Abstract
Recurrence of viral hepatitis after liver transplantation (LT) can progress to graft failure and lead to a decrease in long-term survival. Recently, there have been remarkable improvement in the treatment of chronic hepatitis B (CHB) using potent antiviral agents. Combination of hepatitis B immunoglobulin and potent antiviral therapy has brought marked advances in the management of CHB for liver transplant recipients. Post-transplant antiviral therapy for hepatitis C virus infection is generally reserved for patients showing progressive disease. Acheiving a sustained virological response in patients with LT greatly ameliorates graft and overall survival, however this only occurs in 30% of transplant recipient using pegylated interferon and ribavirin (RBV). Direct acting antivirals such as protease inhibitors, polymerase or other non-structural proteins inhibitors are anticipated to establish the new standard of care for transplant recipients. In liver transplant recipients, hepatitis E virus infection is an uncommon disease. However, it can lead to chronic hepatitis and cirrhosis and may require retransplantation. Recently, 3-month course of RBV monotherapy has been reported as an effective treatment. This review focuses on the recent management and therapeutic approaches of viral hepatitis in liver transplant recipient.
Keywords: Liver transplantation, Viral hepatitis, Recurrence, Management, Antiviral therapy
INTRODUCTION
During the past decade, great progress has been achieved in the field of liver transplantation (LT), with overall 1-year survival rates exceeding 85%.1 In addition to the advances in the surgical procedure, successful management of postoperative complications has contributes to the improved outcome.2 Especially, management of post-transplant viral hepatitis has several evolving issues such as the use of hepatitis B immunoglobulin (HBIG) and potent antiviral agents for the management of hepatitis B virus (HBV) infection, direct acting antivirals (DAAs) for the management of recurrent hepatitis C3,4 and ribavirin (RBV) monotherapy for chronic hepatitis E infection in liver transplant recipients.5 This mini-review will focus on the antiviral management after successful LT.
HBV
The survival for patients undergoing transplantation for HBV is excellent, and HBV ranks the best of all indications for LT. In the past 10 to 15 years, there have been marked improvements in graft and patient survival, and it reflects the advances in management to prevent and control HBV infections after LT. High-dose Hepatitis B immunoglobulin (HBIG) and nucleos(t)ide analogues (NAs), either as monotherapy or in combination, have been most commonly used in Korea.6 However, currently, the combination of long-term antiviral and low-dose HBIG can effectively prevent HBV recurrence in more than 90% of transplant recipients.7,8,9,10 This effective prevention depends on the complimentary mechanisms of HBIG and NAs. Although the mechanism of HBIG is incompletely understood, it possibly acts by binding to and neutralizing circulating virions and will likely inhibit cell-to-cell infection.11 HBIG had little effect on viral replication, as opposed to antivirals that directly inhibit HBV replication in hepatocytes and extrahepatic reservoirs. Combination of HBIG and antiviral therapy varies with regard to the dosing, duration, and routes of HBIG administration.12 Currently, low-dose intramuscular (IM) HBIG in combination with a potent NA is the most cost-effective prophylaxis.13,14,15 Recently, new and potent NAs such as entecavir (ETV) and tenofovir (TDF) are widely used in the post-transplant period in many transplant centers. These higher genetic barrier antivirals increase the efficacy of post-LT prophylaxis and reduce the need for the expensive HBIG preparations at least after the initial post-operative period.16 ETV and TDF had also similar antiviral efficacy when they combined with HBIG.17 The discontinuation of HBIG is generally reserved for patients at low risk for HBV recurrence.18
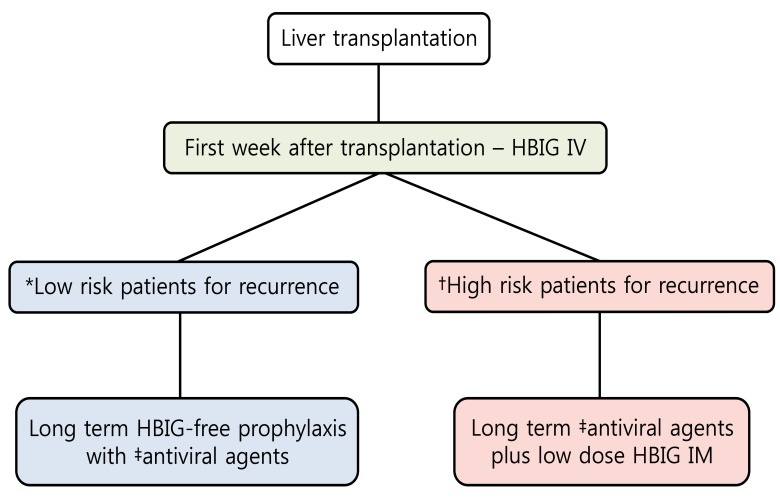
It may be considered over the long term for stable hepatitis B surface antigen (HBsAg) - negative and HBV DNA - negative patients. Long-term treatment with antivirals (single or in combination) can be used as an alternative prophylactic strategy. ETV and TDF should be the first-line options for HBIG-free prophylaxis (Fig.1).
