INTRODUCTION
Caroli's disease (CD) is a rare congenital disorder characterized by non-obstructive segmental saccular dilatation of the large intrahepatic bile ducts.1 Two forms of CD have been described. The pure type is characterized by cystic dilatation of the intrahepatic bile ducts, while the complex type - also designated Caroli's syndrome - is associated with congenital hepatic fibrosis (CHF) and autosomal recessive polycystic kidney disease.2,3,4 CD usually affects the liver diffusely but some localized forms (monolobar CD) affect only a single lobe, predominantly the left lobe.5,6 Complications of Caroli's disease are frequently related to the stagnation of bile resulting from the saccular dilatation of bile ducts, and include intrahepatic lithiasis, recurrent cholangitis, abscess formation, septicemia and amyloidosis. Patients with CD have also been shown to be at risk for intrahepatic cholangiocarcinoma.7,8 A case of intrahepatic cholangiocarcinoma arising in a localized CD is described and the recent literature discussed.
CASE
A 44-year-old female patient was referred to our hospital for further evaluation of an intrahepatic cystic lesion. The hepatic cyst was incidentally identified on computed tomography (CT) five years ago at another hospital. At that time, a septated cystic lesion measuring 4 cm in diameter was noted in segment 2 without dilatation of adjacent intrahepatic bile ducts, and an impression of benign cystadenoma was given. After five years, on triphasic contrast-enhanced CT images, the size of the cystic lesion remained the same but a hypodense round lesion was newly detected near the cystic lesion with dilatation of the left intrahepatic ducts (Fig. 1A). There was no evidence of associated biliary tract anomalies, including choledochal cyst, or polycystic kidney disease by imaging studies. Additional gadoxetic acid-enhanced magnetic resonance images (MRI) showed a T1 hypointense and T2 hyperintense focal lesion adjacent to the cystic lesion and dilated intrahepatic ducts in the left lateral section of the liver, corresponding to the hypodense nodular lesion noted on CT (Fig. 1B). A fluorodeoxyglucose positron emission tomography (FDG-PET) scan showed hypermetabolism in the hepatic cyst (maximum SUV: 3.8) (Fig. 1C).
Figure 1.
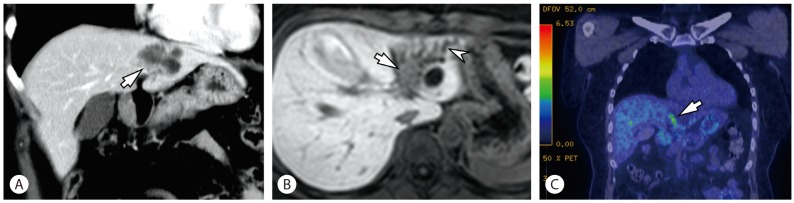
Imaging findings (A-C). (A) A coronal CT image in portal venous phase shows aggregated cystic lesions in the left lateral section of the liver. There is a hypodense round focal lesion (arrow) near the cystic lesion. (B) This focal lesion (arrow) shows hypointensity with dilated adjacent intrahepatic ducts (arrowhead) in hepatobiliary phase of gadoxetic acid-enhanced MRI. (C) A coronal FDG PET-CT image demonstrates focal hypermetabolism (arrow) adjacent to the aggregated cystic lesions.
The initial laboratory findings demonstrated that all liver function tests were within normal limits except for a slightly increased total bilirubin level (1.6 mg/dL). Serum CA19-9 and CEA levels were 12.0 U/ml and 2.0 ng/mL, respectively. Serological tests for hepatitis B and hepatitis C virus were negative.
Considering the possibility of a hepatic malignancy, a left lateral sectionectomy of the liver was performed.
