Abstract
Background/Aims
Spontaneous HBeAg seroconversion occurs frequently in the immune reactive phase in HBeAg-positive chronic hepatitis B (CHB). Therefore, observation for 3-6 months before commencing antiviral therapy is recommended in patients with alanine aminotransferase (ALT) levels that exceed twice the upper limit of normal (ULN). However, HBeAg seroconversion occurs infrequently in patients infected with hepatitis B virus (HBV) genotype C. The aim of the present study was to determine whether the waiting policy is necessary in endemic areas of HBV genotype C infection.
Methods
Ninety patients with HBeAg-positive CHB were followed prospectively without administering antiviral therapy for 6 months. Antiviral therapy was initiated promptly at any time if there was any evidence of biochemical (i.e., acute exacerbation of HBV infection or aggravation of jaundice) or symptomatic deterioration. After 6 months of observation, antiviral therapy was initiated according to the patient's ALT and HBV DNA levels.
Results
Only one patient (1.1%) achieved spontaneous HBeAg seroconversion. Biochemical and symptomatic deterioration occurred before 6 months in 17 patients (18.9%) and 5 patients, respectively. High ALT and HBV DNA levels were both independent risk factors for biochemical deterioration. Of 15 patients with HBV DNA ≥5.1×107 IU/mL and ALT ≥5×ULN, biochemical deterioration occurred in 7 (46.7%), including 1 patient receiving liver transplantation due to liver failure.
Conclusions
Spontaneous HBeAg seroconversion in patients with HBeAg-positive CHB is rare within 6 months. Biochemical deterioration was common and may lead to liver failure. Immediate antiviral therapy should be considered, especially in patients with high ALT and HBV DNA levels in endemic areas of genotype C infection.
Keywords: Hepatitis B virus, chronic hepatitis B; acute exacerbation of hepatitis B, HBV genotype
INTRODUCTION
Hepatitis B virus (HBV) is the most common cause of chronic liver disease including liver cirrhosis and hepatocellular carcinoma.1 Recently, potent oral nucleos(t)ide analogues, including entecavir and tenofovir, have been widely used in the treatment of chronic hepatitis B (CHB).2,3,4,5 The potent antiviral treatment in patients with CHB can suppress the viral replication and prevent progression to cirrhosis, hepatic failure and HCC.6,7,8,9,10,11,12
During the natural course of CHB infection, spontaneous HBeAg seroconversion, defined as loss of HBeAg and detection of anti-HBe in a person who was HBeAg positive and anti-HBe negative,4 occurred frequently in the immune reactive phase in patients with HBeAg-positive CHB ranging from 2-17% per year depending on serum alanime aminotranferase (ALT) levels.13,14,15,16,17,18,19,20,21 Spontaneous HBeAg seroconversion is usually followed by normalization of serum aminotransferase levels and sustained suppression of HBV DNA (<2,000 IU/ml) and is associated with favorable long-term outcomes in patients with CHB.13,22,23 Therefore, in HBeAg-positive CHB patients with elevated serum ALT, most guidelines recommend observation for 3-6 months before antiviral therapy to anticipate spontaneous HBeAg seroconversion.2,3,4,5
However, spontaneous HBeAg seroconversion occurred infrequently in patients with genotype C compared to those with other genotypes.15,16,24 Therefore, the strategy for initiation of antiviral therapy would be different according to the distribution of HBV genotype. The purpose of this study was to investigate the necessity of the waiting strategy before antiviral therapy in HBeAg-positive CHB patients in endemic areas of HBV genotype C infection.
PATIENTS AND METHODS
We prospectively enrolled HBeAg-positive CHB patients with HBV DNA levels of more than 20,000 IU/mL and ALT levels of more than two times the upper limit of normal (ULN) between June 2009 and September 2013 in Jeju National University Hospital. Patients were excluded if they had concurrent hepatitis C or other liver disease (alcoholism, autoimmune liver disease, toxic hepatitis, liver cirrhosis and hepatocelluar carcinoma). Pregnant women and patients who refused this study were also excluded.