Figure 1.
Prophylaxis for prevention of hepatitis B virus recurrence after liver transplantation. HBIG, hepatitis B immunoglobulin; IV, intravenous; IM, intramuscular. *Hepatitis B surface antigen (HBsAg) negative, hepatitis B virus DNA (HBV DNA) negative. †Detectable HBV DNA levels, hepatitis B envelop antigen (HBeAg) positive, presence of drug-resistant HBV. ‡Higher genetic barrier nucleos(t)ide analogs such as entecavir and tenofovir should be the first line option.
HBV reinfection generally occurs during the first 3 years after LT and hardly thereafter.19 Recurrence of HBV infection is identified by the appearance of HBsAg in serum. The HBV replication level is generally high, and there are large amounts of HBV particles in the graft. The recurrence of post transplant HBV infection usually comes from failed prophylaxis, either because of noncompliance or the development of drug-resistant HBV infection. Selection of treatment modality for HBV infection relies on previous therapy (i.e., no therapy, HBIG alone, antiviral alone, or HBIG and antiviral in combination). The optimal treatment strategy to secure long-term HBV suppression is to use higher genetic barrier antiviral agents such as ETV or TDV.20
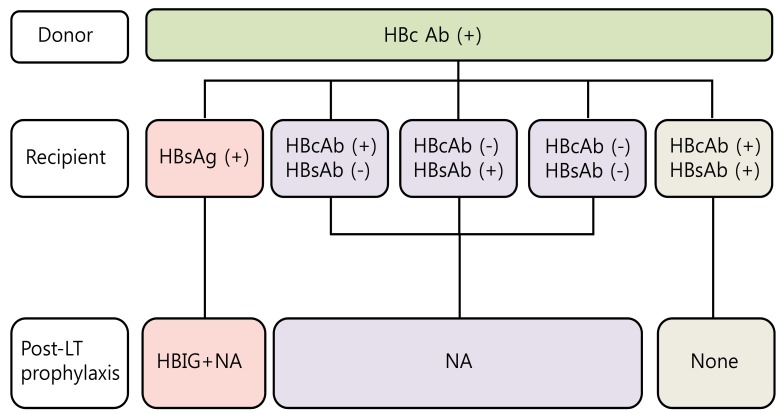
Currently, marginal liver grafts from anti-hepatitis B core (HBc) positive donors are used to overcome the organ shortage. These organs can be an important issue in HBV endemic countries such as Asia and the Mediterranean area. The "occult" HBV infection in the donor liver may be reactivated in the HBsAg negative recipient due to post-LT immunosuppressive therapy and then unfortunately, can progress to de novo HBV infection. HBsAg positive recipients are the optimal candidates from anti-HBc positive donors. HBsAg negative recipients with anti-HBc positive and anti-HBs positive can receive liver grafts from anti-HBc positive donors and may need no prophylaxis at all. However, the anti-HBc and/or anti-HBs negative recipients should receive long term prophylaxis with high genetic barrier NAs (Fig. 2).21
Figure 2.
Algorithm for allocation and management of anti hepatitis B core positive liver grafts. HBc Ab, hepatitis B core antibody; HBsAg, hepatitis B surface antigen; HBsAb, hepatitis B surface antibody; LT, liver transplantation; HBIG, hepatitis B immunoglobulin; NA, nucleos(t)ide analogue.
HCV
The recurrence of hepatitis C virus (HCV) infection is the most common cause of graft loss and death after LT, and covers two-thirds of graft failures.22 All patients who undergo LT with detectable serum HCV RNA experience recurrent HCV infection. Although the course of fibrosis in HCV-infected transplant recipients varies considerably, in the absence of antiviral therapy, the median progression to cirrhosis is 8 to 10 years, whereas an estimated 30% will develop cirrhosis within 5 years of transplantation.22 Decompensation can occur 15% to 30% within the first year of the onset of cirrhosis, and the mortality risk is 40% to 55% within 6 to 12 months of the onset of decompensation. Until now, retransplantation is the only choice for patients with decompensated cirrhosis.
