Abstract
Purpose
We evaluated the tolerability and cardiac safety of docetaxel, cyclophosphamide, and trastuzumab (TCyH) for the treatment of early-stage human epidermal growth factor receptor 2 (HER2)-positive breast cancer and compared to the standard trastuzumab-based chemotherapy regimens doxorubicin with cyclophosphamide followed by paclitaxel and trastuzumab (AC-TH) and docetaxel, carboplatin, and trastuzumab (TCaH).
Methods
We retrospectively reviewed early-stage, resectable, HER2-positive breast cancer patients treated with trastuzumab-based chemotherapy at a single comprehensive cancer center between 2004 and 2011. Patient characteristics, comorbidities, relative dose intensity (RDI) of each regimen, tolerability, and cardiac toxicity were evaluated.
Results
One hundred seventy-seven patients were included in the study (AC-TH, n=114; TCaH, n=39; TCyH, n=24). TCyH was solely administered in the adjuvant setting, whereas two-thirds of the AC-TH and TCaH groups were administered postoperatively. Patients treated with TCyH tended to have a more significant underlying cardiac history, higher Charlson comorbidity index, and were of an earlier stage. All patients treated with TCyH received granulocyte colony stimulating factor primary prophylaxis. No febrile neutropenia or grade ≥3 hematologic toxicity was observed in the TCyH group as compared to the AC-TH and TCaH groups. There were no significant differences in the rates of early termination, hospitalization, dose reduction, or RDI between the regimens. The symptomatic congestive heart failure rate between AC-TH, TCaH, and TCyH groups was not significantly different (4.4% vs. 2.6% vs. 8.3%, respectively, p=0.57). There was also no significant difference in the rate of early trastuzumab termination between patients treated with each regimen.
Conclusion
TCyH is well tolerated and should be investigated as an alternative adjuvant chemotherapy option for patients who are not candidates for standard trastuzumab-containing regimens. Larger clinical trials are necessary to support the wider use of TCyH as an adjuvant regimen.
Keywords: Breast neoplasms, Docetaxel, Human epidermal growth factor receptor 2, Trastuzumab
INTRODUCTION
The human epidermal growth factor receptor 2 (HER2) gene encodes a growth factor receptor that is amplified in approximately 20% to 25% of breast cancers [1]. Amplification of the HER2 gene is a significant predictor of both overall survival and time to relapse [2]. In 1998, the U.S. Food and Drug Administration approved trastuzumab in combination with paclitaxel as a first-line treatment for women with metastatic HER2-positive breast cancer after a significant survival benefit was demonstrated [3]. Since then, large clinical trials have established trastuzumab, in combination with other chemotherapy regimens, as the standard adjuvant therapy for HER2-positive breast cancer [4,5,6,7]. Doxorubicin with cyclophosphamide followed by paclitaxel and trastuzumab (AC-TH) and docetaxel, carboplatin, and trastuzumab (TCaH) are considered the standard of care, and are supported by randomized phase III studies [4,8].
Cardiac toxicity is an overlapping side effect of both trastuzumab and anthracyclines. In prospective studies, 2% to 4% of patients treated with AC-TH develop clinically significant congestive heart failure (CHF) [9,10] compared to 0.4% of TCaH treated patients [8,6]. Therefore, TCaH is an option for HER2-positive patients for whom an anthracycline is contraindicated. However, carboplatin toxicity can be a concern in certain patients. The primary determinant of carboplatin clearance is the glomerular filtration rate (GFR); this parameter is often decreased in elderly patients and may increase the risk of toxicity. Moreover, the widely used GFR calculation formulas, such as the Cockcroft-Gault equation, may not accurately estimate carboplatin clearance [11]. Docetaxel and cyclophosphamide (TCy) has increased in popularity due to its suitability for HER2-negative older women and for patients in whom an anthracycline-based regimen is not preferred. The U.S. Oncology Research Trial 9735 reported that TCy improved disease-free survival (DFS) and overall survival (OS) and was superior to doxorubicin with cyclophosphamide (AC) in a subset analysis of HER2-positive breast cancer treated without trastuzumab [12]. Though not supported by a phase III randomized study or standard guidelines, TCy in combination with trastuzumab (TCyH) has been used in clinical practice [13], including in our institution, for patients who are not good candidates for more aggressive regimens [14].
