Abstract
Purpose
In the present study, we investigated the incidence of cardiotoxicity within 5 years of trastuzumab treatment and evaluated potential risk factors in clinical practice.
Methods
The study cohort included 415 patients diagnosed with early breast cancer (EBC). Cardiotoxicity incidence was evaluated in patients receiving trastuzumab and those who did not. Multivariate Cox proportional hazards regression models were used to estimate hazard ratios and 95% confidence intervals of potential risk factors for trastuzumab-related cardiotoxicity after appropriate adjustments.
Results
Incidence of cardiotoxicity in patients treated with trastuzumab was significantly higher than that in controls (23.7% vs. 10.8%, p<0.001). This result was adjusted for factors that might increase the risk of cardiotoxicity, such as history of coronary artery diseases or the use of anthracyclines for more than four cycles.
Conclusion
Our findings indicated that treatment with trastuzumab was strongly associated with cardiotoxicity in EBC patients.
Keywords: Breast neoplasms, Cardiotoxicity, Trastuzumab
INTRODUCTION
The use of human epidermal growth factor receptor 2 (HER2) monoclonal antibody, trastuzumab, to treat HER2-overexpressing breast cancer has significantly reduced risk of relapse and improved survival in women with early stage and metastatic diseases [1,2]. However, the treatment is associated with an increased risk of cardiotoxicity, which may be attributed to the blockage of HER2 signaling in cardiac myocytes [3]. The reported incidence of cardiotoxicity in metastatic breast cancer patients is 13% and 27% when trastuzumab is coadministered with paclitaxel and anthracyclines, respectively [4]. Several clinical trials of adjuvant trastuzumab have reported cardiotoxicity rates ranging from 4.1% to 10% [5,6,7]. In particular, cardiac dysfunction is the most serious toxicity associated with the use of a trastuzumab-based regimen.
However, other studies have shown that the incidence of trastuzumab-related cardiotoxicity observed in the clinical setting is higher than what reported in clinical trials [3,8]. Farolfi et al. [9] observed a high overall incidence of cardiac events (44%) in a retrospective analysis of trastuzumab-related cardiotoxicity in early breast cancer (EBC) patients of all ages. However, findings of a retrospective study must be interpreted with caution owing to potential bias, as patients receiving trastuzumab therapy might also be treated with anthracyclines or radiation therapy (RT), which often leads to an increased risk of cardiotoxicity. Nevertheless, treatment-induced cardiotoxicity should not be underestimated. Since only a selected population of breast cancer patients is included in clinical trials of trastuzumab, the actual incidence rate of trastuzumab-related cardiotoxicity remains unknown. In addition, although 70% of EBC patients receiving standard treatment achieve satisfactory outcomes, potential treatment-related risk factors should be evaluated to determine whether prophylaxis is necessary to ensure an improved quality of life for these patients.
The aims of this prospective observational study were to assess the incidence of trastuzumab-related cardiotoxicity in EBC patients in clinical practice and to identify potential risk factors.
METHODS
Between January 1, 2008 and December 31, 2012, consecutive women aged 18 years or older diagnosed with invasive breast cancer (stage I-III) were enrolled in this study.
Trastuzumab (Roche, Shanghai, China) was administered intravenously at a loading dose of 8 mg/kg, followed by a maintenance dose of 6 mg/kg, every 3 weeks for 1 year (18 doses total).
Patients receiving prior hormonal therapy, other systemic therapy for breast cancer, or anthracyclines/taxanes for any other malignancies were excluded. Patients diagnosed with heart failure (HF) prior to breast cancer diagnosis or before the start of any planned chemotherapy were also excluded. Left ventricular ejection fraction (LVEF) assessed by two-dimensional (2D) transthoracic echocardiography scan was required to be within the institutional normal range. Patients were followed until the onset of the first cardiotoxicity episode or end of the follow-up period (December 31, 2013), whichever came first.
All EBC patients in this study were assessed for LVEF by 2D echocardiography scan every 6 months after treatment initiation. All scans were performed and interpreted by a team of experienced cardiologists. LVEF was measured by the modified Simpson's method using a GE vivid E-9 (GE Healthcare, Fairfield, USA) or a Philips IE 33 (Philips, Andover, USA). Intraobserver variability was less than 10%.