High HCV RNA,23 HCV genotypes 1 and 4,24,25,26 female gender, older donor age, steatosis of the graft, the degree of human leukocyte antigen (HLA) matching or the interleukin28B (IL28B) genotype of the donor and the recipient27,28,29 are associated with increased risk factors of HCV recurrence.
Post-transplant antiviral therapy is generally reserved for patients with evidence of progressive disease showing the presence of moderate to severe necroinflammation or mild to moderate fibrosis. However, this paradigm will change with the appearance of more efficacious and less toxic antiviral therapy.30 Liver biopsy of the graft is essential before antiviral therapy and it is also useful in monitoring disease severity and progression. It can differentiate recurrent HCV infection from other causes of liver enzyme elevations such as rejection, biliary obstruction or the degree of steatosis.
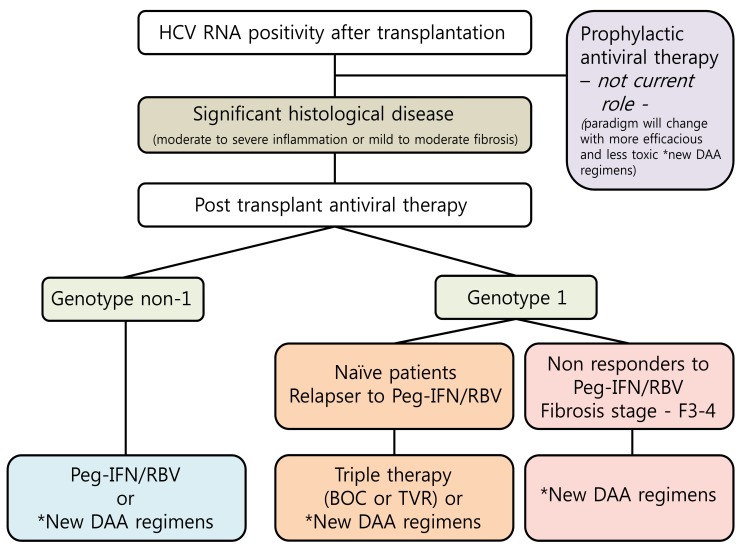
Prophylactic antiviral therapy has no current role in the management of HCV infection after LT.31 The current treatment strategy for recurrent HCV infection after transplantation is to wait for significant fibrosis on the liver graft before initiating antiviral therapy because pegylated interferon (PEG-IFN) based regimens has poor tolerability in early after LT. The optimal management is to achieve a sustained virological response (SVR) with antiviral therapy before LT and eliminate the risk of recurrent HCV infection. A SVR greatly ameliorates graft and overall survival, however this only occurs in 30% of transplant recipient (20-30% in genotype 1 patients and 40-50% in genotype 3 patients) using PEG-IFN and ribavirin (RBV).32 Until 2011, the combination therapy of PEG-IFN and RBV was the only standard therapy. Now the approval of DAAs including protease inhibitors (PI), polymerase or other non-structural proteins inhibitors begins a new era in HCV infection. Although PEG-IFN and RBV therapy is still the standard treatment in non-genotype 1 patients, genotype 1 patients are treated with first generation NS3/4 PI such as boceprevir (BOC) or telaprevir (TVR). SVRs are increased from 45-50% to 60-70% for treatment naive patients in non-transplant patients, and first generation PI are now widely used in most countries that have approved BOC or TVR.33 DAAs are expected to evolve into the new standard treatment for LT recipients infected with genotype 1 virus, although currently, neither DAAs are approved for use in transplant recipients because of safety and tolerance. Data with triple therapy are encouraging in HCV recurrence after LT. Response rates of about 60% at end-of-therapy have been described.34 Although there are great hopes for their use in the field of LT, there are some limitations for safety and tolerance. One limitation is the potential interaction with calcineurin inhibitor (CNI).35 In recent study, the concomitant administration of immunosuppressive therapy with TVR in healthy volunteers resulted in a significant increase in cyclosporin (5-fold) and tacrolimus levels (70-fold) due to the inhibition of the P4503A cytochrome.36 Because of potent drug-drug interaction, the introduction of PI required a close monitoring after LT. Before PI initiation, CNI trough blood concentration has to be stabilized to reach the lowest target rate. Side effects of DAAs are frequent and severe, particularly anemia, infections and acute renal insufficiency. The incidence and severity of anemia are increased by 20% with first-generation DAAs in non-transplant patients.37 Triple therapy leads to the nearly constant use of erythropoietin (EPO), a dose reduction in RBV in almost 75% of patients and the use of blood transfusions.38 These suggest that anemia should be carefully monitored and managed. Proposed algorithm for antiviral strategy for hepatitis C virus infection after liver transplantation is noted in Figure 3.39,40
Figure 3.