The patients were followed without antiviral treatment to anticipate spontaneous HBeAg seroconversion during 6 months. They were followed at 1-3 month intervals or more frequently if clinically needed. At every visit, serial liver function tests, including ALT and bilirubin, and HBV DNA were measured. HBeAg and anti-HBe were analyzed by third-generation microparticle enzyme immunoassays using commercial enzyme immunoassay kits (Abbott, North Chicago, IL, USA). Serum HBV DNA levels were measured by the Cobas Amplicor HBV Monitor (detection limit, 60 IU/mL) (Roche Molecular Diagnostics, Pleasanton, CA, USA) before December 2009 or the Cobas AmpliPrep/Cobas TaqMan® HBV Test, v2.0 (detection limit, 20 IU/mL) (Roche Molecular Diagnostics) after DeDecember 2009.
Antiviral therapy was initiated promptly at any time if there was any evidence of biochemical (acute exacerbation of hepatitis B or aggravation of jaundice, defined by bilirubin ≥2 mg/dL) or symptomatic deterioration which disturbed the patients' daily life, and the patients wanted to receive antiviral therapy. Acute exacerbation of hepatitis B was defined as elevations of serum ALT levels to more than 10 times the ULN and more than twice the baseline value.4 In patients with biochemical deterioration, other causes of acute hepatitis were excluded.
Data analysis was performed using SPSS version 12.0 for windows (SPSS Corp, Chicago, IL, USA). The data were analyzed by the Student's t-test for continuous variables and the chi-square test for categorizing variables. A logistic regression analysis was used to identify the independent predictive factors for biochemical or symptomatic deterioration. P values less than 0.05 were considered significant.
The study protocol was approved by the ethics committees of our institution and written informed consents were obtained from the patients.
RESULTS
Patient characteristics
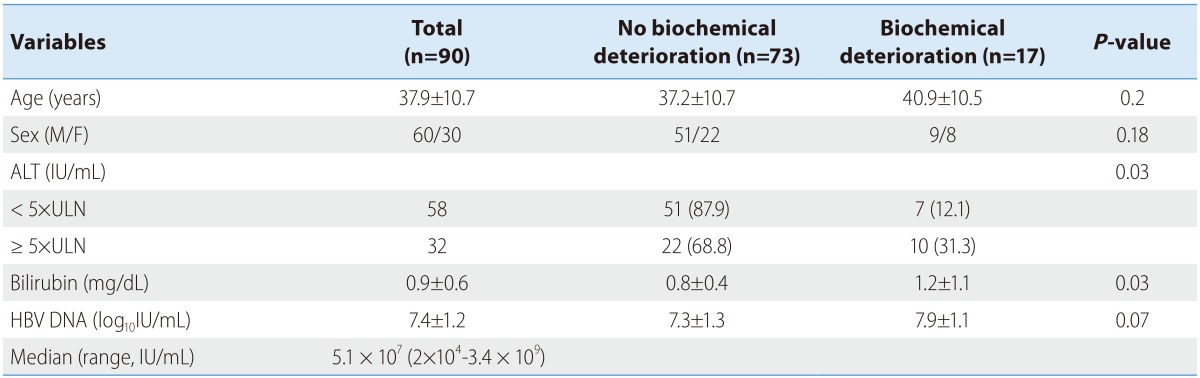
During the study period, 119 consecutive patients fulfilled the inclusion criteria. Among them, 29 patients were excluded because of exclusion criteria (n=19) and were lost to follow-up after the first visit (n=10). Therefore, 90 patients with HBeAg-positive CHB were analyzed. Baseline characteristics of the 90 patients are shown in Table 1. The mean age of the patients was 37.9 years and 60 patients (67.0%) were male. Fifty-four patients (60%) were vertically infected.
Table 1.