PATHOLOGIC FINDINGS
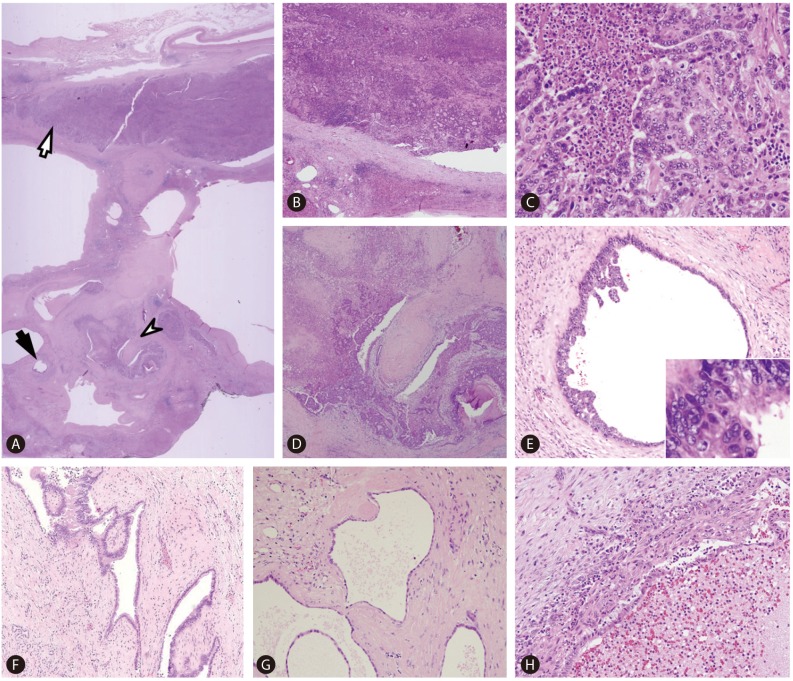
Macroscopically, marked cystic dilatation of the larger intrahepatic bile ducts were seen, surrounded by thick fibrous walls (Fig. 2). Some of the larger cystic spaces were filled with yellowish friable solid masses. The surrounding hepatic parenchyma showed atrophic change, and some smaller peripheral bile ducts appeared accentuated on gross examination due to involvement by ascending cholangitis and periductal fibrosis.
Figure 2.
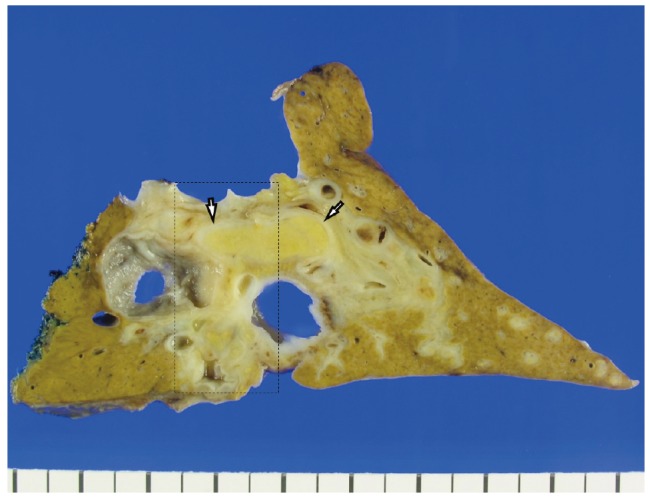
Gross findings. An overview of a representative section demonstrates multiple cystically dilated large intrahepatic bile ducts, some of which are filled with bright yellow solid lesions (white arrows). The smaller peripheral ducts on the right appear accentuated, due to ascending cholangitis and periductal fibrosis. The boxed area is seen in more detail in Figure 3.
On histological examination, the affected large intrahepatic bile ducts were markedly dilated and surrounded by various degrees of fibrosis and chronic active inflammation (Fig. 3A). Most of the ducts were lined by flattened cuboidal or columnar epithelium, and some dilated ducts demonstrated polypoid fibrovascular projections characteristic of ductal plate malformation (DPM) (Fig. 3D, 3F, 3G). Ascending cholangitis with periductal fibrosis was seen in some smaller peripheral bile duct branches, and the bile duct mucosa were ulcerated and the lumens filled with inflammatory exudate (Fig. 3H). There was no histological evidence of associated congenital hepatic fibrosis.
Figure 3.