Proposed algorithm for antiviral strategy for hepatitis C virus infection after liver transplantation. HCV, hepatitis C virus; RNA, ribonucleic acid; IFN, interferon; RBV, ribavirin; F, firbrosis; BOC, boceprevir; TOC, telaprevir; DAA, direct-acting antiviral agent. *Second generation NS3/4A protease, HCV polymerase or NS5a inhibitors.
In the near future, newer pangenotypic, IFN-free DAAs with improved SVR rates and better tolerability will potentially result in fewer HCV patients coming forward as liver transplant candidates and also result in improved mortality and morbidity post-LT.
HEV
Hepatitis E virus (HEV) infection can evolve to chronic hepatitis in immunocompromised patients. In liver transplant recipients, HEV infection is considered as an uncommon disease. The prevalence may be as high as 1% to 2%.41 However, when the cause of graft hepatitis is unclear, a high suspicion is required. There are 4 genotypes (HEV1-HEV4) that are part of a single serotype.42 HEV1 and HEV2 are found only in humans, with the main transmission being fecal-oral route from contaminated water. HEV3 and HEV4 can infect humans, pigs, and other mammals. HEV3 and HEV4 are primarily swine viruses that are transmitted to humans by direct contact or by the ingestion of infected meat, with humans acting as accidental hosts.43 Until now, all reported cases of HEV infection after LT have been HEV genotype 3.
HEV genotype 3 causes self-limiting infection in non-immunocompromised patients.44,45 However, it can progress to chronic hepatitis and cirrhosis in patients who have received solid-organ transplants,45 and may require retransplantation.46 This retransplantation in patients with HEV viremia in the liver may induce chronic HEV infection again.47 Hence, viral clearance should be a goal in patients with chronic HEV infection, to avoid potential progression of liver fibrosis and extrahepatic manifestations. The current gold standard for the diagnosis of HEV infection is the detection of HEV RNA in serum, stools, or both.
Until recently, there is no specific treatment for patients with a chronic HEV infection. As clearance of the virus depends on the development of both humoral and cellular immunity, lowering the immunosuppressive medication has been recommended.48 A reduction in immunosuppressive therapy, mainly immunosuppressants that target T cells, has resulted in HEV clearance in nearly 30% of solid-organ transplant recipients with chronic hepatitis.46,49,50,51,52
In LT patients, PEG-IFN therapy has been found to efficiently treat chronic HEV infection.53,54 However, the optimal dosing, the duration of treatment and the role of the pretreatment viral load as a predictor of treatment success remains unclear. Recently, Kamar et al reported the effects of RBV monotherapy for solid-organ transplant recipients with prolonged HEV viremia.5 The mechanisms by which RBV achieves HEV clearance are unknown. RBV inhibits the replication of a wide range of RNA and DNA viruses.55 The overall rate of SVR was 78% (46 of 59 patients) in this study, and six of the 10 patients who had a recurrence were re-treated. Finally, 50 of the 59 patients (85%) had clearance of HEV viremia. Anemia was the main identified side effect and required a reduction in RBV dose in 29% of the patients, the use of EPO in 54%, and blood transfusions in 12%. A 3-month course seems to be an appropriate duration for this therapy, though a longer therapy can be given to heavily immunosuppressed patients and those who still have viremia 1 month after the initiation of therapy. Prospective studies are required to determine the most beneficial dose and duration of ribavirin therapy.
CONCLUSION
Viral reactivation is common in liver transplant recipients, and early detection of viral hepatitis may be tricky because many possible causes need to be differentiated such as chronic rejection, biliary complication, recurrence of underlying non-infectious liver diseases and opportunistic systemic infections. Recently, the appearance of potent antiviral agents improved the management of HBV infection after LT and new DAAs are expected to evolve into the new standard of care for recurrent HCV infection, and RBV monotherapy is considered as better approach for HEV infection after LT recipients. The effective management of viral hepatitis in post transplant recipient is essential for successful long term survival. Team approach may be warranted for the optimal care of post-transplant viral hepatitis.