Baseline characteristics of the study patients
During follow-up, only one (1.1%) of 90 HBeAg-positive CHB patients achieved spontaneous HBeAg seroconversion at 2 months. In another patient, HBeAg loss, but not anti-HBe positive occurred at 3 months. However, in this patient, persistent elevation of HBV DNA above 2,000 IU/mL and elevation of ALT levels was observed over 6 months.
Biochemical deterioration was observed in 17 patients (18.9%; acute exacerbations in 8, aggravation of jaundice in 2, and both in 7 patients) and symptomatic deteriorations without biochemical deterioration occurred in 5 patients before 6 months.
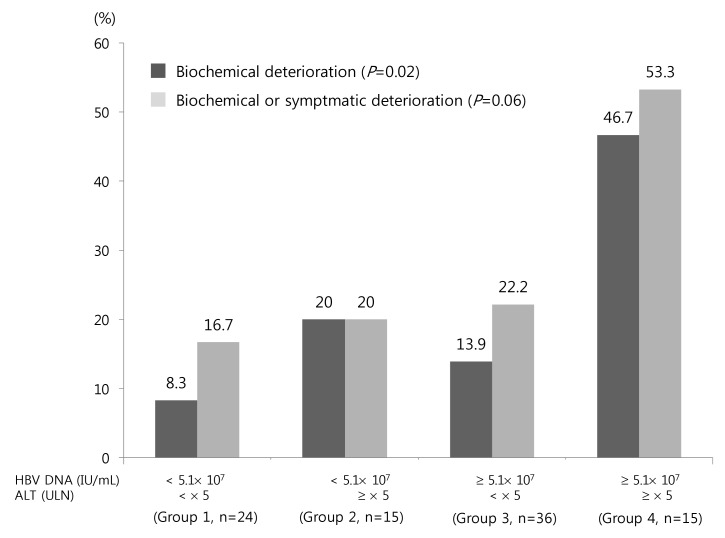
Biochemical deterioration occurred more often in patients with high ALT, high bilirubin and high HBV DNA levels in univariate analysis (Table 1). Among these, high serum ALT and HBV DNA levels were two independent risk factors for biochemical deterioration (Table 2). Serum HBV DNA levels were dichotomized at the levels of 5.1×107 IU/mL (median value). We categorized the patients into the following 4 groups: Group 1 (n=24), low HBV DNA (<5.1×107 IU/mL) and low ALT (< ×5 ULN); group 2 (n=15), low HBV DNA and high ALT (≥×5 ULN); group 3 (n=36); group 4 (n=15), high HBV DNA (≥5.1×107 IU/mL) and high ALT. The percentages of biochemical or symptomatic deteriorations in each group are demonstrated in Fig. 1.
Table 2.
Independent risk factors for biochemical deterioration in patients with HBeAg-positive CHB
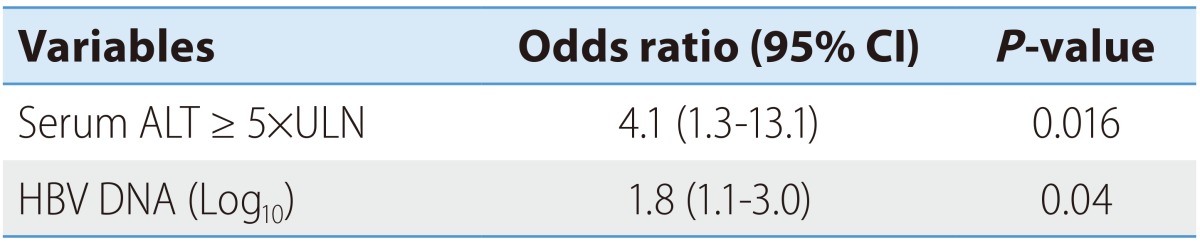
ALT, alanine aminotransferase; CI, confidence interval.
Figure 1.
