Abstract
Mycobacterium bovis infection in wildlife and feral species is a potential source of infection for livestock and a threat to protected and endangered species. The aim of this study was to identify Spanish wild animal species infected with M. bovis through bacteriological culture and spacer oligonucleotide typing (spoligotyping) of isolates for epidemiological purposes. This study included samples from red deer (Cervus elaphus), fallow deer (Dama dama), wild boar (Sus scrofa), Iberian lynx (Lynx pardina), hare (Lepus europaeus), and cattle (Bos taurus). They were collected in several geographical areas that were selected for their unique ecological value and/or known relationships between wildlife and livestock. In the areas included in this survey, M. bovis strains with the same spoligotyping pattern were found infecting several wild species and livestock, which indicates an epidemiological link. A locally predominant spoligotype was found in these areas. Better understanding of the transmission and distribution of disease in these populations will permit more precise targeting of control measures.
Mycobacterium bovis, the etiological agent of bovine tuberculosis, can infect a wide range of domestic and wild animals (16, 22, 32, 38). The importance of the infection in wild animals focuses on three aspects: (i) the role of some wild species in maintaining tuberculosis and acting as a reservoir of infection for livestock, (ii) the morbidity and mortality that the infection can cause in protected and endangered species, and (iii) the possibility of its impact on public health.
In a report from the Office International des Epizooties, 22% of countries have detected bovine tuberculosis in wildlife (deer, elk, wild boar, feral goat, buffalo, possum, ferret, mink, hedgehog, lion, cheetah, kudu, baboon, and seal) in the last 10 years (30). However, wild species do not reach the status of maintenance host for M. bovis in all countries. In most cases, they become infected when the challenge level is high, but when infection is eliminated from the natural host (cattle), it also disappears from the other animal species. The risk that these reservoirs of infection pose for domestic animals and humans is quite variable, depending on the specific epidemiological situation for the species and the environment (32).
However, the potential role of wild animals in the maintenance and spread of M. bovis infection in domestic livestock is of particular importance in countries where eradication programs have substantially reduced the incidence of bovine tuberculosis but sporadic outbreaks still occur. The best-known examples are the European badger (Meles meles) in the United Kingdom and the Republic of Ireland (19, 29, 35) and the possum (Trichosurus vulpecula) in New Zealand (8). The potential for a badger population to become endemically infected with M. bovis and to act as a source of infection for cattle was experimentally proved (29). The involvement of badgers and possums has been based principally on the observed incidence of infection in these animals inhabiting affected areas, coupled with the finding that intervention studies that removed badgers or possums were shown to result in a consequential decrease in the number of tuberculosis-infected cattle and reinfections (8, 37). Although badgers did contribute to cattle tuberculosis, the available data made it impossible to quantify the contribution in the United Kingdom (27).
In Michigan in the United States, self-sustaining infection in white-tailed deer (Odocoileus virginianus) has served as the presumptive source of infection for cattle herds (44) and carnivores (5). The best-studied maintenance host of M. bovis in Africa is the African buffalo (Syncerus caffer), which has spread the infection to predators (26).
There are some circumstances in Spain that may favor infection transmission between species. First, there is a wide variety of wild animal species, and many of them are susceptible to infection by M. bovis. Second, game has become an essential part of the economy of a sustainable agriculture, which in some areas has led to overgrown populations, also related to the lack of natural predators. Third, specific farming practices (extensive management) allow grazing cattle to overlap wildlife habitats. These circumstances are present in some areas of central, southern, and western Spain. In 2002, the prevalence of herds with positive intradermal tuberculin (IDTB) tests in these regions ranged from 7.7 to 10.6%, in comparison to 0.07 to 0.6% in some areas of the northern part of the country.
A combination of traditional disease-tracing investigation and molecular typing is needed to understand the epidemiology of tuberculosis and can provide valuable insight into the importance of different hosts in the maintenance and spread of the infection. Some genetic elements of M. bovis can be used as strain-specific markers, but there is no consensus as to which method is best suited for this purpose (2, 13, 14, 42).
