<0.05) of the cell growth rate compared to the controls (K). The MCF-7 cells proliferation was statistically analyzed using Kruskal-Wallis test and Dunn post-test (mean±SEM).
OD=optical density; a, b, c, d=p<0.05. *p<0.05.
Figure 6.
MTT assay (n=6) to evaluate the modulation of MCF-7 cells growth after the treatment with increasing concentration (10-10-10-4 M) of capsazepine at different experimental time points (2, 24, 48, and 72 hours). All the samples treated with increasing concentrations of capsazepine demonstrated a significant decrease (p<0.05) of the cell growth rate compared to the controls (K). The MCF-7 cells proliferation was statistically analyzed using Kruskal-Wallis test and Dunn post-test (mean±SEM).
OD=optical density; a, b, c, d=p<0.05. *p<0.05.
Figure 7.
MTT assay (n=6) to evaluate the modulation of MCF-7 cells growth after the treatment with increasing concentration (10-10-10-6 M) of resiniferatoxin (RTX) at different experimental time points (2, 24, 48, and 72 hours). All the samples treated with increasing concentrations of RTX demonstrated a significant decrease (p<0.05) of the cell growth rate compared to the controls (K). The MCF-7 cells proliferation was statistically analyzed using Kruskal-Wallis test and Dunn post-test (mean±SEM).
OD=optical density; a, b, c, d=p<0.05. *p<0.05.
Figure 8.
MTT assay (n=6) to evaluate the modulation of MCF-7 cells growth after the treatment with increasing concentration (10-10-10-6 M) of 5-iodoresiniferatoxin (5-I-RTX) at different experimental time points (2, 24, 48, and 72 hours). All the samples treated with increasing concentrations of 5-I-RTX demonstrated a significant decrease (p<0.05) of the cell growth rate compared to the controls (K). The MCF-7 cells proliferation was statistically analyzed using Kruskal-Wallis test and Dunn post-test (mean±SEM).
OD=optical density; a, b, c, d=p<0.05. *p<0.05.
DISCUSSION
Although the importance of TRP channels in different solid tumors has been recently outlined, their role in breast cancer has been insufficiently investigated. In this study, we demonstrated the presence of TRPV1 in MCF-7 cells by means of two different techniques (binding assays and Western blotting). Binding assays revealed the presence of measurable and saturable concentrations of TRPV1. The apparent molecular weight of the protein detected by Western blot is consistent with that described in other cell types [19]. The presence and functionality of TRPV1 in breast cancer cell lines had already been studied, and it was suggested that TRPV1 could represent a promising therapeutic target for antineoplastic strategies [21]. Other researchers took into consideration the expression of different TRP channels in human breast ductal adenocarcinoma, human breast cancer epithelial primary culture, and the MCF-7 cell line; their results underscored the importance of the role of TRPV6 in neoplastic cell invasion and migration [22].
In our study, competition assays indicated that, among agonists and antagonists, anandamide and 5-I-RTX bind with the highest affinity to TRPV1. This finding is somewhat consistent with the results found by Rigoni et al. [23], who found that 5-I-RTX, a halogenated version of RTX, has no agonist activity but retains nearly the same potency as RTX, was 20 times more potent than the antagonist capsazepine.
TRPV1 receptors expressed in MCF-7 were functionally active, as all of the vanilloid TRPV1 receptor agonists (anandamide, RTX, and capsaicin) induced a dose-dependent increase in intracellular calcium concentration that was abolished by the vanilloid TRPV1 receptor antagonists capsazepine and 5-I-RTX. These results are consistent with those obtained in prostate cancer cells [19].
When considering calcium uptake, anandamide and capsaicin showed similar potency in activating TRPV1, whereas RTX was the most potent among tested agonists. Although it has been suggested that significant differences emerge when comparing the efficacy of anandamide in native and recombinant receptor systems and between tissues, according to our results, the compound appears to be a full agonist of TRPV1 that is expressed in MCF-7, as demonstrated by its E max value [24]. Similarly, RTX, besides showing a lower degree of affinity than anandamide, has proven to be the most potent TRPV1 agonist.