Biochemical or symptomatic deterioration in patients with HBeAg-positive CHB according to serum HBV DNA and ALT levels. The patients were categorized into the following four groups: group 1 (n=24), low HBV DNA (<5.1×107 IU/mL) and low ALT (<5×ULN); group 2 (n=15), low HBV DNA and high ALT (≥5×ULN); group 3 (n=36), high HBV DNA (≥5.1×107 IU/mL) and low ALT; and group 4 (n=15), high HBV DNA and high ALT.
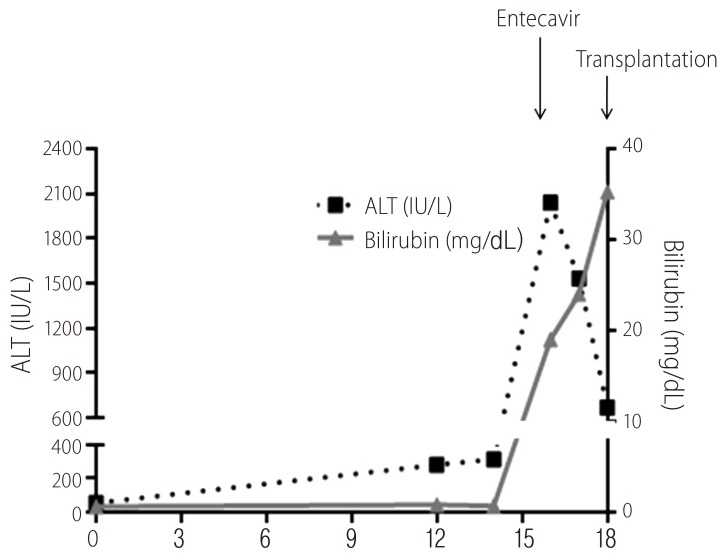
In 17 patients with biochemical deterioration and 5 patients with symptomatic deterioration (n=22), immediate antiviral therapy was recommended before 6 months of follow up. Twenty patients received antiviral therapy (entecavir or tenofovir) promptly (mean 3 months, range 1-5 months). However, in 2 patients with acute exacerbation of hepatitis B during follow-up, antiviral therapy was initiated at 8 and 9 months as the patients wanted to delay the treatment for several months anticipating spontaneous HBeAg seroconversion. Among 17 patients with biochemical deterioration during follow-up, a patient received living-related liver transplantation because of acute-on-chronic liver failure25 even introducing entecavir therapy (Fig. 2).
Figure 2.
Clinical course of a 62-year-old female patient with HBeAg-positive CHB who received liver transplantation because of acute-on-chronic liver failure. This patient was followed at weekly intervals over a 6-month period without any antiviral therapy before week 0, at which point her serum ALT, bilirubin, and HBV DNA levels were 55 IU/mL, 0.6 mg/dL, and 3.1×108 IU/mL, respectively; at 12 weeks these levels had increased to 282 IU/mL, 0.8 mg/dL, and 2.8×109 IU/mL. It was requested that the patient be followed without antiviral therapy even though her ALT levels were elevated to more than twice the ULN. At 14 weeks her serum ALT and bilirubin levels were 314 IU/mL and 0.7 mg/dL, respectively. At 16 weeks, the patient visited the emergency room because of severe anorexia and nausea. Her serum ALT, bilirubin, and HBV DNA levels at that point were 2,039 IU/mL, 19 mg/dL, and 3.85×108 IU/mL, respectively. Entecavir was introduced immediately. However, at 18 weeks her serum bilirubin level had increased to 35.3 mg/dL and she had developed hepatic encephalopathy. The patient made a complete recovery following emergent liver transplantation.
In remaining 68 patients without biochemical or symptomatic deterioration within 6 months, 56 patients received antiviral therapy at or after 6 months of follow-up (mean 12.9 months, range 6-48 months), 6 patients did not satisfy the treatment indication until the last follow-up, and 6 patients were lost to follow-up after 6 months. As a total, 78 of 84 patients (93%) who were followed, received antiviral therapy.