Spacer oligonucleotide typing (spoligotyping), described by Kamerbeek et al. in 1997 (26), is a PCR-based method that reveals the polymorphism of the direct repeat region by detecting the presence or absence of specific spacer sequences. In a previous report, M. bovis spoligotypes related to cattle isolates were also found in M. bovis isolates from four wild boars and two red deer, suggesting transmission (1). This finding led us to embark on this comprehensive study.
The aim of the current study was to identify Spanish wild animal species infected with M. bovis through bacteriological culture and spoligotyping with two objectives: (i) comparison of the patterns of M. bovis strains from wild and domestic animals, and (ii) insight into possible inter- and intraspecies transmission among wild animals in the same habitat.
MATERIALS AND METHODS
Sample collection.
The study included samples from wild red deer (Cervus elaphus) (n = 108), fallow deer (Dama dama) (n = 89), wild boar (Sus scrofa) (n = 96), Iberian lynx (Lynx pardina) (n = 4), and hare (Lepus europaeus) (n = 8) and from domestic cattle (Bos taurus) (n = 179). Sources and sampling periods are summarized in Table 1. The samples were collected in geographical areas of Spain selected for their unique ecological value or because the relationships between wild animals and livestock are known. These sampling areas are several hundred kilometers apart and are located in Andalucía, Extremadura, Castilla-La Mancha, and Madrid (south, west, central-south, and central Spain, respectively).
TABLE 1.
Results of bacteriological culture for M. bovis and spoligotyping patterns of isolates
| Area | No. on Table 3 | Sampling period | Animal species | No. of animals | No. of positive cultures | Spoligotypes (no. of isolates) |
|---|---|---|---|---|---|---|
| Doñana National Park (west Andalucia) | 1 | 1996-2002 | Red deer | 35 | 12 | spb-52 (10), spb-54 (2) |
| Fallow deer | 40 | 14 | spb-52 (13), spb-54 (1) | |||
| Wild boar | 44 | 27 | spb-40 (1), spb-52 (20), spb-53 (1), spb-54 (4), spb-64 (1) | |||
| Iberian lynx | 4 | 3 | spb-52 (3) | |||
| Cattle | 13 | 9 | spb-52 (7), spb-53 (1), spb-101 (1) | |||
| Monte de El Pardo (northwest Madrid) | 2 | 1998-2002 | Red deer | 19 | 10 | spb-16 (9), spb-23 (1) |
| Fallow deer | 49 | 46 | spb-7 (2), spb-16 (41), spb-23 (3) | |||
| Wild boar | 18 | 8 | spb-16 (7), spb-23 (1) | |||
| Game property 1 (north Extremadura) | 3 | 1999-2000 | Red deer | 32 | 3 | spb-7 (2), spb-75 (1) |
| Wild boar | 2 | 2 | spb-75 (2) | |||
| Game property 2 (north Extremadura) | 4 | 1999-2001 | Red deer | 20 | 0 | |
| Wild boar | 6 | 4 | spb-13 (1), spb-94 (3) | |||
| Cattle property 1 (north Extremadura) | 5 | 1998 | Wild boar | 5 | 2 | spb-7 (1), spb-8 (1) |
| Cattle | 49 | 19 | spb-7 (3), spb-8 (14), 2 (NDa) | |||
| Cattle property 2 (south Castilla-La Mancha) | 6 | 2000, 2002 | Wild boar | 8 | 5 | spb-13 (3), spb-19 (1), spb-89 (1) |
| Cattle | 17 | 1 | spb-13 (1) | |||
| Cattle and game property (east Castilla-La Mancha) | 7 | 2001-2002 | Red deer | 2 | 1 | spb-9 (1) |
| Wild boar | 13 | 3 | spb-9 (3) | |||
| Hare | 8 | 0 | ||||
| Cattle | 100 | 21 | spb-7 (10), spb-9 (1), spb-34 (1), spb-98 (9) |
ND, not done.