Abbreviations
- CHB
chronic hepatitis B
- CNI
calcineurin inhibitor
- DAAs
direct acting antivirals
- EPO
erythropoietin
- ETV
entecavir
- HBc
hepatitis B core
- HBIG
hepatitis B immunoglobulin
- HBeAg
hepatitis B envelop antigen
- HBsAg
hepatitis B surface antigen
- HCV
hepatitis C virus
- HEV
hepatitis E virus
- HLA
human leukocyte antigen
- IL28B
interleukin28B
- LT
liver transplantation
- NA
nucleos(t)ide analogue
- PEG-IFN
pegylated interferon
- PI
protease inhibitors
- RBV
ribavirin
- SVR
sustained virological response
- TDF
tenofovir
Footnotes
The authors have no conflicts to disclose.
References
- 1.Lo CM. Liver transplantation in 2012: Transplantation for liver cancer--more with better results. Nat Rev Gastroenterol Hepatol. 2013;10:74–76. doi: 10.1038/nrgastro.2012.257. [DOI] [PubMed] [Google Scholar]
- 2.Starzl TE, Fung JJ. Themes of liver transplantation. Hepatology. 2010;51:1869–1884. doi: 10.1002/hep.23595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burger D, Back D, Buggisch P, Buti M, Craxi A, Foster G, et al. Clinical management of drug-drug interactions in HCV therapy: challenges and solutions. J Hepatol. 2013;58:792–800. doi: 10.1016/j.jhep.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 4.Coilly A, Furlan V, Roche B, Barau C, Noel C, Bonhomme-Faivre L, et al. Practical management of boceprevir and immunosuppressive therapy in liver transplant recipients with hepatitis C virus recurrence. Antimicrob Agents Chemother. 2012;56:5728–5734. doi: 10.1128/AAC.01151-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamar N, Izopet J, Tripon S, Bismuth M, Hillaire S, Dumortier J, et al. Ribavirin for chronic hepatitis E virus infection in transplant recipients. N Engl J Med. 2014;370:1111–1120. doi: 10.1056/NEJMoa1215246. [DOI] [PubMed] [Google Scholar]
- 6.Hwang S, Ahn CS, Song GW, Kim KH, Moon DB, Oh HB, et al. Posttransplantation prophylaxis with primary high-dose hepatitis B immunoglobulin monotherapy and complementary preemptive antiviral add-on. Liver Transpl. 2011;17:456–465. doi: 10.1002/lt.22226. [DOI] [PubMed] [Google Scholar]
- 7.Loomba R, Rowley AK, Wesley R, Smith KG, Liang TJ, Pucino F, et al. Hepatitis B immunoglobulin and Lamivudine improve hepatitis B-related outcomes after liver transplantation: meta-analysis. Clin Gastroenterol Hepatol. 2008;6:696–700. doi: 10.1016/j.cgh.2008.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao W, Wu X, Xiu D. Lamivudine or lamivudine combined with hepatitis B immunoglobulin in prophylaxis of hepatitis B recurrence after liver transplantation: a meta-analysis. Transpl Int. 2009;22:387–394. doi: 10.1111/j.1432-2277.2008.00784.x. [DOI] [PubMed] [Google Scholar]
- 9.Katz LH, Paul M, Guy DG, Tur-Kaspa R. Prevention of recurrent hepatitis B virus infection after liver transplantation: hepatitis B immunoglobulin, antiviral drugs, or both? Systematic review and meta-analysis. Transpl Infect Dis. 2010;12:292–308. doi: 10.1111/j.1399-3062.2009.00470.x. [DOI] [PubMed] [Google Scholar]
- 10.Dan YY, Wai CT, Yeoh KG, Lim SG. Prophylactic strategies for hepatitis B patients undergoing liver transplant: a cost-effectiveness analysis. Liver Transpl. 2006;12:736–746. doi: 10.1002/lt.20685. [DOI] [PubMed] [Google Scholar]
- 11.Shouval D, Samuel D. Hepatitis B immune globulin to prevent hepatitis B virus graft reinfection following liver transplantation: a concise review. Hepatology. 2000;32:1189–1195. doi: 10.1053/jhep.2000.19789. [DOI] [PubMed] [Google Scholar]
- 12.Cholongitas E, Goulis J, Akriviadis E, Papatheodoridis GV. Hepatitis B immunoglobulin and/or nucleos(t)ide analogues for prophylaxis against hepatitis b virus recurrence after liver transplantation: a systematic review. Liver Transpl. 2011;17:1176–1190. doi: 10.1002/lt.22354. [DOI] [PubMed] [Google Scholar]