Antiviral therapy was initiated in 17, 14, 31 and 16 patients during follow-up in group 1, 2, 3 and 4, respectively. In each group, antiviral therapy was initiated at a mean of 8.9 months (3-32 months), 18.2 months (1-48 months), 9.8 months (2-36 months) and 6.1 months (1-18 months) in group 1, 2, 3 and 4, respectively.
DISCUSSION
The current study showed two important findings. First, the possibility of HBeAg seroconversion within 6 months is negligible in the endemic areas of HBV genotype C infection even in patients with elevated serum ALT levels. Second, biochemical deterioration occurred frequently during short-term follow-up, especially in patients with high ALT and high HBV DNA levels.
Previous studies showed that spontaneous HBeAg seroconversion occurred more often in patients with old age, high ALT, non-genotype C and low HBV DNA.15,16,17,18,19,26 In a Hong Kong study,17 HBeAg seroconversion rate at 5 years was 72.4% of the patients with ALT levels more than 2 times the ULN on clinical presentation. In addition, the possibility of HBeAg seroconversion within 3 months in patients with peak ALT levels 1.5-2 times the ULN, >2-5 times the ULN, and > 5 times the ULN were 27.2%, 35.6%, and 46.4%, respectively.17 In a Korean study, HBeAg seroconversion occurred in 35.5 % within 6 months in patients with ALT levels more than 5 times the ULN.18 Therefore, current guidelines for the treatment of CHB recommend observation for 3-6 months to anticipate spontaneous HBeAg seroconversion in HBeAg-positive CHB patients with HBV DNA> 20,000 IU/mL, and serum ALT more than 2 times the ULN.2,3,4,5
The geographic distribution of HBV genotypes are different.27 Almost all Korean patients with CHB are infected with HBV genotype C.18,28,29 Spontaneous HBeAg seroconversion occurred infrequently in patients with genotype C compared to those with other genotypes.15,16,24 A previous study showed that the cumulative rates of spontaneous HBeAg seroconversion at 1, 3, and 5 years were 6%, 24%, and 46% in genotype B patients and 0%, 18%, and 34% in genotype C patients, respectivly.15 It has been also reported that spontaneous HBeAg seroconversion occurred one decade later in genotype C patients compared to genotype B patients.15,16,24 It did not occur within 6 months, even in patients with ALT levels more than 4-5 times the ULN15,19 in patients with genotype C. In addition, the difference in the rate of HBeAg seroconversion was predominantly noted in patients who had elevated ALT levels on presentation. In patients with genotype B, HBeAg seroconversion was dependent on serum ALT levels on presentation. However, in patients with HBV genotype C, HBeAg seroconversion was not dependent on serum ALT levels.
In general, high serum ALT levels are considered a surrogate marker for vigorous host immune response caused by cytotoxic T-cell-mediated immune hepatolysis and eventual immune clearance of HBV.30,31 However, HBeAg seroconversion occurred only in 1.1% within 6 months of follow-up in this study. Previous studies15,19 and this data suggests that some abortive immune clearance mechanism might exist in patients infected with HBV genotype C.