Doñana National Park has been classified as World Heritage Site by the United Nations Educational, Scientific, and Cultural Organization. Located in western Andalucía, it covers 50,720 ha and is notable for the great diversity of its ecosystems. Doñana National Park also maintains a number of grazing cattle and horses as a traditional activity for local farmers. There was a continuous increase in livestock in the 1990s motivated by agricultural subsidies. Samples consisted of animals found dead or terminally ill for protected species; in the case of hoofed species, samples from animals randomly selected for health monitoring were also collected. Samples from 13 cows that used to graze in the park and that reacted to the IDTB test were included in the study.
Monte de el Pardo is a natural park of 14,474 ha close to the city of Madrid. Its entire perimeter is protected with a stone or wire fence. From 2000 to 2001, 529 red deer, 4,020 fallow deer, and 639 wild boars were slaughtered in this park for population control. Animals were inspected by the staff of the Public Health Service of Madrid, and 1.51, 1.39, and 0.63%, respectively, showed visible lesions compatible with tuberculosis. There is no livestock in El Pardo, but it is bordered on the north and west by many cattle-grazing properties.
Game properties 1 and 2 are privately owned properties on the boundaries of Monfragüe Natural Park (northern Extremadura). Both of them are devoted to hunting, and wild animals are not in contact with the cattle. The sampling was a random survey of mature male and female red deer and wild boars that were shot by hunters.
Sampling in cattle property 1 (northern Extremadura) and in cattle property 2, Ciudad Real (southern Castilla-La Mancha), was performed because a high percentage of livestock, which are raised extensively there, reacted to the IDTB test. Samples from IDTB reactors and wild boars hunted in these properties were also studied.
The last sampling area is a large cattle and game property located in Albacete (eastern Castilla-La Mancha). Bullfighting cattle are bred with extensive management system and occasional supplements. The property is also exploited for game. Red deer, wild boars, and hares were sampled. Cattle were mustered for the eradication campaigns, and those that reacted to IDTB and animals that were slaughtered for other reasons were also analyzed.
In this study we also included the results for seven red deer, two fallow deer, and nine wild boars that were diagnosed with tuberculosis at our department. These animals were shot between 1996 and 2001, and most of them came from areas close to Madrid. The origins and years of collection of these 18 animals are detailed in Table 2.
TABLE 2.
Spoligotyping results for wildlife samples received at our laboratory for diagnosis of tuberculosis
| Species | Geographical location | Letter on Table 3 | Yr | No. of animals | Spoligo- type |
|---|---|---|---|---|---|
| Red deer | Toledo | a | 1996 | 1 | spb-8 |
| Jaén | b | 1997 | 1 | spb-7 | |
| Southwest Madrid | c | 1999 | 1 | spb-7 | |
| Southwest Madrid | c | 1999 | 1 | spb-16 | |
| Ávila | d | 2000 | 2 | spb-7 | |
| Southwest Madrid | c | 2001 | 1 | spb-16 | |
| Fallow deer | Ávila | d | 2000 | 1 | spb-7 |
| South Madrid | e | 2001 | 1 | spb-56 | |
| Wild boar | North Madrid | f | 1997 | 1 | spc-5a |
| Southwest Madrid | c | 1999 | 1 | spb-8 | |
| Southwest Madrid | c | 1999 | 1 | spb-8 | |
| Southwest Madrid | c | 2000 | 1 | spb-9 | |
| Northwest Madrid | g | 2001 | 1 | spb-16 | |
| Southwest Madrid | c | 2001 | 1 | spc-3a | |
| South Madrid | e | 2001 | 3 | spb-19 |
This spoligotype shows the characteristics of M. caprae.
Necropsies were performed by veterinarians, with different sets of sterile instruments used for each animal. Samples usually consisted of retropharyngeal, mediastinal, bronchial, mandibular, and mesenteric lymph nodes, lung, and liver collected at the post mortem examination. The samples received from four dead lynxes were a lesion at the elbow joint from an adult male (this first case has already been reported [4, 39]), a lung with granulomatous lesions from a female who died in captivity in 2000, lesions from the lung, kidney, and mesenteric lymph node from a male found dead in 2001, and the lung and liver from a male killed in traffic in 2002. All samples were stored at −20°C until culture.
Bacteriology.