Our results also indicate that the number of viable cells, as measured by the MTT assay, significantly decreases after exposure to RTX, capsaicin, capsazepine, and 5-I-RTX. It has been suggested that receptor-mediated antitumor activity is associated with induction of apoptosis following activation of TRPV1 and changes in cell calcium influx [21]. Calcium is an important intracellular messenger that regulates many physiological functions, such as cellular differentiation, proliferation, and apoptosis. Abnormal functioning of calcium signaling pathways may occur during carcinogenesis. It has been shown, for example, that anandamide induces apoptosis of cervical carcinoma cells [25].
On the other hand, there is evidence that changes in TRPV1 expression may occur in different neoplastic diseases, such as prostatic, colonic, pancreatic, and bladder cancers, as previously mentioned. Kalogris et al. [26] found that TRPV1 is down-regulated in urothelial cancer specimens, and the decrease in TRPV1 mRNA expression correlates with tumor progression. It has also been observed that prolonged exposure to agonists causes desensitization of TRPV1 by promoting cell surface receptor internalization with a clathrin-independent pathway [27]. Therefore, we cannot exclude the possibility that the inhibitory effects on cell viability caused by TRPV1 ligands in our study may be due to receptor desensitization.
RTX was the only compound able to induce time-dependent inhibition of MCF-7 cell growth rate and the most efficacious among tested agonists. The high efficiency of RTX is the reason why this compound has been extensively studied as a TRPV1-targeting therapeutic drug in different pathological conditions such as bone cancer pain [28]. It has been demonstrated that RTX causes inhibition of mitochondrial function and induces apoptosis in pancreatic cancer cells, presumably via endogenous TRPV1 channels, suggesting the use of vanilloids to treat pancreatic cancer [29].
According to our results, TRPV1 antagonists caused the same inhibitory effect on MCF-7 viability as agonists. The inhibitory effect on cell viability induced by specific TRPV1 antagonists could be ascribed to a functional block of the receptor and, consequently, to a modification of cell activities regulated by TRPV1. Furthermore, it has been recently demonstrated that capsazepine sensitizes colorectal cancer cells to apoptosis by decreasing the expression of antiapoptotic proteins (e.g., survivin) and increasing the expression of proapoptotic proteins (e.g., p53) [30].
Because the MTT assay does not provide insight into the molecular mechanisms involved in TRPV1 responses, additional research is needed to elucidate breast cancer cell viability upon TRPV1 agonist and antagonist stimulation. Consequently, the present data should be considered preliminary, although they do indicate that TRPV1 ligands may have potential for anticancer drugs. Considerable effort has been made to fight cancer cells, and targeted therapy seems to be a promising strategy; in this regard, ion channels belonging to the TRP channel superfamily could play an important role [29]. Considering these findings, studies on TRPV1-mediated effects on human breast cancer cells should be encouraged.
Footnotes
The authors declare that they have no competing interests.