A study in Korea reported that HBeAg seroconversion occurred in 35.5 % within 6 months in patients with ALT levels more than 5 times the ULN.18 We could not explain the exact mechanism of the discrepancy between the previous studies15,18,19 and our data. Previous study18 showed that non-vertical infection of HBV was one of the independent predictive factors for HBeAg seroconversion. In their study, 31% of the subjects were vertically infected.18 In our study, 60% of the subjects were vertically infected. The difference of the proportion of vertical infection in subject might partially explain the difference in HBeAg seroconversion rate within 6 months. Yoon et. al also reported that HBeAg seroconversion within 6 months in patients with elevated ALT (mean 179 IU/mL) levels was less than 3%.32
Acute exacerbation of hepatitis B may lead to hepatic decompensation and death from liver failure.20,33,34,35,36 In this study, acute exacerbation of hepatitis B or aggravation of jaundice developed in 17 patients (18.9%) and one patient received liver transplantation even after introducing antiviral because of acute-on-chronic liver failure following acute excerbation of hepatitis B. Jeng et al. reported that hepatic decompensation occurred in 5.1%, especially in patients with serum HBV DNA>1.55×109 copies/mL (around 3×108 IU/mL) in CHB patients with abrupt increase in ALT more than 5 times the ULN.36 In this study, in patints with high ALT ( ≥×5 ULN) and high viral load (HBV DNA ≥ 5.1×107 IU/mL), acute exacerbation of hepatitis B or aggravation of jaundice developed in 46.7% of the patients. Lok et al. also reported that acute exacerbationof hepatitis B occurred frequently in patient with ALT levels more than 5 times the ULN.35 In addtion, Kim et al. resported that patients with serum HBV DNA levels more than 2×106 IU/mL had liitle possibility of spontaneous HBeAg seroconversion.18 After spontaneous HBeAg seroconversion, those with genotype C were more likely to revert to the HBeAg-positive state24 and reactivation of hepatitis B18,37 occurred in 50-80% of patients during long-term follow-up.18,37 In addition, reactivation of hepatitis B occurred frequently in patients with genotype C compared with those with genotype C and serum ALT levels more than 5 times the ULN.37
Considering previous results18,35,36,37 and the results of our study, serum ALT and HBV DNA cut-off levels for immediate antiviral therapy might be 5 times the ULN and 5.1×107 IU/mL, respectively.
In conclusion, spontaneous HBeAg seroconversion in patients with HBeAg-positive CHB in the endemic area of genotype C infection was rare within 6 months. Biochemical deterioration was common. Therefore, immediate antiviral therapy should be considered, especially in patients with high ALT and HBV DNA levels.
Acknowledgements
This work was supported by a research grant from Jeju National University Hospital.
Abbreviations
- ALT
alanine aminotransferase
- CHB
chronic hepatitis B
- HBV
hepatitis B virus
- ULN
upper limit of normal
Footnotes
The authors have no conflicts to disclose.
References
- 1.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 2.Korean Association for the Study of the Liver. KASL Clinical Practice Guidelines: Management of chronic hepatitis B. Clin Mol Hepatol. 2012;18:109–162. doi: 10.3350/cmh.2012.18.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 5.Liaw YF, Kao JH, Piratvisuth T, Chan HLY, Chin R, Liu C, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6:531–561. doi: 10.1007/s12072-012-9365-4. [DOI] [PubMed] [Google Scholar]