Samples from each animal were pooled, homogenized with sterile distilled water, and decontaminated with 0.35% hexadecylpyridinium chloride for 30 min (10), centrifuged at 3,500 rpm (1,068 × g) for 30 min, and cultured onto Coletsos and 0.2% (wt/vol) pyruvate-enriched Löwenstein-Jensen media (bioMérieux España and Biomedics, Madrid, Spain) at 37°C. The isolates were identified as M. bovis by staining for acid-alcohol fastness, colony morphology, and PCR amplification of Mycobacterium genus-specific 16S rRNA fragment and MPB70 (49) and IS6110 (25) sequences.
Fingerprinting.
The spoligotyping method was performed as described by Kamerbeek et al. (26). PCR of the direct repeat locus was performed with heat-treated cell suspensions. The amplified product was detected by hybridization of the biotin-labeled PCR product onto a spoligotyping membrane (Isogen Bioscience BV, Maarssen, The Netherlands). Purified sterile water and clinical isolates of M. tuberculosis and M. bovis were included as controls in every batch of tests.
The spoligotyping results were compared with those obtained with M. bovis strains isolated from cattle and other domestic animals in our database. This database comprises 115 spoligotyping patterns from about 900 Spanish M. tuberculosis complex isolates. For the type nomenclature, patterns were allocated a prefix and a number; the prefix spb was used for classical M. bovis isolates, and spc was used for patterns with the characteristics of M. caprae (3) (formerly M. bovis subsp. caprae [34]). Numbering follows the order in which they were found and does not indicate any specific relationship among them.
RESULTS
A total of 323 wild animals (five species) and 179 cattle were sampled and examined by culture to detect tuberculosis infection. In general terms, red deer and fallow deer showed granulomatous lesions in the lung and associated lymph nodes but with some differences. Tuberculosis lesions in fallow deer involved the whole lung, which appeared completely covered with caseous nodules of different sizes; however, in red deer they were usually limited to a small area of the lung. Sporadically, lesions were found in other organs (liver and spleen). Wild boars showed lesions compatible with tuberculosis at the mandibular and/or retropharyngeal lymph node and in the lung and associated lymph nodes. Occasionally, hepatic lesions were also found in the inspected animals.
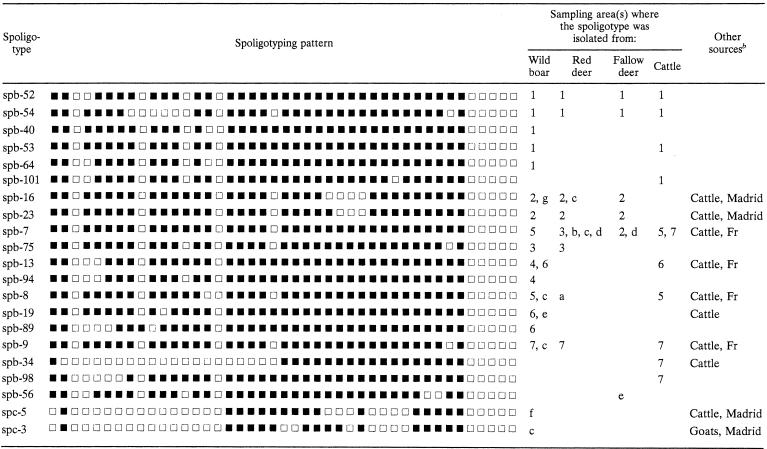
M. bovis was isolated from samples from 156 wild animals (33 red deer, 62 fallow deer, 58 wild boars, and 3 Iberian lynxes) included in this survey. Two other wild boars were found to be infected with M. caprae. The results from culture and spoligotyping are shown in Tables 1 and 2. The results were compared with those obtained from livestock (Table 3).
TABLE 3.
Schematic representation of spoligotyping patterns identified among wildlife and areas where the patterns were isolateda
Six spoligotypes were identified among the isolates from Doñana National Park. Spoligotype spb-52 was the most prevalent type, as it was found in 43 of 53 (81.1%) positive animals from the three wild Artiodactyla species, and it was isolated through all 7 years of sampling. This type also affected three dead lynxes, one found in 1999, a second case in 2000, and a third in 2002. The lungs were the affected organs in two lynxes. Nine M. bovis isolates were obtained from cattle, seven of them also belonging to the spb-52 type.