A phase II study of TCyH in 493 early-stage HER2-positive breast cancer patients has shown that this regimen is well tolerated. The study reported that at 2 years, the DFS and OS were 97.8% and 99.2%, respectively [15]. The tolerability of TCyH compared to other chemotherapy regimens, such as AC-TH and TCaH, commonly used in clinical practice has not been well studied. Therefore, we reviewed our experience of the safety and tolerability of the TCyH regimen in our institution, and compared it with other standard trastuzumab-containing chemotherapy regimens (AC-TH and TCaH) in early-stage, resectable, HER2-positive breast cancer patients.
METHODS
Patients
After obtaining approval from the Roswell Park Cancer Institutional Review Board (IRB), we retrospectively reviewed the medical records of all of the HER2-positive breast cancer patients in the pharmacy database who were treated with adjuvant or neoadjuvant trastuzumab-containing chemotherapy at our institution between January 2004 and December 2011. The following information was abstracted: patient characteristics, comorbidities, Charlson comorbidity index (CCI) [16], Eastern Cooperative Oncology Group performance status (ECOG PS), stage at diagnosis, estrogen receptor status, progesterone receptor status, HER2 status, neo/adjuvant treatment, chemotherapy regimen, type of surgery (mastectomy vs. lumpectomy), and radiation treatment. The relative dose intensity (RDI) of chemotherapies was calculated from the amount of drug administered per unit of time and is expressed as a percentage of the standard regimen [17]. Patients who received trastuzumab-containing chemotherapy regimens for local recurrence or metastatic cancer were excluded. The study was carried out according to the principles set out in the Declaration of Helsinki 1964, and the relevant IRB had approved the study (IRB approval number: EDR 219112).
Treatments and data collection
All patients were treated at Roswell Park Cancer Institute. Patients in the AC-TH group received doxorubicin (60 mg/m2) and cyclophosphamide (600 mg/m2) every 2 or 3 weeks for four cycles, followed by weekly paclitaxel (80 mg/m2) for 12 weeks. Trastuzumab was administered with the first dose of paclitaxel at a dose of 4 mg/kg body weight, followed by 2 mg/kg body weight weekly during chemotherapy. Patients in the TCaH group received docetaxel (75 mg/m2) plus carboplatin (area under the curve, 6 mg/mL/min), given every 3 weeks for six cycles. Patients in TCyH group received docetaxel (75 mg/m2) plus cyclophosphamide (600 mg/m2) for four cycles. In the TCaH and TCyH groups, trastuzumab was administered with the first cycle of chemotherapy at a dose of 8 mg/kg body weight, followed by 6 mg/kg body weight with the subsequent cycles of chemotherapy. After chemotherapy was completed, all patients received trastuzumab 6 mg/kg body weight every 3 weeks to complete 1 year of trastuzumab treatment [4,8,15]. Only patients treated with one of the described regimens were included in this study. All patients treated with AC every 2 weeks (dose dense AC-TH) received 6 mg pegfilgrastim as primary prophylaxis beginning at the first cycle. Patients with high risk of febrile neutropenia treated with standard AC-TH (every 3 weeks) received granulocyte-colony stimulating factor (G-CSF) primary prophylaxis, according to standard recommendations, at the physician's discretion [18]. Patients treated with TCaH and TCyH also received 6 mg pegfilgrastim primary prophylaxis, as is standard in our institution [14].