- 6.Schiff ER, Lee SS, Chao YC, Kew Yoon S, Bessone F, Wu SS, et al. Long-term treatment with entecavir induces reversal of advanced fibrosis or cirrhosis in patients with chronic hepatitis B. Clin Gastroenterol Hepatol. 2011;9:274–276. doi: 10.1016/j.cgh.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 7.Chang TT, Liaw YF, Wu SS, Schiff E, Han KH, Lai CL, et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52:886–893. doi: 10.1002/hep.23785. [DOI] [PubMed] [Google Scholar]
- 8.Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468–475. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
- 9.Hosaka T, Suzuki F, Kobayashi M, Seko Y, Kawamura Y, Sezaki H, et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98–107. doi: 10.1002/hep.26180. [DOI] [PubMed] [Google Scholar]
- 10.Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 11.Hosaka T, Suzuki F, Kobayashi M, Seko Y, Kawamura Y, Sezaki H, et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98–107. doi: 10.1002/hep.26180. [DOI] [PubMed] [Google Scholar]
- 12.Wong GL, Chan HL, Mak CW, Lee SK, Ip ZM, Lam AT, et al. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology. 2013;58:1537–1547. doi: 10.1002/hep.26301. [DOI] [PubMed] [Google Scholar]
- 13.Fattovich G, Rugge M, Brollo L, Pontisso P, Noventa F, Guido M, et al. Clinical, virologic and histologic outcome following seroconversion from HBeAg to anti-HBe in chronic hepatitis type B. Hepatology. 1986;6:167–172. doi: 10.1002/hep.1840060203. [DOI] [PubMed] [Google Scholar]
- 14.Lok AS, Lai CL, Wu PC, Leung EK, Lam TS. Spontaneous hepatitis B e antigen to antibody seroconversion and reversion in Chinese patients with chronic hepatitis B virus infection. Gastroenterology. 1987;92:1839–1843. doi: 10.1016/0016-5085(87)90613-5. [DOI] [PubMed] [Google Scholar]
- 15.Chu CJ, Hussain M, Lok AS. Hepatitis B virus genotype B is associated with earlier HBeAg seroconversion compared with hepatitis B virus genotype C. Gastroenterology. 2002;122:1756–1762. doi: 10.1053/gast.2002.33588. [DOI] [PubMed] [Google Scholar]
- 16.Chu CM, Liaw YF. Genotype C hepatitis B virus infection is associated with a higher risk of reactivation of hepatitis B and progression to cirrhosis than genotype B: a longitudinal study of hepatitis B e antigen-positive patients with normal aminotransferase levels at baseline. J Hepatol. 2005;43:411–417. doi: 10.1016/j.jhep.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Yuen MF, Yuan HJ, Hui CK, Wong DK, Wong WM, Chan AO, et al. A large population study of spontaneous HBeAg seroconversion and acute exacerbation of chronic hepatitis B infection: implications for antiviral therapy. Gut. 2003;52:416–419. doi: 10.1136/gut.52.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HS, Kim HJ, Shin WG, Kim KH, Lee JH, Kim HY, et al. Predictive factors for early HBeAg seroconversion in acute exacerbation of patients with HBeAg-positive chronic hepatitis B. Gastroenterology. 2009;136:505–512. doi: 10.1053/j.gastro.2008.10.089. [DOI] [PubMed] [Google Scholar]
- 19.Kao JH, Chen PJ, Lai MY, Chen DS. Hepatitis B virus genotypes and spontaneous hepatitis B e antigen seroconversion in Taiwanese hepatitis B carriers. J Med Virol. 2004;72:363–369. doi: 10.1002/jmv.10534. [DOI] [PubMed] [Google Scholar]
- 20.Liaw YF, Chu CM, Su IJ, Huang MJ, Lin DY, Chang-Chien CS. Clinical and histological events preceding hepatitis B e antigen seroconversion in chronic type B hepatitis. Gastroenterology. 1983;84:216–219. [PubMed] [Google Scholar]
- 21.Lim YS, Han S, Heo NY, Shim JH, Lee HC, Suh DJ. Mortality, liver transplantation, and hepatocellular carcinoma among patients with chronic hepatitis B treated with entecavir vs lamivudine. Gastroenterology. 2014;147:152–161. doi: 10.1053/j.gastro.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 22.Hsu YS, Chien RN, Yeh CT, Sheen IS, Chiou HY, Chu CM, et al. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology. 2002;35:1522–1527. doi: 10.1053/jhep.2002.33638. [DOI] [PubMed] [Google Scholar]