Microscopic findings (A-H). (A) Scanning power photomicrograph demonstrating an overview of the boxed area of Figure 2. Cystically dilated bile ducts are seen, and a large bile duct (white arrow) is plugged with a cholangiocarcinoma, corresponding to the bright yellow solid lesion on gross examination. The neoplastic lesion is a gland-forming moderately differentiated adenocarcinoma with intraductal growth (B, C). Another bile duct (white arrowhead) in (A) demonstrates features of ductal plate malformation and involvement by cholangiocarcinoma. This area is seen in detail in (D); the fibrovascular intraluminal projection is evident. A smaller peripheral duct (black arrow) in (A) is lined by biliary intraepithelial neoplasia (BilIN) 3, and is seen in detail in (E). A micropapillary architecture is seen in addition to the high-grade nuclear atypia (inset). Features of ductal plate malformation were seen in other large and small bile ducts (F, G). (H) demonstrates a smaller bile duct that was affected by ascending cholangitis: the mucosa is ulcerated and the lumen is filled with inflammatory exudate. [Hematoxylin-eosin, original magnification ×1.25 (A), ×40 (B, D), ×100 (E, F, H), ×200 (G), ×400 (C, inset of E)].
Some of the biliary epithelium lining the dilated ducts showed dysplastic changes compatible with biliary intraepithelial neoplasia (BilIN) grade 3, characterized by increased nuclear-to-cytoplasmic ratio, hyperchromatic or vesicular nuclei and prominent nucleoli, and focal micropapillary growths were seen (Fig. 3E). Some dilated intrahepatic ducts were entirely filled with solid growths of neoplastic biliary epithelium with variable-sized tubular spaces, compatible with moderately differentiated intraductal growth type cholangiocarcinoma, and invasion of the bile duct wall by the tumor was also noted (Fig. 3B, 3C).
DISCUSSION
CD is a rare condition that belongs to the group of fibrocystic liver diseases, including CHF, von Meyenburg complexes and choledochal cysts. Fibrocystic liver diseases are believed to arise from the abnormal or arrested development of the embryonic ductal plates, as evidenced by the presence of persistent ductal plate-like structures in these conditions.1 The ductal plate of the human fetal liver is a double-layered, wreath-like, keratin 19 (K19)-expressing bile duct structure surrounding venous branches and directly juxtaposed to the limiting plate of the hepatocytic parenchyme. After further remodeling, the ductal structures are normally separated from the limiting plate and incorporated into the portal tracts, subsequently forming mature bile ducts and ductules. The remodeling process of the ductal plates begins at the hilum and progresses to the periphery of the liver, and arrested or deranged remodeling of the ductal plate results in DPM.
CD usually involves the entire liver, but several cases of localized, monolobar CD have been reported in the literature. Histologically, the affected intrahepatic ducts are cystically dilated, and are lined by biliary epithelium which may be hyperplastic and ulcerated. Various degrees of fibrosis and chronic inflammation may be observed around the affected ducts. Acute cholangitis is frequently observed, with neutrophilic infiltrates or microabscesses. When associated with CHF, fibrotic portal tracts containing small ductal structures with features of DPM are observed.2,9,10
Although common complications of CD include bile stasis, intrahepatic stone formation and ascending cholangitis,4 CD is notable for its association with cholangiocarcinoma: the risk of cholangiocarcinoma development has been reported to be as high as 100-fold greater in patients with CD than in the general population.11 Generally, chronic inflammation has been suggested to be a common pathogenic pathway for biliary carcinogenesis, and cholangiocarcinomas are considered to develop through a multistep carcinogenic process, from precursor lesions including BilIN and intraductal papillary neoplasms of the bile duct (IPN-B).12 Therefore, it is not surprising that the biliary epithelium lining the cystically dilated bile ducts in CD - which are chronically inflamed and exposed to bile sludge - would be prone to neoplastic transformation.13
An interesting recent finding is the degradation of basement membrane matrix proteins of the intrahepatic bile ducts in CHF and CD.14 The expression of laminin and type IV collagen was found to be reduced in these conditions, and overexpression of plasminogen and tissue-type plasminogen activator (tPA) was demonstrated in the biliary epithelium of CHF and CD, suggesting that increased plasmin levels may contribute to the degradation of basement membrane of intrahepatic bile ducts. Notably, the reduction of basement membrane matrix proteins was also demonstrated in foci of carcinoma in situ of CD and CHF. Therefore, it could be speculated that once carcinoma in situ (or BilIN3) develops in the setting of CD or CHF, the neoplastic cells are able to invade the basement membrane more easily, resulting in an earlier development of invasive intrahepatic cholangiocarcinoma in these patients.