Tolerability
We recorded the following events: the early termination of chemotherapy, delays (of at least 7 days), dose reductions (of at least 20%), and hospitalizations (of at least 24 hours). Febrile neutropenia was defined as a body temperature ≥38.2℃ and an absolute neutrophil count <0.5×109/L on the same day of or day after the fever [19]. Neutropenia and anemia were defined according to the Common Terminology Criteria for Adverse Events (V.4) [20]. Toxicity was assessed retrospectively from medical records of each patient visit up to 3 to 4 weeks after the last dose of chemotherapy
Cardiac safety was defined as grade III or IV CHF, cardiac death, a left ventricular ejection fraction (LVEF) decrease of ≥10% from baseline, and/or a LVEF decline leading to a delay or discontinuation of trastuzumab [8,21].
Statistical analyses
The primary objective was to determine the safety and tolerability of TCyH compared to AC-TH and TCaH, including the incidence of febrile neutropenia, hospitalization, delay in chemotherapy, dose reduction, RDI (≥85% vs. <85%), grade 3-4 neutropenia, anemia, and thrombocytopenia [22,23]. Secondary analyses investigated the cardiac safety of the AC-TH, TCaH, and TCyH regimens.
The data was analyzed using Stata software, version 11.0 (Stata Inc., College Station, USA). All continuous variable data was tested for normal distribution and is reported as the mean±standard deviation for normally distributed data and the median (25-75 interquartile range) for nonparametric data. Group differences between several samples were calculated using the analysis of variance and Kruskal-Wallis equality of populations rank test for parametric and nonparametric continuous variables, respectively. The differences between groups of categorical variables were compared using the Pearson chi-square or Fisher exact test. Differences were considered to be statistically significance when p<0.05. Multivariate analysis was not performed due to the small sample size of the study, which could result in overparameterization and uninterpretable outcomes.
RESULTS
Patient characteristics