Trastuzumab-related cardiotoxicity was defined as follows: an absolute LVEF reduction greater than 15% of baseline, LVEF <50%, or development of symptoms attributable to cardiac dysfunction [9,10]. The decision to terminate or continue trastuzumab treatment was at the clinical oncologists and cardiologists' discretion. Other clinicopathological data, including the date of breast cancer diagnosis, age at diagnosis, body mass index (BMI), estrogen receptor (ER) status, progesterone receptor (PR) status, history of hypertension, coronary artery disease (CAD), and diabetes mellitus, site of breast cancer, type of treatment, and previous therapeutic regimen or RT were retrieved from the electronic medical records.
The present study was approved by the Institutional Review Board (approval number: KY200812).
Statistical analysis
Differences in the distribution of baseline characteristics between the two patient groups were evaluated using the chisquare test. Survival was assessed using the Kaplan-Meier method, and time-to-event rates were calculated from the date of chemotherapy initiation to the onset of the first cardiac event or censoring time, whichever came first. Stratified survival analysis according to the use of trastuzumab and anthracyclines was also performed with differences determined by the log-rank test.
A Cox regression model with trastuzumab as a time-dependent variable was used to calculate the hazards of cardiotoxicity for patients receiving trastuzumab treatment compared to those who did not. Variables included in the model were age, BMI, ER status, PR status, stage of disease, tumor site, RT, anthracyclines use, taxanes use, cyclophosphamide use, and history of hypertension, CAD, or diabetes mellitus. The risk factors independently associated with cardiotoxicity in patients treated with trastuzumab were also determined. Results were expressed as hazard ratios (HR) and 95% confidence intervals (CI).
All computer programming and statistical analyses were performed using the Predictive Analytic Software (PASW) Statistics 20.0 (IBM Inc., Armonk, USA). A p-value of less than 0.05 was considered statistically significant.
RESULTS
Patient characteristics
Of 415 consecutive breast cancer patients (stage I-III), 211 (50.8%) received trastuzumab treatment, whereas 204 (49.2%) who did not were analyzed as controls. The median age at diagnosis was 49.8 years (range, 25-74 years). Patients treated with trastuzumab were slightly younger those who were not (age <50 years, 51.7% vs. 41.7%, p=0.041). Patients receiving trastuzumab were less likely to present with comorbidities than those who did not. Moreover, a negative hormonal receptor status was more commonly observed in patients receiving trastuzumab (57.8% vs. 37.8%, p<0.001). Patients treated with trastuzumab were less likely to receive anthracyclines (20.9% vs. 33.8%, p<0.001), but more likely to be treated with taxanes (13.7% vs. 6.9%, p<0.001). No differences in the use of cyclophosphamide (52.6% vs. 43.6%, p=0.135) and RT (55.9% vs. 49.0%, p=0.159) were observed between the two patient groups. Overall, patients receiving trastuzumab were younger with better pretreatment physical condition and less cardiovascular comorbidities. Patient characteristics are listed in Table 1.
Table 1.