Abbreviations
- BilIN
biliary intraepithelial neoplasia
- CD
Caroli's disease
- CHF
congenital hepatic fibrosis
- DPM
ductal plate malformation
- IPN-B
intraductal papillary neoplasm of bile duct
- K19
keratin 19
Footnotes
The authors have no conflicts to disclose.
References
- 1.Desmet VJ. Congenital diseases of intrahepatic bile ducts: variations on the theme "ductal plate malformation". Hepatology. 1992;16:1069–1083. doi: 10.1002/hep.1840160434. [DOI] [PubMed] [Google Scholar]
- 2.Summerfield JA, Nagafuchi Y, Sherlock S, Cadafalch J, Scheuer PJ. Hepatobiliary fibropolycystic diseases. A clinical and histological review of 51 patients. J Hepatol. 1986;2:141–156. doi: 10.1016/s0168-8278(86)80073-3. [DOI] [PubMed] [Google Scholar]
- 3.Ko JS, Yi NJ, Suh KS, Seo JK. Pediatric liver transplantation for fibropolycystic liver disease. Pediatr Transplant. 2012;16:195–200. doi: 10.1111/j.1399-3046.2012.01661.x. [DOI] [PubMed] [Google Scholar]
- 4.Yonem O, Bayraktar Y. Clinical characteristics of Caroli's disease. World J Gastroenterol. 2007;13:1930–1933. doi: 10.3748/wjg.v13.i13.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giovanardi RO. Monolobar Caroli's disease in an adult. Case report. Hepatogastroenterology. 2003;50:2185–2187. [PubMed] [Google Scholar]
- 6.Roda E, Sama C, Festi D, Aldini R, Mazzella G, Roda A, et al. Caroli's disease. description of a rare clinical case with monolobar localization. Am J Gastroenterol. 1979;71:621–626. [PubMed] [Google Scholar]
- 7.Chapman RW. Risk factors for biliary tract carcinogenesis. Ann Oncol. 1999;10(Suppl 4):308–311. [PubMed] [Google Scholar]
- 8.Shimonishi T, Sasaki M, Nakanuma Y. Precancerous lesions of intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2000;7:542–550. doi: 10.1007/s005340070002. [DOI] [PubMed] [Google Scholar]
- 9.Sato Y, Ren XS, Nakanuma Y. Caroli's disease: current knowledge of its biliary pathogenesis obtained from an orthologous rat model. Int J Hepatol. 2012;2012:107945. doi: 10.1155/2012/107945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terada T, Moriki T. Monolobar ductal plate malformation disease of the liver. Pathol Int. 2010;60:407–412. doi: 10.1111/j.1440-1827.2010.02535.x. [DOI] [PubMed] [Google Scholar]
- 11.Kim RD, Book L, Haafiz A, Schwartz JJ, Sorensen JB, Gonzalez-Peralta RP. Liver transplantation in a 7-month-old girl with Caroli's disease. J Pediatr Surg. 2011;46:1638–1641. doi: 10.1016/j.jpedsurg.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Nakanuma Y, Curado MP, Franceschi S, Gores G, Paradis V, Sripa B. Intrahepatic cholangiocarcinoma. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumours of the digestive system. 4th ed. Lyon: IARC; 2010. pp. 217–224. [Google Scholar]
- 13.Fozard JB, Wyatt JI, Hall RI. Epithelial dysplasia in Caroli's disease. Gut. 1989;30:1150–1153. doi: 10.1136/gut.30.8.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yasoshima M, Sato Y, Furubo S, Kizawa K, Sanzen T, Ozaki S, et al. Matrix proteins of basement membrane of intrahepatic bile ducts are degraded in congenital hepatic fibrosis and Caroli's disease. J Pathol. 2009;217:442–451. doi: 10.1002/path.2472. [DOI] [PubMed] [Google Scholar]