We identified 189 patients treated with adjuvant or neoadjuvant trastuzumab using our institutional database. We excluded 12 patients (three patients due to incomplete data and nine for a nonstandard chemotherapy regimen); in total, 177 patients were included. All patients were confirmed to have HER2-positive breast cancer by either immunohistochemistry or fluorescence in situ hybridization. Chemotherapy regimens were selected according to physician preference. There were 114 patients who received AC-TH (dose-dense AC-TH 64 patients, every 21 days AC-TH 50 patients), 39 patients who received TCaH, and 24 patients who received TCyH. The patient characteristics are summarized in Table 1. The patients treated with TCyH reported significantly more cardiac history and higher CCI scores compared to the AC-TH and TCaH group patients (cardiac history, 17% vs. 1% vs. 5%, respectively, p<0.01; and CCI ≥2, 50% vs. 25% vs. 13%, respectively, p<0.01). All of the patients treated with TCyH received primary G-CSF support [14]. Primary G-CSF prophylaxis was administered to 38 of 39 patients treated with TCaH; one patient refused treatment. No neoadjuvant TCyH was given. There was no significant difference in the race, ECOG PS, hypertension, diabetes, hormone receptor status, or surgery type of the groups.
Table 1.
Patient characteristics
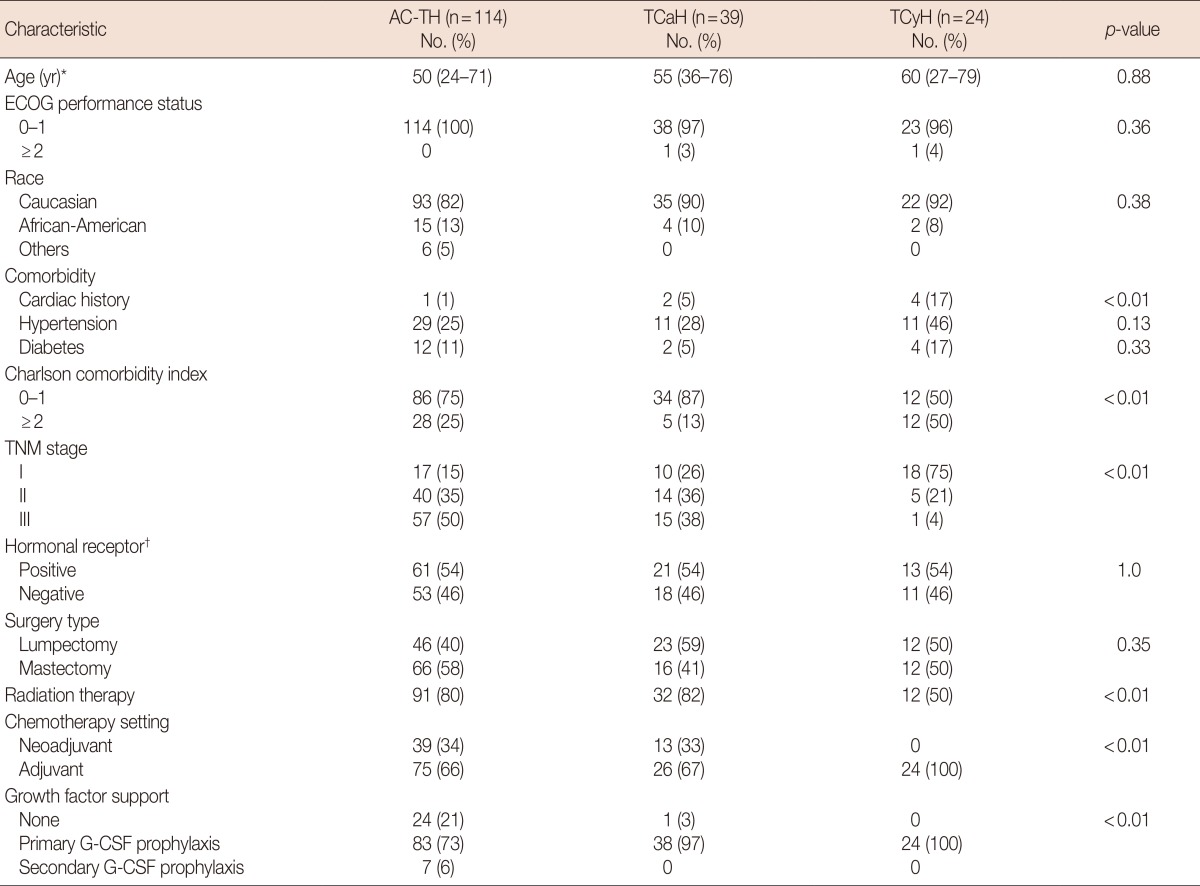
AC-TH=doxorubicin with cyclophosphamide followed by paclitaxel and trastuzumab; TCaH=docetaxel, carboplatin, and trastuzumab; TCyH=docetaxel, cyclophosphamide, and trastuzumab; ECOG=Eastern Cooperative Oncology Group; G-CSF=granulocyte-colony stimulating factor.
*Median (range); †Estrogen and/or progesterone receptors.
Chemotherapy tolerability
The median follow-up period was 43.7 months (range, 7.7-97.2 months). The majority of patients were able to complete the TCyH (87%), AC-TH (87%), and TCaH (95%) regimens (Table 2). Early termination of chemotherapy occurred in 20 patients, 15 in AC-TH group, two in TCaH group, and three in TCyH group. The reasons for early termination of TCyH were intolerance (two patients) and disease progression during adjuvant chemotherapy (one patient). Two patients in the TCaH group discontinued docetaxel due to a rash; in both patients, nab-paclitaxel was substituted and the chemotherapy was completed. Fifteen patients in the AC-TH group required early termination of chemotherapy for the following toxicities: grade 3-4 neuropathy (five patients), grade 3 fatigue (five patients), patient preference (three patients), neutropenia before the last cycle (two patients), and diverticulitis (one patient).
Table 2.