Patient characteristic according to trastuzumab use
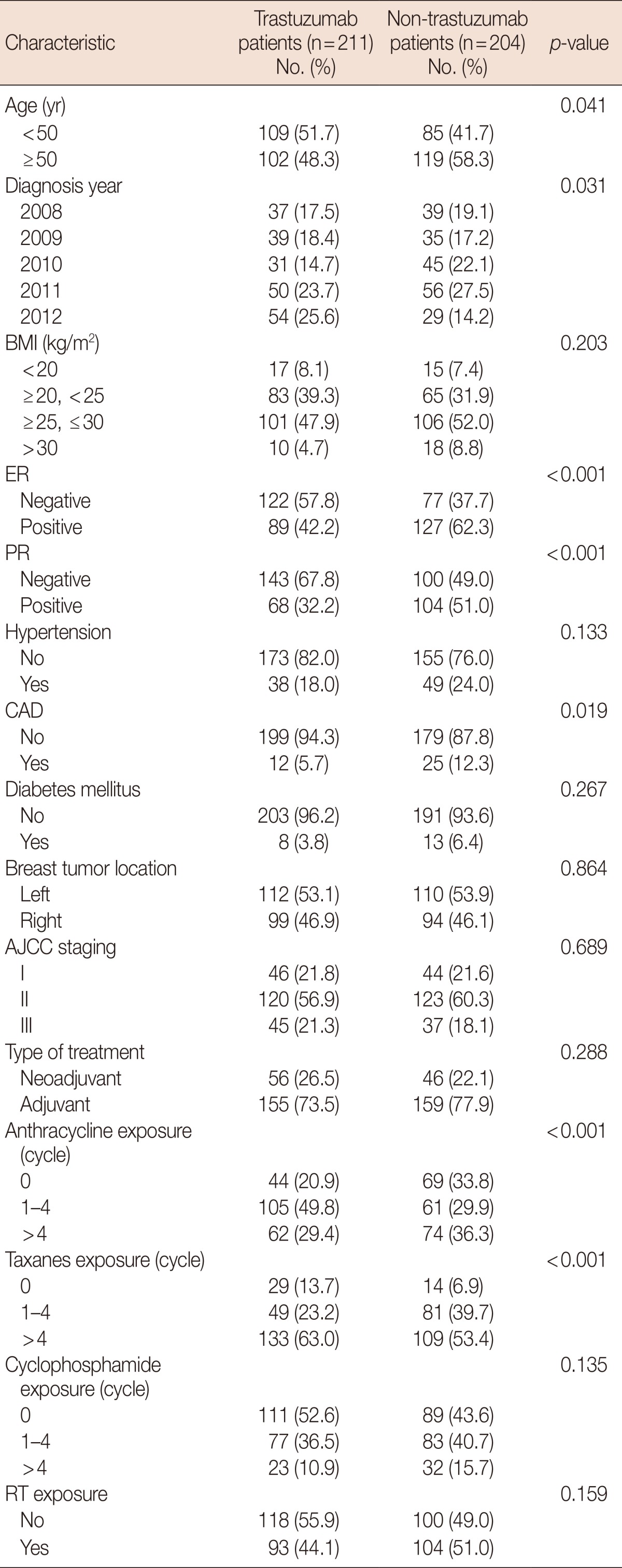
BMI=body mass index; ER=estrogen receptor; PR=progesterone receptor; CAD=coronary artery disease; AJCC=American Joint Committee on Cancer; RT=radiotherapy.
Trastuzumab-related cardiotoxicity
A total of 72 patients (17.4%) experienced cardiotoxicity during the course of treatment. The incidence rate of cardiotoxicity was 23.7% in EBC patients treated with trastuzumab and 10.8% in those who were not (p<0.001).
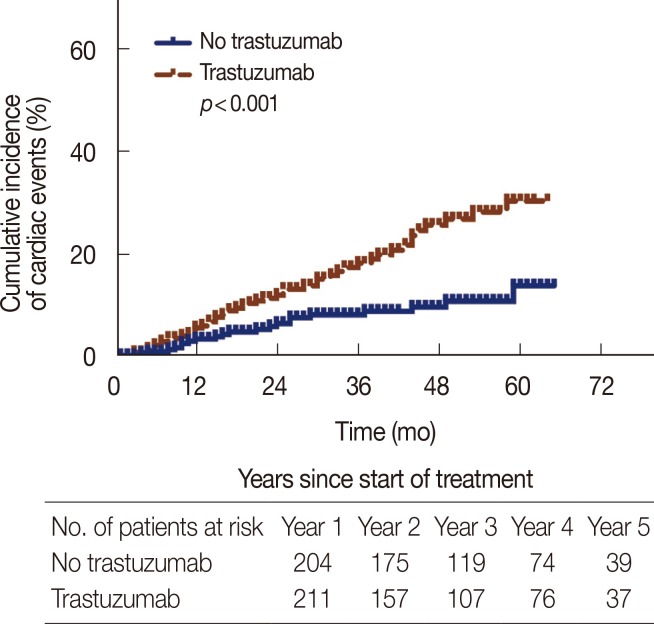
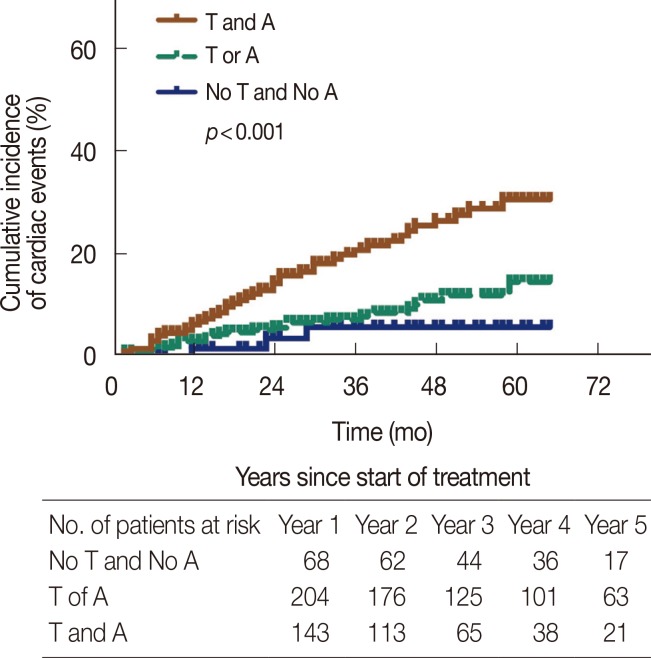
The cumulative incidence of adverse cardiac events for the first 5 years of follow-up is shown in Figure 1. Kaplan-Meier analysis revealed a relatively higher cumulative incidence of cardiac events in patients receiving trastuzumab treatment (p<0.001), but the incidence in the first year of follow-up was similar between the two groups. When patients were stratified according to the use of anthracyclines, those treated with both trastuzumab and anthracyclines had the highest cumulative incidence of cardiac events (29.4%) as shown in Figure 2.
Figure 1.
Comparison of cumulative incidence of cardiotoxicity events in early breast cancer between patients treated with and without trastuzumab.
Figure 2.
Cumulative incidence of cardiotoxicity events according to the use of trastuzumab and/or anthracyclines in early breast cancer patients. T and A=trastuzumab and anthracyclines; No T and No A=no trastuzumab and no anthracyclines; T or A=trastuzumab or anthracyclines.