The population of M. bovis in Monte de El Pardo was quite homogeneous, as only three spoligotyping patterns were found; 57 of 64 M. bovis isolates (89%) were spb-16, isolated from 1998 to 2002 in the three wild species sampled for this study. This spoligotype is frequently isolated from cattle in the northern area of Madrid. Red deer and wild boars were the species affected during the first years of survey in the park, but in recent hunting seasons, fallow deer has been the species most frequently diagnosed with tuberculosis.
M. bovis from cattle and hunted wild boars from cattle property 1 had the same spoligotyping patterns; 38.8% of the cattle inspected of this property were found to be infected with M. bovis, most of them with type spb-8, and this type was also found in one wild boar. Three isolates from cattle were typed as spb-7, as was the isolate from the other wild boar. The same spoligotype (spb-13) was found in three wild boars and one cow from cattle property 2.
M. bovis strains from one red deer and wild boars from sampling area 7 were compared to M. bovis strains obtained from 21 cattle from the same property. The wild animals were infected with M. bovis type spb-9, also found in one cow. Types spb-7, spb-34, and spb-98 were found only in the livestock.
Eighteen more wild animals were diagnosed in our department as having tuberculosis. The results are shown in Table 2. Type spb-16, common in Monte de El Pardo and the farms in northern Madrid, was found in two red deer and a wild boar from southwest and northwest Madrid, respectively.
A wild boar hunted in the northern part of Madrid was infected with M. caprae, as revealed by the special characteristics of spoligotyping (lack of spacers 1, 3 to 16, 28, 30 to 33, and 39 to 43) and the sequencing of the pncA gene, which had CAC (His) at codon 57. This unusual pattern, spc-5, was also found in two cows from two farms located in this area. A caprine spoligotyping pattern was also found in the Mycobacterium isolate from a wild boar from southwest Madrid; this type, spc-3, is common in caprine herds in this area.
M. avium subsp. avium and atypical mycobacteria were cultured from a small number of animals (a red deer and a wild boar from Monte de El Pardo, three red deer from game property 1, and one fallow deer from Doñana National Park). In these cases, samples consisted of hemorrhagic lymph nodes without granulomatous lesions.
DISCUSSION
This study combines conventional disease-tracing investigation and molecular typing in an attempt to understand the epidemiology of bovine tuberculosis in Spain, specifically, the transmission of the infection between wildlife and domestic animals. In this survey, spoligotyping demonstrated that wildlife species (red deer, fallow deer, wild boar, and Iberian lynx) and domestic animals from the same geographical area are infected with the same M. bovis strains. This fact reveals an epidemiological connection; either the two populations are infecting each other or there is a common source of infection, although fingerprinting per se does not determine the direction of transmission.
The M. bovis populations isolated from wild animals during this survey were highly homogeneous. In each specific area, spoligotyping identified a local type that is prevalent for both the number of infected animals and presence throughout the years, although a small group of spoligotypes can also be found. This finding also reflects the main pattern found among local cattle and may indicate an enhanced ability of the strains to disseminate. In general terms, most spoligotyping patterns are regionally distributed (scarce types are confined to a particular location), while a small number of spoligotyping patterns are widespread in Spain, affecting wild animals from different geographical areas that are several hundred kilometers apart. This was the case for spb-7 and spb-8; these types were found frequently in cattle, and trade between farms could be the reason for their spread. Spb-7 is the second most predominant spoligotype in France, and it is also observed in Great Britain (pattern GB54), but at a low level (24).
The question now is to ascertain the role that these wildlife species play in the epidemiology of bovine tuberculosis in Spain. Classification of wild animal species as maintenance or spillover hosts has been a controversial issue, based on the location of tuberculous lesions coupled with ecological factors such as population density, behavioral characteristics, and interaction in the same habitat. According to these criteria, the results obtained in this study indicate that deer and wild boar may be maintenance hosts of M. bovis in Spain.