- 23.Hui CK, Leung N, Shek TW, Yao H, Lee WK, Lai JY, et al. Sustained disease remission after spontaneous HBeAg seroconversion is associated with reduction in fibrosis progression in chronic hepatitis B Chinese patients. Hepatology. 2007;46:690–698. doi: 10.1002/hep.21758. [DOI] [PubMed] [Google Scholar]
- 24.Livingston SE, Simonetti JP, Bulkow LR, Homan CE, Snowball MM, Cagle HH, et al. Clearance of hepatitis B e antigen in patients with chronic hepatitis B and genotypes A, B, C, D, and F. Gastroenterology. 2007;133:1452–1457. doi: 10.1053/j.gastro.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Sarin SK, Kumar A, Almeida JA, Chawla YK, Fan ST, Garg H, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL) Hepatol Int. 2009;3:269–282. doi: 10.1007/s12072-008-9106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMahon BJ, Holck P, Bulkow L, Snowball M. Serologic and clinical outcomes of 1536 Alaska Natives chronically infected with hepatitis B virus. Ann Intern Med. 2001;135:759–768. doi: 10.7326/0003-4819-135-9-200111060-00006. [DOI] [PubMed] [Google Scholar]
- 27.Miyakawa Y, Mizokami M. Classifying hepatitis B virus genotypes. Intervirology. 2003;46:329–338. doi: 10.1159/000074988. [DOI] [PubMed] [Google Scholar]
- 28.Song BC, Cui XJ, Kim H. Hepatitis B virus genotypes in Korea: an endemic area of hepatitis B virus infection. Intervirology. 2005;48:133–137. doi: 10.1159/000081740. [DOI] [PubMed] [Google Scholar]
- 29.Bae SH, Yoon SK, Jang JW, Kim CW, Nam SW, Choi JY, et al. Hepatitis B virus genotype C prevails among chronic carriers of the virus in Korea. J Korean Med Sci. 2005;20:816–820. doi: 10.3346/jkms.2005.20.5.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chu CM, Liaw YF. Intrahepatic distribution of hepatitis B surface and core antigens in chronic hepatitis B virus infection. Hepatocyte with cytoplasmic/membranous hepatitis B core antigen as a possible target for immune hepatocytolysis. Gastroenterology. 1987;92:220–225. doi: 10.1016/0016-5085(87)90863-8. [DOI] [PubMed] [Google Scholar]
- 31.Tsai SL, Chen PJ, Lai MY, Yang PM, Sung JL, Huang JH, et al. Acute exacerbations of chronic type B hepatitis are accompanied by increased T cell responses to hepatitis B core and e antigens. Implications for hepatitis B e antigen seroconversion. J Clin Invest. 1992;89:87–96. doi: 10.1172/JCI115590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoon JH, Rhee PL, Lee HS, Kim CY. Spontaneous HBeAg clearance rate and its affecting factors inpatients with chronic hepatitis B in Korea. Korean J Gastroenterol. 1992;24:1313–1319. [Google Scholar]
- 33.Sheen IS, Liaw YF, Tai DI, Chu CM. Hepatic decompensation associated with hepatitis B e antigen clearance in chronic type B hepatitis. Gastroenterology. 1985;89:732–735. doi: 10.1016/0016-5085(85)90566-9. [DOI] [PubMed] [Google Scholar]
- 34.Davis GL, Hoofnagle JH, Waggoner JG. Spontaneous reactivation of chronic hepatitis B virus infection. Gastroenterology. 1984;86:230–235. [PubMed] [Google Scholar]
- 35.Lok AS, Lai CL. Acute exacerbations in Chinese patients with chronic hepatitis B virus (HBV) infection. Incidence, predisposing factors and etiology. J Hepatol. 1990;10:29–34. doi: 10.1016/0168-8278(90)90069-4. [DOI] [PubMed] [Google Scholar]
- 36.Jeng WJ, Sheen IS, Liaw YF. Hepatitis B virus DNA level predicts hepatic decompensation in patients with acute exacerbation of chronic hepatitis B. Clin Gastroenterol Hepatol. 2010;8:541–545. doi: 10.1016/j.cgh.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 37.Chu CM, Liaw YF. Predictive factors for reactivation of hepatitis B following hepatitis B e antigen seroconversion in chronic hepatitis B. Gastroenterology. 2007;133:1458–1465. doi: 10.1053/j.gastro.2007.08.039. [DOI] [PubMed] [Google Scholar]