Potential risk factors
A multivariate time-to-event analysis (Table 2) revealed that among all EBC patients, those treated with trastuzumab were 3.9 times more likely to develop cardiotoxicity, after adjusting for other factors (HR, 3.90; 95% CI, 2.31-6.58). In addition, the development of a cardiac event was significantly associated with anthracycline usage (p=0.008). However, administration of anthracyclines for no more than four cycles (less than 200 mg/m2 of pirarubicin or 300 mg/m2 of epirubicin) did not increase the risk of cardiotoxicity (HR, 1.08; 95% CI, 0.50-2.34). Patients who received a cumulative dose of more than four cycles of anthracyclines had a 2.5-fold increased risk of cardiotoxicity (HR, 2.59; 95% CI, 1.27-5.29) compared to those who did not receive the treatment. Other variables associated with an increased risk of cardiotoxicity included history of CAD (HR, 2.54; 95% CI, 1.16-5.56) and RT (HR, 1.74; 95% CI, 1.07-2.83).
Table 2.
Cox regression model using trastuzumab as a time-dependent variable among early breast cancer patients
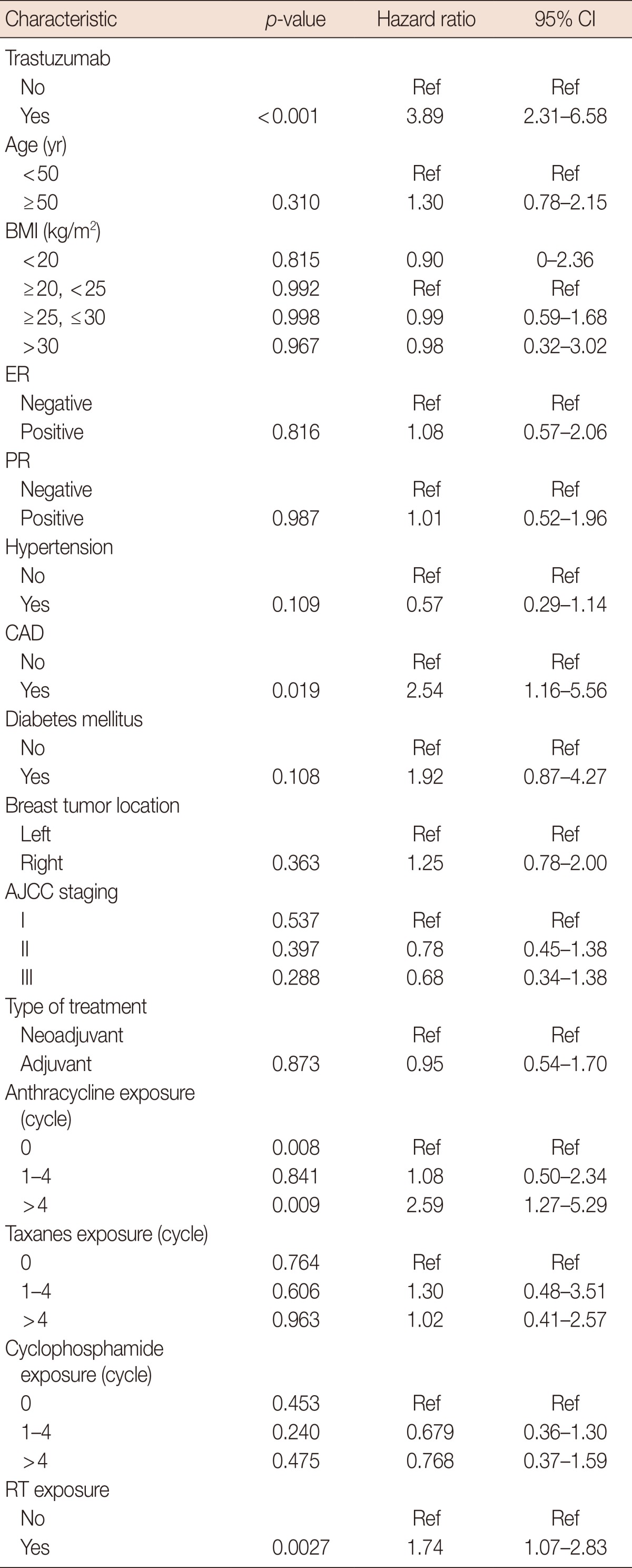
CI=confidence interval; Ref=reference; BMI=body mass index; ER=estrogen receptor; PR=progesterone receptor; CAD=coronary artery disease; AJCC=American Joint Committee on Cancer; RT=radiotherapy.
Cox multivariate analysis showed similar results for all patients and those treated with trastuzumab. History of CAD, anthracycline usage, and RT were risk factors for the development of cardiotoxicity. The risk of cardiotoxicity was more strongly associated with history of