The lesions in the deer included in this study were mainly located in the thoracic cavity, which has been described as the most common site of infection in maintenance hosts of M. bovis (16), as this may also provide an indication of the probable means of the spread of the infection. Internationally, the prevalence of M. bovis in wild Cervidae has been reported to reach 5% (7, 38). Deer appear to be more infectious to other species than cattle and may act as a vector infecting wildlife (32, 47). There were epidemiological evidence of their ability to initiate new foci of infection in other wildlife species, i.e., coyotes (Canis latrans) and raccoons (Procyon lotor) in Michigan (5). In the Republic of Ireland, Costello et al. identified the same spoligotyping patterns among deer, cattle, and badgers (13).
M. bovis infection in wild swine has been reported in Australia, New Zealand, and Italy. In the Northern Territory of Australia, the old swamp buffaloes that died at the end of the dry season provided infected carcasses for scavenging. It was concluded that swine in the Northern Territory were almost certainly dead-end hosts which rarely transmitted the infection to other species (9, 31). However, studies in New Zealand found that 33% of infected feral pigs had either lung or bronchial lesions, suggesting that M. bovis can be transmitted between animals by aerosols (48). In Italy, spoligotyping provided good strain differentiation when it was applied to monitoring the transmission of M. bovis between cattle and wild boars; the authors believed that boars were the end host for M. bovis because, in that case, the lesions were limited to the lymph nodes of the head (43).
In our survey, the wild boars showed lesions in several body sites; the presence of tuberculous lesions in the lung and associated lymph nodes usually reveals transmission by the aerogenous route, while lesions in the retropharyngeal and mandibular lymph nodes could reveal infection by ingestion of contaminated offal or scavenging on carcasses. The behavior of wild boars can be closely related to this dual source of infection. They are also active animals, able to pass under wire fences. These facts demonstrate that in Spain, wild boars are not a dead-end host of tuberculosis.
We also report the isolation of M. caprae (3, 34) from two wild boars with lesions compatible with tuberculosis. In both cases, they were geographically linked with isolates with the same typing patterns from cattle or goats.
It is likely that infection in wildlife first came from cattle, i.e., no cases of tuberculosis were detected in Doñana National Park before the uncontrolled increase in the cattle population. This observation agrees with evidence suggesting the introduction of tuberculosis into Kruger National Park via an infected cattle herd (46). The campaign eradicate tuberculosis in cattle relies on the IDTB test and removal of reactors, but the sensitivity of the tuberculin test (at standard interpretation) is only 90% (33). The performance of tuberculin tests can be affected by specific situations, as seasonal undernutrition combined with the effects of stress due to mustering of semiwild herds. Thus, failure to diagnose cattle tuberculosis results in the persistence of the infection. Transmission of infection from cattle to wildlife (and vice versa) may happen at several points. First, the territories of wild and domesticated animals overlap because they share pasture and drinking ponds. Some factors, such as crowding of animals at watering ponds, may facilitate close contact and thus may lead to increased respiratory transmission of infection (1, 12). Second, persistence in infected animals after death may be a source of infection for scavengers, providing the large dose of microorganisms needed for infection by the alimentary route. It has been described that M. bovis survived for 2 weeks in the carcass of a tuberculous badger lying on pasture (29) and 6 weeks in infected tissue from a buffalo under South African conditions (46). Third, survival in the environment is a factor, as M. bovis can survive for 4 weeks in soil in 80% shade (17).
Once established in a wild population, M. bovis is probably able to persist by transmission among wild animals (during feeding, grooming, or mating). The fence around Monte de El Pardo allows only sporadic contacts with domestic animals from neighboring farms, which does not seem to explain the high prevalence of the infection in wildlife. The finding of the same spoligotype within this locality over several years may reflect self-maintenance of the M. bovis strains. This transmission among wild animals could also explain the infection of wild animals from areas without livestock, as it happened in game properties 1 and 2.