Summary of acute complications of chemotherapy in combination with trastuzumab
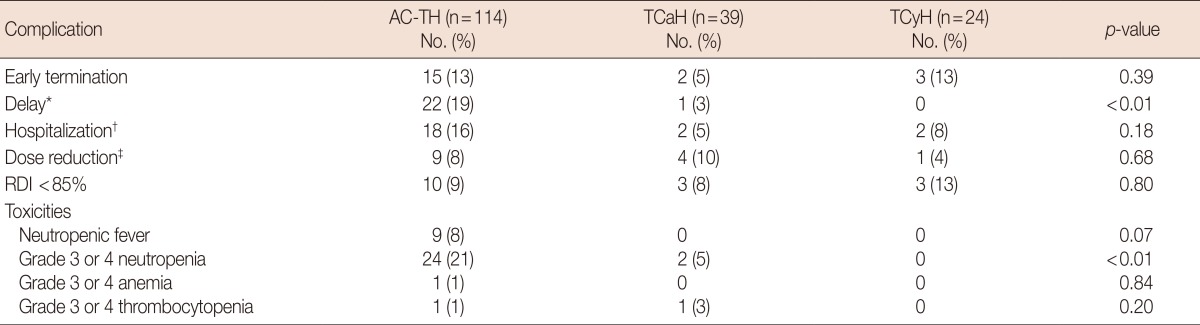
AC-TH=doxorubicin with cyclophosphamide followed by paclitaxel and trastuzumab; TCaH=docetaxel, carboplatin, and trastuzumab; TCyH=docetaxel, cyclophosphamide, and trastuzumab; RDI=relative dose intensity.
*Delay ≥7 days due to toxicity; †Hospitalization >24 hours; ‡Dose reduction ≥20%.
There was no significant difference in the hospitalization rate or rate of dose reduction between the regimens. Nine of 20 patients were hospitalized due to febrile neutropenia. There were no instances of febrile neutropenia in the TCyH or TCaH groups, likely reflecting the use of pegfilgrastim primary prophylaxis in all of the TCyH patients and 38 of the 39 TCaH patients. Two TCyH patients were hospitalized for 3 days due to pneumonia and chronic obstructive pulmonary disease exacerbation. In the TCaH group, two patients were hospitalized for 6 and 2 days, respectively, for a urinary tract infection and diarrhea. The TCyH group had no delays in chemotherapy administration, although the AC-TH and TCaH groups did (0% vs. 19% vs. 3%, respectively, p<0.01). There was no significant difference between the three regimens in terms of RDI <85%. There were no chemotherapy-related deaths observed in the study.
Cardiac safety
The incidence of CHF did not differ between the AC-TH, TCaH, and TCyH groups (4.4% vs. 2.6% vs. 8.3%, respectively, p=0.57) (Table 3). Early termination of trastuzumab was not significantly different between the regimens, nor was the incidence of asymptomatic LVEF decline ≥10%. The median absolute LVEF decline was also similar between the AC-TH, TCaH, and TCyH groups (5% vs. 4% vs. 4%, respectively, p=0.24). There was one cardiac death observed in a patient treated with AC-TH. This 57-year-old patient had a normal baseline LVEF with no prior cardiac history. She received standard AC-TH every 3 weeks. After 2 doses of trastuzumab, she developed CHF and unfortunately expired despite the discontinuation of trastuzumab.
Table 3.
Cardiac safety profile according to treatment
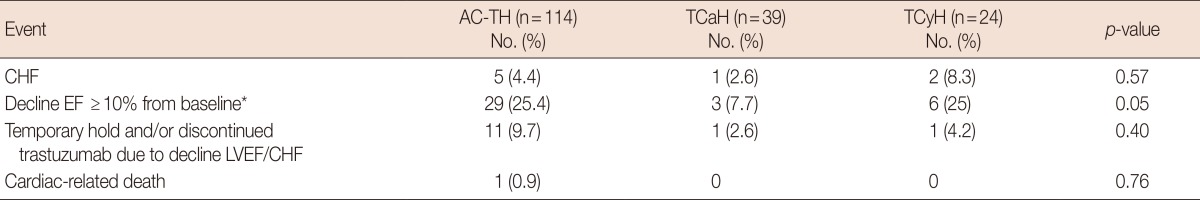
AC-TH=doxorubicin with cyclophosphamide followed by paclitaxel and trastuzumab; TCaH=docetaxel, carboplatin, and trastuzumab; TCyH=docetaxel, cyclophosphamide, and trastuzumab; CHF=congestive heart failure; EF=ejection fraction; LVEF=left ventricular ejection fraction.