CAD among patients treated with trastuzumab than in those who were not (HR, 3.28 vs. 2.54). In our study, patients who received RT to the left breast did not have a higher rate of cardiotoxicity than those who did to right side (17.5% vs. 17.6%, p>0.050). The complete model is presented in Table 3.
Table 3.
Cox proportional hazards model evaluating factors associated with cardiotoxicity among early breast cancer patients
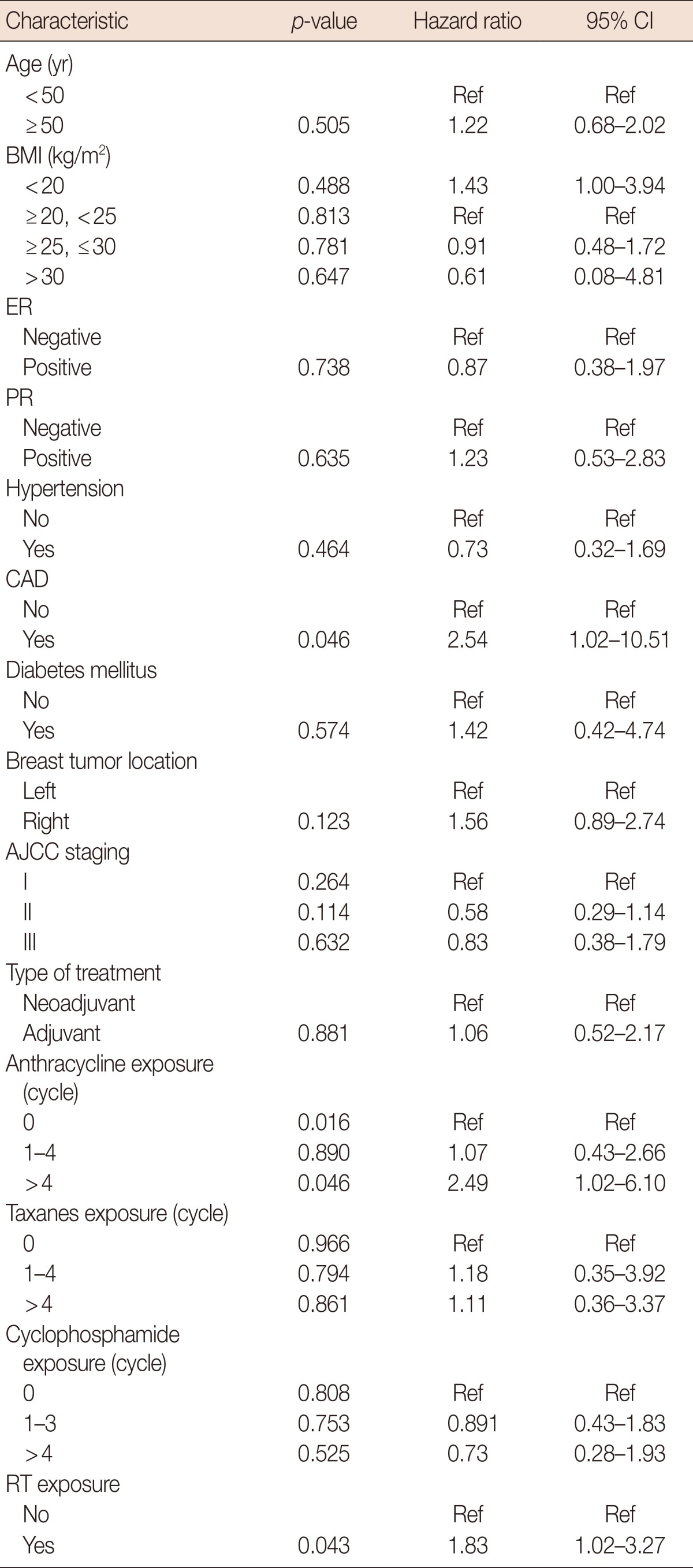
CI=confidence interval; Ref=reference; BMI=body mass index; ER=estrogen receptor; PR=progesterone receptor; CAD=coronary artery disease; AJCC=American Joint Committee on Cancer; RT=radiotherapy.
DISCUSSION
The present study prospectively evaluated the rate of cardiotoxicity and explored the underlying risk factors associated with trastuzumab-related cardiac side effects in clinical practice. In our study, the cardiotoxicity rate among EBC patients was higher than what observed in previous clinical trials [10,11]. Our patients who received trastuzumab experienced a higher cardiotoxicity rate at 23.7%. However, the reported rate of cardiotoxicity was only 7.1% in the Herceptin Adjuvant trial [1], and the incidence of decreased LVEF was 9.4% in the Breast Cancer International Research Group (BCIRG) 006 trial [11]. Thus, there was an absolute increase in cardiotoxicity risk, ranging from 10% to 15%, in our results compared to those of the above-mentioned clinical trials. However, our high-risk estimation was similar to recently published data. In a U.S. population-based retrospective cohort