- 13.Gane EJ, Angus PW, Strasser S, Crawford DH, Ring J, Jeffrey GP, et al. Lamivudine plus low-dose hepatitis B immunoglobulin to prevent recurrent hepatitis B following liver transplantation. Gastroenterology. 2007;132:931–937. doi: 10.1053/j.gastro.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Han SH, Ofman J, Holt C, King K, Kunder G, Chen P, et al. An efficacy and cost-effectiveness analysis of combination hepatitis B immune globulin and lamivudine to prevent recurrent hepatitis B after orthotopic liver transplantation compared with hepatitis B immune globulin monotherapy. Liver Transpl. 2000;6:741–748. doi: 10.1053/jlts.2000.18702. [DOI] [PubMed] [Google Scholar]
- 15.Hooman N, Rifai K, Hadem J, Vaske B, Philipp G, Priess A, et al. Antibody to hepatitis B surface antigen trough levels and half-lives do not differ after intravenous and intramuscular hepatitis B immunoglobulin administration after liver transplantation. Liver Transpl. 2008;14:435–442. doi: 10.1002/lt.21343. [DOI] [PubMed] [Google Scholar]
- 16.Papatheodoridis GV, Cholongitas E, Archimandritis AJ, Burroughs AK. Current management of hepatitis B virus infection before and after liver transplantation. Liver Int. 2009;29:1294–1305. doi: 10.1111/j.1478-3231.2009.02085.x. [DOI] [PubMed] [Google Scholar]
- 17.Cholongitas E, Papatheodoridis GV. High genetic barrier nucleos(t)ide analogue(s) for prophylaxis from hepatitis B virus recurrence after liver transplantation: a systematic review. Am J Transplant. 2013;13:353–362. doi: 10.1111/j.1600-6143.2012.04315.x. [DOI] [PubMed] [Google Scholar]
- 18.Fox AN, Terrault NA. The option of HBIG-free prophylaxis against recurrent HBV. J Hepatol. 2012;56:1189–1197. doi: 10.1016/j.jhep.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 19.Roche B, Feray C, Gigou M, Roque-Afonso AM, Arulnaden JL, Delvart V, et al. HBV DNA persistence 10 years after liver transplantation despite successful anti-HBS passive immunoprophylaxis. Hepatology. 2003;38:86–95. doi: 10.1053/jhep.2003.50294. [DOI] [PubMed] [Google Scholar]
- 20.Roche B, Samuel D. Treatment of patients with HBV-related decompensated cirrhosis and liver transplanted patients. Clin Liver Dis. 2013;17:451–473. doi: 10.1016/j.cld.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Cholongitas E, Papatheodoridis GV. Review of the pharmacological management of hepatitis B viral infection before and after liver transplantation. World J Gastroenterol. 2013;19:9189–9197. doi: 10.3748/wjg.v19.i48.9189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berenguer M, Prieto M, Rayon JM, Mora J, Pastor M, Ortiz V, et al. Natural history of clinically compensated hepatitis C virus-related graft cirrhosis after liver transplantation. Hepatology. 2000;32:852–858. doi: 10.1053/jhep.2000.17924. [DOI] [PubMed] [Google Scholar]
- 23.Charlton M, Seaberg E, Wiesner R, Everhart J, Zetterman R, Lake J, et al. Predictors of patient and graft survival following liver transplantation for hepatitis C. Hepatology. 1998;28:823–830. doi: 10.1002/hep.510280333. [DOI] [PubMed] [Google Scholar]
- 24.Gane EJ, Portmann BC, Naoumov NV, Smith HM, Underhill JA, Donaldson PT, et al. Long-term outcome of hepatitis C infection after liver transplantation. N Engl J Med. 1996;334:815–820. doi: 10.1056/NEJM199603283341302. [DOI] [PubMed] [Google Scholar]
- 25.Zekry A, Whiting P, Crawford DH, Angus PW, Jeffrey GP, Padbury RT, et al. Liver transplantation for HCV-associated liver cirrhosis: predictors of outcomes in a population with significant genotype 3 and 4 distribution. Liver Transpl. 2003;9:339–347. doi: 10.1053/jlts.2003.50063. [DOI] [PubMed] [Google Scholar]