*Absolute EF decline from baseline.
DISCUSSION
This is a retrospective, single U.S. institution study conducted to evaluate the tolerability and safety of the TCyH regimen compared to other standard trastuzumab-containing regimens. Though our study did not indicate any significant differences in the tolerability of each regimen, the patient characteristics were different. The patients treated with TCyH were older, had more comorbidities (including cardiac history), and were diagnosed with an earlier stage disease than patients who received AC-TH or TCaH. This is consistent with the results of a retrospective study of 200 patients treated with adjuvant trastuzumab- containing regimens at U.S. cancer centers [13]. This study also surveyed 65 medical oncologists evaluating adjuvant trastuzumab-containing regimen selection for HER2-positive breast cancer in Wisconsin, United States. For T1bN0M0 tumors, more than half of the physicians who participated in the survey preferred an adjuvant TCaH regimen. Interestingly, comparable numbers of physicians chose observation, AC-TH, and TCyH regimens for this stage, despite there being limited data regarding the TCyH regimen. Another population-based, retrospective study by the Integrated Cancer Research Network Health Systems revealed that women who received anthracycline- based chemotherapy were younger, diagnosed at later stages, and had fewer comorbidities [24].
In this study, 8% of patients treated with the AC-TH regimen had febrile neutropenia, while febrile neutropenia was not observed in either the TCyH or TCaH groups, likely due to G-CSF primary prophylaxis. It is unclear whether primary prophylaxis with G-CSF support should be considered for the TCyH regimen. A single arm phase II study of the TCyH regimen reported 6.2% febrile neutropenia without the use of primary prophylaxis [15]. This incidence of febrile neutropenia is similar to a phase III randomized study of adjuvant TCy regimen [12,25]. Other studies have reported a higher rate of febrile neutropenia, between 23% and 46%, in patients treated with the TCy regimen without primary prophylaxis [14,26,27]. Due to the conflicting data from adjuvant TCy regimen studies, we recommend considering primary prophylaxis, particularly in the elderly or in patients with comorbidities, based on our institutional experience and other studies reporting a higher incidence of febrile neutropenia [14].
We did not find any statistically significant differences in the cardiac safety of the AC-TH, TCaH, and TCyH chemotherapy regimens. However, the numerical differences between each regimen likely reflect physician selection. The majority of patients with more comorbidities, especially preexisting cardiac disease, received TCyH chemotherapy in our study. The rate of symptomatic CHF in the TCyH and TCaH groups in this study was 2.6% and 8.3%, respectively. This is higher than the previously reported 0.4% in prospective clinical trials from which patients with an abnormal baseline LVEF or preexisting cardiac disease were excluded [6,15]. Nevertheless, the 4% symptomatic CHF rate in the AC-TH arm was similar to previous prospective studies [4,7], likely reflecting the selection of fit patients with less prior cardiac history for this regimen.
This study is limited due to its retrospective nature and the small sample size drawn from a single institution. Though the TCyH regimen is well tolerated, its efficacy remains unclear compared to other standard trastuzumab-containing regimens. A single arm phase II study of early breast cancer patients treated with adjuvant TCyH reported DFS of 97.8% and OS of 99.2% at 2 years. To the best of our knowledge, no randomized study of the TCyH regimen has been reported. Therefore, adjuvant TCyH should not be considered standard for all HER2-positive breast cancer patients. Standard trastuzumab-containing regimens, such as AC-TH, TCaH, or 5-fluorouracil, epirubicin, and cyclophosphamide followed by docetaxel or paclitaxel plus trastuzumab and pertuzumab are preferred [28,29]. However, the need to develop better tolerated regimens for women with comorbidities or low risk HER2-positive breast cancer is recognized [15]. A recent single arm phase II study of low risk early-stage breast cancer patients treated with adjuvant paclitaxel and trastuzumab indicated that the regimen was very well tolerated and reported a 3 year DFS of 98.7% [30]. Currently, the TCyH regimen should only be considered for patients with comorbidities who are not candidates for standard trastuzumab-containing regimens.