study of 12,500 invasive breast cancer patients, including 554 women treated with a trastuzumab-based regimen, the 5-year cumulative incidence of HF and/or cardiomyopathy for those receiving adjuvant trastuzumab only and those treated with trastuzumab plus anthracycline was 12.1% (95% CI, 5.3-18.3) and 20.1% (95% CI, 14.0-25.6), respectively [12]. In an Italian cohort that included 499 patients receiving adjuvant trastuzumab, treatment-related cardiotoxicity was reported in 133 patients (27.0%), which was also higher than results from other clinical trials [3]. Overall, although the results of these studies were equivocal, various clinical trials might have underestimated trastuzumab-related cardiotoxicity because some patients were excluded owing to suboptimal cardiac function or other comorbidities, leading to a lower observable incidence of cardiotoxicity
Findings from previous clinical trials often provide essential information for the revision of prescription warning labels and the design of safety monitoring in following studies. However, medical management of EBC is usually comprehensive and includes more than one treatment modality, such as the combination of trastuzumab, anthracyclines, and adjuvant RT, all of which imply potential risk for cardiotoxicity. The combination of traditional chemotherapy and new drugs might complicate cardiotoxicity mechanisms and increase its likelihood [13]. Furthermore, various treatment regimens are often selected by the treating oncologist for patients with different physical and psychosocial condition.