- 26.Wali MH, Heydtmann M, Harrison RF, Gunson BK, Mutimer DJ. Outcome of liver transplantation for patients infected by hepatitis C, including those infected by genotype 4. Liver Transpl. 2003;9:796–804. doi: 10.1053/jlts.2003.50164. [DOI] [PubMed] [Google Scholar]
- 27.Gane EJ. The natural history of recurrent hepatitis C and what influences this. Liver Transpl. 2008;14(Suppl 2):S36–S44. doi: 10.1002/lt.21646. [DOI] [PubMed] [Google Scholar]
- 28.Charlton MR, Thompson A, Veldt BJ, Watt K, Tillmann H, Poterucha JJ, et al. Interleukin-28B polymorphisms are associated with histological recurrence and treatment response following liver transplantation in patients with hepatitis C virus infection. Hepatology. 2011;53:317–324. doi: 10.1002/hep.24074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukuhara T, Taketomi A, Motomura T, Okano S, Ninomiya A, Abe T, et al. Variants in IL28B in liver recipients and donors correlate with response to peg-interferon and ribavirin therapy for recurrent hepatitis C. Gastroenterology. 2010;139:1577–1585. 1585 e1571–1585 e1573. doi: 10.1053/j.gastro.2010.07.058. [DOI] [PubMed] [Google Scholar]
- 30.Wiesner RH, Sorrell M, Villamil F. Report of the first International Liver Transplantation Society expert panel consensus conference on liver transplantation and hepatitis C. Liver Transpl. 2003;9:S1–S9. doi: 10.1053/jlts.2003.50268. [DOI] [PubMed] [Google Scholar]
- 31.Lucey MR, Terrault N, Ojo L, Hay JE, Neuberger J, Blumberg E, et al. Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl. 2013;19:3–26. doi: 10.1002/lt.23566. [DOI] [PubMed] [Google Scholar]
- 32.Berenguer M. Systematic review of the treatment of established recurrent hepatitis C with pegylated interferon in combination with ribavirin. J Hepatol. 2008;49:274–287. doi: 10.1016/j.jhep.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Barritt ASt, Fried MW. Maximizing opportunities and avoiding mistakes in triple therapy for hepatitis C virus. Gastroenterology. 2012;142:1314–1323 e1311. doi: 10.1053/j.gastro.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pungpapong S, Aqel BA, Koning L, Murphy JL, Henry TM, Ryland KL, et al. Multicenter experience using telaprevir or boceprevir with peginterferon and ribavirin to treat hepatitis C genotype 1 after liver transplantation. Liver Transpl. 2013;19:690–700. doi: 10.1002/lt.23669. [DOI] [PubMed] [Google Scholar]
- 35.Kiser JJ, Burton JR, Anderson PL, Everson GT. Review and management of drug interactions with boceprevir and telaprevir. Hepatology. 2012;55:1620–1628. doi: 10.1002/hep.25653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garg V, van Heeswijk R, Lee JE, Alves K, Nadkarni P, Luo X. Effect of telaprevir on the pharmacokinetics of cyclosporine and tacrolimus. Hepatology. 2011;54:20–27. doi: 10.1002/hep.24443. [DOI] [PubMed] [Google Scholar]
- 37.Hezode C. Boceprevir and telaprevir for the treatment of chronic hepatitis C: safety management in clinical practice. Liver Int. 2012;32(Suppl 1):32–38. doi: 10.1111/j.1478-3231.2011.02707.x. [DOI] [PubMed] [Google Scholar]
- 38.Romero-Gomez M, Berenguer M, Molina E, Calleja JL. Management of anemia induced by triple therapy in patients with chronic hepatitis C: challenges, opportunities and recommendations. J Hepatol. 2013;59:1323–1330. doi: 10.1016/j.jhep.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 39.Coilly A, Roche B, Samuel D. Current management and perspectives for HCV recurrence after liver transplantation. Liver Int. 2013;33(Suppl 1):56–62. doi: 10.1111/liv.12062. [DOI] [PubMed] [Google Scholar]