References
- 1.Veronesi B, Oortgiesen M. The TRPV1 receptor: target of toxicants and therapeutics. Toxicol Sci. 2006;89:1–3. doi: 10.1093/toxsci/kfj034. [DOI] [PubMed] [Google Scholar]
- 2.Chou MZ, Mtui T, Gao YD, Kohler M, Middleton RE. Resiniferatoxin binds to the capsaicin receptor (TRPV1) near the extracellular side of the S4 transmembrane domain. Biochemistry. 2004;43:2501–2511. doi: 10.1021/bi035981h. [DOI] [PubMed] [Google Scholar]
- 3.Salazar H, Jara-Oseguera A, Hernández-García E, Llorente I, Arias-Olguín II, Soriano-García M, et al. Structural determinants of gating in the TRPV1 channel. Nat Struct Mol Biol. 2009;16:704–710. doi: 10.1038/nsmb.1633. [DOI] [PubMed] [Google Scholar]
- 4.Everaerts W, Sepúlveda MR, Gevaert T, Roskams T, Nilius B, De Ridder D. Where is TRPV1 expressed in the bladder, do we see the real channel? Naunyn Schmiedebergs Arch Pharmacol. 2009;379:421–425. doi: 10.1007/s00210-008-0391-7. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Wang DH. Increased GFR and renal excretory function by activation of TRPV1 in the isolated perfused kidney. Pharmacol Res. 2008;57:239–246. doi: 10.1016/j.phrs.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kauer JA, Gibson HE. Hot flash: TRPV channels in the brain. Trends Neurosci. 2009;32:215–224. doi: 10.1016/j.tins.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Lin YS, Lin RL, Bien MY, Ho CY, Kou YR. Sensitization of capsaicin-sensitive lung vagal afferents by anandamide in rats: role of transient receptor potential vanilloid 1 receptors. J Appl Physiol (1985) 2009;106:1142–1152. doi: 10.1152/japplphysiol.91229.2008. [DOI] [PubMed] [Google Scholar]
- 8.Caterina MJ. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R64–R76. doi: 10.1152/ajpregu.00446.2006. [DOI] [PubMed] [Google Scholar]
- 9.Nilius B. TRP channels in disease. Biochim Biophys Acta. 2007;1772:805–812. doi: 10.1016/j.bbadis.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Marincsák R, Tóth BI, Czifra G, Márton I, Rédl P, Tar I, et al. Increased expression of TRPV1 in squamous cell carcinoma of the human tongue. Oral Dis. 2009;15:328–335. doi: 10.1111/j.1601-0825.2009.01526.x. [DOI] [PubMed] [Google Scholar]
- 11.Czifra G, Varga A, Nyeste K, Marincsák R, Tóth BI, Kovács I, et al. Increased expressions of cannabinoid receptor-1 and transient receptor potential vanilloid-1 in human prostate carcinoma. J Cancer Res Clin Oncol. 2009;135:507–514. doi: 10.1007/s00432-008-0482-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santoni G, Caprodossi S, Farfariello V, Liberati S, Gismondi A, Amantini C. Antioncogenic effects of transient receptor potential vanilloid 1 in the progression of transitional urothelial cancer of human bladder. ISRN Urol. 2012;2012:458238. doi: 10.5402/2012/458238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, Barritt GJ. TRPM8 in prostate cancer cells: a potential diagnostic and prognostic marker with a secretory function? Endocr Relat Cancer. 2006;13:27–38. doi: 10.1677/erc.1.01093. [DOI] [PubMed] [Google Scholar]
- 14.Youlden DR, Cramb SM, Dunn NA, Muller JM, Pyke CM, Baade PD. The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality. Cancer Epidemiol. 2012;36:237–248. doi: 10.1016/j.canep.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Scatchard G. The attractions of proteins for small molecules and ions. Ann N Y Acad Sci. 1949;51:660–672. [Google Scholar]
- 16.Tóth A, Blumberg PM, Chen Z, Kozikowski AP. Design of a high-affinity competitive antagonist of the vanilloid receptor selective for the calcium entry-linked receptor population. Mol Pharmacol. 2004;65:282–291. doi: 10.1124/mol.65.2.282. [DOI] [PubMed] [Google Scholar]