Several studies have reported that anthracycline usage, high BMI, hypertension, antihypertensive therapy, and old age are risk factors for the development of cardiotoxicity [6,8,14,15]. Our study involved a large cohort of EBC patients of all ages with a number of identifiable risk factors for cardiotoxicity. The rate of cardiotoxicity increased with the use of anthracyclines, to even a higher rate in those receiving more than four cycles (200 mg/m2 of pirarubicin or 300 mg/m2 of epirubicin). Cardiac comorbidities such as CAD and RT were also associated with trastuzumab-related cardiotoxicity.
Our results indicated that adjuvant anthracyclines at a lower dose did not increase the risk of cardiotoxicity compared to no anthracycline treatment. In addition, taxanes might be more appropriate for patients receiving trastuzumab treatment than anthracyclines to reduce the likelihood of cardiotoxicity. However, almost all current adjuvant therapy regimens include anthracyclines to enhance treatment efficacy and improve survival outcomes [9]. Nonetheless, it is widely recognized that anthracycline-induced cardiotoxicity is dose-dependent; therefore, a lower dose (less than four cycles) could be a safe and effective option for EBC patients receiving trastuzumab [16]. A prospective randomized clinical trial is warranted to define the role of combination therapy in this setting.
We observed a possible association between RT, which is also an essential part of adjuvant treatment for EBC patients, and the risk of trastuzumab-related cardiotoxicity. Patients treated with adjuvant RT were almost twice as likely to develop cardiotoxicity compared to those who were not in our study. Preclinical data have suggested a radio-sensitizing effect by trastuzumab in breast cancer cells, but whether such a finding is valid in normal cells remains unknown [13]. Although the addition of RT to trastuzumab treatment might raise concerns about cardiac safety, there are limited published data on concurrent treatment with adjuvant RT and trastuzumab. According to two phase III trials (NSABP B-31 and NCCTG N9831), trastuzumab and RT did not increase the risk of cardiac toxicity regardless of the breast tumor treatment side [6,17]. However, RT-associated cardiovascular toxicity is progressive. Long-term cardiac follow-up is therefore essential in these patients [18].
In our experience, elderly patients showed a slight, non-significant increase in cardiotoxicity risk. It has been implicated that old age may be a potential risk factor for cardiotoxicity [12]. An association between age and cardiac toxicity was observed in the NCTG N9831 trial, in which patients aged ≥60 years accounted for 15% of the study population [17]. However, such an association was not reported in the BCIRG 006 trial [11]. Moreover, Farolfi et al. [9] retrospectively assessed the incidence of cardiotoxicity in EBC patients of all age and found that the risk of cardiotoxicity among younger patients was unexpectedly higher than that in the elderly. These contradictory results might be attributed to differences in the number of patients, time points of analysis, eligibility criteria, and definition of cardiotoxicity. Our study enrolled a relatively small proportion of elderly patients with 71 patients (17%) older than 60 years of age, which might limit our statistical power for comparison. A large-scale population-based study is needed to investigate the significance of age in the development of cardiotoxicity.
The present investigation was an observational study that included patients who might not be eligible for clinical trials. Although clinical trials could provide more relevant estimates for eligible candidates, many ineligible patients still receive treatment in community practice. Thus, while clinical trials may offer better internal validity than observational studies because they can reduce bias from confounding factors through randomization, their external validity is limited. Our results highlighted the importance of generalizability when applying clinical trial findings to community settings, and thus provided important insights.
Our study had a number of limitations. First, the fairly small sample size reduced the statistical power of detection for potential cardiotoxicity risk factors. Second, the evaluation of cardiac function by 2D echocardiography was deemed inadequate to predict and monitor cardiac damage, leaving LVEF as the only parameter for assessment of cardiac function in this study. Therefore, a more comprehensive imaging technique is necessary in future studies.