- 40.Coilly A, Roche B, Duclos-Vallee JC, Samuel D. Management of HCV transplant patients with triple therapy. Liver Int. 2014;34(Suppl 1):46–52. doi: 10.1111/liv.12406. [DOI] [PubMed] [Google Scholar]
- 41.Haagsma EB, Niesters HG, van den Berg AP, Riezebos-Brilman A, Porte RJ, Vennema H, et al. Prevalence of hepatitis E virus infection in liver transplant recipients. Liver Transpl. 2009;15:1225–1228. doi: 10.1002/lt.21819. [DOI] [PubMed] [Google Scholar]
- 42.Hepgul N, Kodate N, Anderson JE, Henderson M, Ranjith G, Hotopf M, et al. Understanding clinical risk decision making regarding development of depression during interferon-alpha treatment for hepatitis-C: a qualitative interview study. Int J Nurs Stud. 2012;49:1480–1488. doi: 10.1016/j.ijnurstu.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 43.Meng XJ. From barnyard to food table: the omnipresence of hepatitis E virus and risk for zoonotic infection and food safety. Virus Res. 2011;161:23–30. doi: 10.1016/j.virusres.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kamar N, Bendall R, Legrand-Abravanel F, Xia NS, Ijaz S, Izopet J, et al. Hepatitis E. Lancet. 2012;379:2477–2488. doi: 10.1016/S0140-6736(11)61849-7. [DOI] [PubMed] [Google Scholar]
- 45.Hoofnagle JH, Nelson KE, Purcell RH. Hepatitis E. N Engl J Med. 2012;367:1237–1244. doi: 10.1056/NEJMra1204512. [DOI] [PubMed] [Google Scholar]
- 46.Kamar N, Garrouste C, Haagsma EB, Garrigue V, Pischke S, Chauvet C, et al. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology. 2011;140:1481–1489. doi: 10.1053/j.gastro.2011.02.050. [DOI] [PubMed] [Google Scholar]
- 47.Haagsma EB, van den Berg AP, Porte RJ, Benne CA, Vennema H, Reimerink JH, et al. Chronic hepatitis E virus infection in liver transplant recipients. Liver Transpl. 2008;14:547–553. doi: 10.1002/lt.21480. [DOI] [PubMed] [Google Scholar]
- 48.Kamar N, Selves J, Mansuy JM, Ouezzani L, Peron JM, Guitard J, et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med. 2008;358:811–817. doi: 10.1056/NEJMoa0706992. [DOI] [PubMed] [Google Scholar]
- 49.Kamar N, Bendall RP, Peron JM, Cintas P, Prudhomme L, Mansuy JM, et al. Hepatitis E virus and neurologic disorders. Emerg Infect Dis. 2011;17:173–179. doi: 10.3201/eid1702.100856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheung MC, Maguire J, Carey I, Wendon J, Agarwal K. Review of the neurological manifestations of hepatitis E infection. Ann Hepatol. 2012;11:618–622. [PubMed] [Google Scholar]
- 51.Kamar N, Weclawiak H, Guilbeau-Frugier C, Legrand-Abravanel F, Cointault O, Ribes D, et al. Hepatitis E virus and the kidney in solid-organ transplant patients. Transplantation. 2012;93:617–623. doi: 10.1097/TP.0b013e318245f14c. [DOI] [PubMed] [Google Scholar]
- 52.Kamar N, Abravanel F, Selves J, Garrouste C, Esposito L, Lavayssiere L, et al. Influence of immunosuppressive therapy on the natural history of genotype 3 hepatitis-E virus infection after organ transplantation. Transplantation. 2010;89:353–360. doi: 10.1097/TP.0b013e3181c4096c. [DOI] [PubMed] [Google Scholar]
- 53.Kamar N, Rostaing L, Abravanel F, Garrouste C, Esposito L, Cardeau-Desangles I, et al. Pegylated interferon-alpha for treating chronic hepatitis E virus infection after liver transplantation. Clin Infect Dis. 2010;50:e30–e33. doi: 10.1086/650488. [DOI] [PubMed] [Google Scholar]
- 54.Haagsma EB, Riezebos-Brilman A, van den Berg AP, Porte RJ, Niesters HG. Treatment of chronic hepatitis E in liver transplant recipients with pegylated interferon alpha-2b. Liver Transpl. 2010;16:474–477. doi: 10.1002/lt.22014. [DOI] [PubMed] [Google Scholar]
- 55.Patterson JL, Fernandez-Larsson R. Molecular mechanisms of action of ribavirin. Rev Infect Dis. 1990;12:1139–1146. doi: 10.1093/clinids/12.6.1139. [DOI] [PubMed] [Google Scholar]