In conclusion, TCyH is well tolerated. The rate of cardiac events seen in nonanthracycline chemotherapy combinations with trastuzumab are higher in clinical practice than in clinical trials, likely reflecting the higher burden of comorbidities and cardiac history in nonclinical trial populations. TCyH is an alternative adjuvant chemotherapy option for patients who are not candidates for standard trastuzumab-containing regimens. Larger clinical trials, with longer follow-up periods, are warranted to further investigate TCyH as an adjuvant regimen.
ACKNOWLEDGEMENTS
The authors thank Ms. Grazyna Riebandt who contributed by way of study coordination, and sample preparation.
Footnotes
The authors declare that they have no competing interests.
References
- 1.Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol. 2010;28:92–98. doi: 10.1200/JCO.2008.19.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 3.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 4.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 5.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 6.Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez EA, Romond EH, Suman VJ, Jeong JH, Davidson NE, Geyer CE, Jr, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29:3366–3373. doi: 10.1200/JCO.2011.35.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valero V, Forbes J, Pegram MD, Pienkowski T, Eiermann W, von Minckwitz G, et al. Multicenter phase III randomized trial comparing docetaxel and trastuzumab with docetaxel, carboplatin, and trastuzumab as first-line chemotherapy for patients with HER2-gene-amplified metastatic breast cancer (BCIRG 007 study): two highly active therapeutic regimens. J Clin Oncol. 2011;29:149–156. doi: 10.1200/JCO.2010.28.6450. [DOI] [PubMed] [Google Scholar]
- 9.Procter M, Suter TM, de Azambuja E, Dafni U, van Dooren V, Muehlbauer S, et al. Longer-term assessment of trastuzumab-related cardiac adverse events in the Herceptin Adjuvant (HERA) trial. J Clin Oncol. 2010;28:3422–3428. doi: 10.1200/JCO.2009.26.0463. [DOI] [PubMed] [Google Scholar]
- 10.Russell SD, Blackwell KL, Lawrence J, Pippen JE, Jr, Roe MT, Wood F, et al. Independent adjudication of symptomatic heart failure with the use of doxorubicin and cyclophosphamide followed by trastuzumab adjuvant therapy: a combined review of cardiac data from the National Surgical Adjuvant breast and Bowel Project B-31 and the North Central Cancer Treatment Group N9831 clinical trials. J Clin Oncol. 2010;28:3416–3421. doi: 10.1200/JCO.2009.23.6950. [DOI] [PubMed] [Google Scholar]
- 11.Donahue A, McCune JS, Faucette S, Gillenwater HH, Kowalski RJ, Socinski MA, et al. Measured versus estimated glomerular filtration rate in the Calvert equation: influence on carboplatin dosing. Cancer Chemother Pharmacol. 2001;47:373–379. doi: 10.1007/s002800000260. [DOI] [PubMed] [Google Scholar]
- 12.Jones S, Holmes FA, O'Shaughnessy J, Blum JL, Vukelja SJ, McIntyre KJ, et al. Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US Oncology Research Trial 9735. J Clin Oncol. 2009;27:1177–1183. doi: 10.1200/JCO.2008.18.4028. [DOI] [PubMed] [Google Scholar]
- 13.Rocque G, Onitilo A, Engel J, Pettke E, Boshoven A, Kim K, et al. Adjuvant therapy for HER2+ breast cancer: practice, perception, and toxicity. Breast Cancer Res Treat. 2012;131:713–721. doi: 10.1007/s10549-011-1862-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ngamphaiboon N, O'Connor TL, Advani PP, Levine EG, Kossoff EB. Febrile neutropenia in adjuvant docetaxel and cyclophosphamide (TC) with prophylactic pegfilgrastim in breast cancer patients: a retrospective analysis. Med Oncol. 2012;29:1495–1501. doi: 10.1007/s12032-011-0035-5. [DOI] [PubMed] [Google Scholar]