In conclusion, our results suggested that clinical trial findings might not truly reflect the actual cardiotoxicity risk among EBC patients treated with trastuzumab in community practice. When treating EBC patients with history of CAD and prior anthracycline or RT, physicians should be aware of potential cardiotoxicity risk and thus timely initiate treatment. The use of prophylactic cardio-protective agents and careful cardiac monitoring might reduce the incidence of adverse cardiac events and improve long-term survival outcomes in patients with high risk of cardiotoxicity.
Footnotes
This study was supported by the Capital Health Research Foundation for Development (grant number: SF2011-5006-02).
The authors declare that they have no competing interests.
References
- 1.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 3.Tarantini L, Gori S, Faggiano P, Pulignano G, Simoncini E, Tuccia F, et al. Adjuvant trastuzumab cardiotoxicity in patients over 60 years of age with early breast cancer: a multicenter cohort analysis. Ann Oncol. 2012;23:3058–3063. doi: 10.1093/annonc/mds127. [DOI] [PubMed] [Google Scholar]
- 4.Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215–1221. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 5.Perez EA, Suman VJ, Davidson NE, Sledge GW, Kaufman PA, Hudis CA, et al. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol. 2008;26:1231–1238. doi: 10.1200/JCO.2007.13.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romond EH, Jeong JH, Rastogi P, Swain SM, Geyer CE, Jr, Ewer MS, et al. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2012;30:3792–3799. doi: 10.1200/JCO.2011.40.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huszno J, Leś D, Sarzyczny-Słota D, Nowara E. Cardiac side effects of trastuzumab in breast cancer patients: single centere experiences. Contemp Oncol Pozn. 2013;17:190–195. doi: 10.5114/wo.2013.34624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chavez-MacGregor M, Zhang N, Buchholz TA, Zhang Y, Niu J, Elting L, et al. Trastuzumab-related cardiotoxicity among older patients with breast cancer. J Clin Oncol. 2013;31:4222–4228. doi: 10.1200/JCO.2013.48.7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farolfi A, Melegari E, Aquilina M, Scarpi E, Ibrahim T, Maltoni R, et al. Trastuzumab-induced cardiotoxicity in early breast cancer patients: a retrospective study of possible risk and protective factors. Heart. 2013;99:634–639. doi: 10.1136/heartjnl-2012-303151. [DOI] [PubMed] [Google Scholar]
- 10.Martín M, Esteva FJ, Alba E, Khandheria B, Pérez-Isla L, García-Sáenz JA, et al. Minimizing cardiotoxicity while optimizing treatment efficacy with trastuzumab: review and expert recommendations. Oncologist. 2009;14:1–11. doi: 10.1634/theoncologist.2008-0137. [DOI] [PubMed] [Google Scholar]
- 11.Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowles EJ, Wellman R, Feigelson HS, Onitilo AA, Freedman AN, Delate T, et al. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst. 2012;104:1293–1305. doi: 10.1093/jnci/djs317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zambelli A, Della Porta MG, Eleuteri E, De Giuli L, Catalano O, Tondini C, et al. Predicting and preventing cardiotoxicity in the era of breast cancer targeted therapies: novel molecular tools for clinical issues. Breast. 2011;20:176–183. doi: 10.1016/j.breast.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Du XL, Xia R, Burau K, Liu CC. Cardiac risk associated with the receipt of anthracycline and trastuzumab in a large nationwide cohort of older women with breast cancer, 1998-2005. Med Oncol. 2011;28(Suppl 1):S80–S90. doi: 10.1007/s12032-010-9717-7. [DOI] [PubMed] [Google Scholar]
- 15.Tan-Chiu E, Yothers G, Romond E, Geyer CE, Jr, Ewer M, Keefe D, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol. 2005;23:7811–7819. doi: 10.1200/JCO.2005.02.4091. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz RG, Jain D, Storozynsky E. Traditional and novel methods to assess and prevent chemotherapy-related cardiac dysfunction noninvasively. J Nucl Cardiol. 2013;20:443–464. doi: 10.1007/s12350-013-9707-1. [DOI] [PubMed] [Google Scholar]
- 17.Halyard MY, Pisansky TM, Dueck AC, Suman V, Pierce L, Solin L, et al. Radiotherapy and adjuvant trastuzumab in operable breast cancer: tolerability and adverse event data from the NCCTG Phase III Trial N9831. J Clin Oncol. 2009;27:2638–2644. doi: 10.1200/JCO.2008.17.9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]