- 17.Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 18.Ho WY, Yeap SK, Ho CL, Rahim RA, Alitheen NB. Development of multicellular tumor spheroid (MCTS) culture from breast cancer cell and a high throughput screening method using the MTT assay. PLoS One. 2012;7:e44640. doi: 10.1371/journal.pone.0044640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanchez MG, Sanchez AM, Collado B, Malagarie-Cazenave S, Olea N, Carmena MJ, et al. Expression of the transient receptor potential vanilloid 1 (TRPV1) in LNCaP and PC-3 prostate cancer cells and in human prostate tissue. Eur J Pharmacol. 2005;515:20–27. doi: 10.1016/j.ejphar.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 20.Bodó E, Kovács I, Telek A, Varga A, Paus R, Kovács L, et al. Vanilloid receptor-1 (VR1) is widely expressed on various epithelial and mesenchymal cell types of human skin. J Invest Dermatol. 2004;123:410–413. doi: 10.1111/j.0022-202X.2004.23209.x. [DOI] [PubMed] [Google Scholar]
- 21.Ligresti A, Moriello AS, Starowicz K, Matias I, Pisanti S, De Petrocellis L, et al. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J Pharmacol Exp Ther. 2006;318:1375–1387. doi: 10.1124/jpet.106.105247. [DOI] [PubMed] [Google Scholar]
- 22.Dhennin-Duthille I, Gautier M, Faouzi M, Guilbert A, Brevet M, Vaudry D, et al. High expression of transient receptor potential channels in human breast cancer epithelial cells and tissues: correlation with pathological parameters. Cell Physiol Biochem. 2011;28:813–822. doi: 10.1159/000335795. [DOI] [PubMed] [Google Scholar]
- 23.Rigoni M, Trevisani M, Gazzieri D, Nadaletto R, Tognetto M, Creminon C, et al. Neurogenic responses mediated by vanilloid receptor-1 (TRPV1) are blocked by the high affinity antagonist, iodo-resiniferatoxin. Br J Pharmacol. 2003;138:977–985. doi: 10.1038/sj.bjp.0705110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossi T, Castelli M, Zandomeneghi G, Ruberto A, Benassi L, Magnoni C, et al. Selectivity of action of glycyrrhizin derivatives on the growth of MCF-7 and HEP-2 cells. Anticancer Res. 2003;23(5A):3813–3818. [PubMed] [Google Scholar]
- 25.Contassot E, Tenan M, Schnüriger V, Pelte MF, Dietrich PY. Arachidonyl ethanolamide induces apoptosis of uterine cervix cancer cells via aberrantly expressed vanilloid receptor-1. Gynecol Oncol. 2004;93:182–188. doi: 10.1016/j.ygyno.2003.12.040. [DOI] [PubMed] [Google Scholar]
- 26.Kalogris C, Caprodossi S, Amantini C, Lambertucci F, Nabissi M, Morelli MB, et al. Expression of transient receptor potential vanilloid-1 (TRPV1) in urothelial cancers of human bladder: relation to clinicopathological and molecular parameters. Histopathology. 2010;57:744–752. doi: 10.1111/j.1365-2559.2010.03683.x. [DOI] [PubMed] [Google Scholar]
- 27.Sanz-Salvador L, Andrés-Borderia A, Ferrer-Montiel A, Planells-Cases R. Agonist- and Ca2+-dependent desensitization of TRPV1 channel targets the receptor to lysosomes for degradation. J Biol Chem. 2012;287:19462–19471. doi: 10.1074/jbc.M111.289751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menéndez L, Juárez L, García E, García-Suárez O, Hidalgo A, Baamonde A. Analgesic effects of capsazepine and resiniferatoxin on bone cancer pain in mice. Neurosci Lett. 2006;393:70–73. doi: 10.1016/j.neulet.2005.09.046. [DOI] [PubMed] [Google Scholar]
- 29.Santoni G, Farfariello V. TRP channels and cancer: new targets for diagnosis and chemotherapy. Endocr Metab Immune Disord Drug Targets. 2011;11:54–67. doi: 10.2174/187153011794982068. [DOI] [PubMed] [Google Scholar]
- 30.Sung B, Prasad S, Ravindran J, Yadav VR, Aggarwal BB. Capsazepine, a TRPV1 antagonist, sensitizes colorectal cancer cells to apoptosis by TRAIL through ROS-JNK-CHOP-mediated upregulation of death receptors. Free Radic Biol Med. 2012;53:1977–1987. doi: 10.1016/j.freeradbiomed.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]