- 15.Jones SE, Collea R, Paul D, Sedlacek S, Favret AM, Gore I, Jr, et al. Adjuvant docetaxel and cyclophosphamide plus trastuzumab in patients with HER2-amplified early stage breast cancer: a single-group, open-label, phase 2 study. Lancet Oncol. 2013;14:1121–1128. doi: 10.1016/S1470-2045(13)70384-X. [DOI] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Hryniuk W, Bush H. The importance of dose intensity in chemotherapy of metastatic breast cancer. J Clin Oncol. 1984;2:1281–1288. doi: 10.1200/JCO.1984.2.11.1281. [DOI] [PubMed] [Google Scholar]
- 18.Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, Balducci L, et al. 2006 Update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–3205. doi: 10.1200/JCO.2006.06.4451. [DOI] [PubMed] [Google Scholar]
- 19.Vogel CL, Wojtukiewicz MZ, Carroll RR, Tjulandin SA, Barajas-Figueroa LJ, Wiens BL, et al. First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo-controlled phase III study. J Clin Oncol. 2005;23:1178–1184. doi: 10.1200/JCO.2005.09.102. [DOI] [PubMed] [Google Scholar]
- 20.Common terminology criteria for adverse events (CTCAE) version 4.0. U.S. Department of Health and Human Services; 2009. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdfPublished June 14th, 2010. [Google Scholar]
- 21.Gianni L, Dafni U, Gelber RD, Azambuja E, Muehlbauer S, Goldhirsch A, et al. Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: a 4-year follow-up of a randomised controlled trial. Lancet Oncol. 2011;12:236–244. doi: 10.1016/S1470-2045(11)70033-X. [DOI] [PubMed] [Google Scholar]
- 22.Shayne M, Culakova E, Wolff D, Poniewierski MS, Dale DC, Crawford J, et al. Dose intensity and hematologic toxicity in older breast cancer patients receiving systemic chemotherapy. Cancer. 2009;115:5319–5328. doi: 10.1002/cncr.24560. [DOI] [PubMed] [Google Scholar]
- 23.Wildiers H, Reiser M. Relative dose intensity of chemotherapy and its impact on outcomes in patients with early breast cancer or aggressive lymphoma. Crit Rev Oncol Hematol. 2011;77:221–240. doi: 10.1016/j.critrevonc.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Bowles EJ, Wellman R, Feigelson HS, Onitilo AA, Freedman AN, Delate T, et al. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst. 2012;104:1293–1305. doi: 10.1093/jnci/djs317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones SE, Savin MA, Holmes FA, O'Shaughnessy JA, Blum JL, Vukelja S, et al. Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol. 2006;24:5381–5387. doi: 10.1200/JCO.2006.06.5391. [DOI] [PubMed] [Google Scholar]
- 26.Chan A, Fu WH, Shih V, Coyuco JC, Tan SH, Ng R. Impact of colony-stimulating factors to reduce febrile neutropenic events in breast cancer patients receiving docetaxel plus cyclophosphamide chemotherapy. Support Care Cancer. 2011;19:497–504. doi: 10.1007/s00520-010-0843-8. [DOI] [PubMed] [Google Scholar]
- 27.Vandenberg T, Younus J, Al-Khayyat S. Febrile neutropenia rates with adjuvant docetaxel and cyclophosphamide chemotherapy in early breast cancer: discrepancy between published reports and community practice-a retrospective analysis. Curr Oncol. 2010;17:2–3. doi: 10.3747/co.v17i2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joensuu H, Kellokumpu-Lehtinen PL, Bono P, Alanko T, Kataja V, Asola R, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354:809–820. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 29.National Comprehensive Cancer Network guidelines: breast cancer version 1. National Comprehensive Cancer Network; 2014. [Accessed January 16th, 2014]. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. [Google Scholar]
- 30.Tolaney SM, Barry WT, Dang CT, Yardley DA, Moy B, Marcom PK, et al. A phase II of adjuvant paclitaxel (T) and trastuzumab (H) (APT trial) for node-negative, HER2-positive breast cancer (BC) Cancer Res. 2013;73(24 Suppl):S1-04. [Google Scholar]