Research on the epidemiology of wildlife disease was also undertaken because of the concern that infectious diseases may affect the survival of endangered species. The Iberian lynx is considered the most endangered feline in the world (36). It has been classified as critically endangered C2a(i) in the IUCN red list of threatened species. In 1990, the population was estimated to be 1000 animals, in small groups in south-western Spain (41); during the last decade, the total population has dropped to 500 individuals. Three Iberian lynxes were found infected with M. bovis, and the spoligotyping pattern was identical to the predominant pattern found in Artyodactyla species in the National Park. Lesions were found in the respiratory tract, although scavenging on tuberculous carcasses, probably on fallow deer because this is part of the lynx′s diet (15), was the most likely source of infection. M. bovis infection has been reported in other wild felids, as lions (Panthera leo) and cheetah (Acionyx jubatus) in the Kruger National Park (27), and the bobcat (Felix rufus) in United States (5). Consumption of tuberculous buffaloes and deer, respectively, was assumed as likely source of infection. Extensive lesions at the lungs were also described in the African carnivores, probably caused by infection within pride by inhalation. However, Iberian lynx is not a gregarious species, and are in close contact only at mating season. The second case could be a reactivation of an old infection, as the female lynx had spent last years in captivity, with no contact with other animals and was feed on products suitable for human consumption.
Wild animals may also act as a source of infection for human beings. During the hunting season from 2000 to 2001, Spanish hunters took approximately 117,305 wild boar, 70,863 red deer, 5,431 fallow deer, and 6,427 roe deer (Capreolus capreolus) (J. L. Garrido, personal communication). There is a danger of transmission of infection by direct contact between infected animals and hunters as well as from infected food products. Regarding direct contact, people who handle sick animals or infected carcasses are at risk through aerosol contamination when the carcass is open and cut or through entry of organisms via cuts in the skin (18, 20, 40). A special (although scarce) risk is infected animals without visible lesions. In our study, three wild boars and three red deer with no visible lesions were M. bovis positive by culture. Meat from infected animals may contain viable M. bovis and represents a hazard for consumers, particularly when meat is eaten uncooked (sausages). Post mortem inspection to detect lesions and confiscation of the affected organs or whole carcasses reduce the danger of infection and are compulsory in Spain. In this sense, it is important that hunters become aware of their role in fighting tuberculosis, first, by reporting animals with lesions and facilitating the inspection of hunted animals, and second, by collaborating in the elimination of tuberculous animals to reduce environmental contamination.
The results obtained in this study raise several questions which need to be addressed. The first question is the estimation of the true prevalence of M. bovis infection in Spanish wildlife and whether tuberculosis has become endemic in wild animal populations. It is not possible to infer the true prevalence of the infection from our survey: hunted animals are a biased population sample, weighted towards adult males, and therefore unrepresentative of the population with regard to both sex and age, and the numbers of samples collected each year did not allow monitoring of changes in prevalence. The prevalence of tuberculosis in the clinical samples included in this study is likely to be underestimated, as in most cases only some lymph nodes were available for culture, and the retropharyngeal node, one of the most commonly affected organs, could not be inspected in all animals. Despite these limitations, we can affirm that M. bovis infection is widespread among Spanish wild animals, because it has been found in several animal species and in unrelated geographical areas. This fact, assuming that wild animals have only limited contact with cattle and coupled with the finding of a prevalent typing pattern in each location, indicates that the infection has become endemic in wildlife populations.
The second and most important question concerns the development of rational strategies to control or eradicate M. bovis infection in wild animal populations. Vaccination of wildlife and feral reservoirs against tuberculosis has been proposed as a viable strategy for the control of the disease (6, 12, 21), but many issues need to be addressed before it becomes a realistic option. However, this option deserves the effort, and development of a vaccine against tuberculosis for use in deer (23), badger (45), and possum (11) populations has already been initiated.
At present, the only control measures available for wildlife hosts are removal of the infected animals and population control. The detection of infected animals is hampered by the difficulties of handling and the lack of appropriate diagnostic tools, and control of populations (culling) shocks public opinion, and in some cases, species are statutorily protected. A realistic and convenient option would be intensification of the eradication campaigns in cattle from the affected areas, coupled with limiting contact between domestic and wild species by fencing wildlife in nature reserves. This simple strategy has been implemented in sampling area 7, and tuberculosis has been eradicated from the cattle herd that had been chronically infected.
In summary, spoligotyping has identified local prevalent patterns of M. bovis that were found infecting domestic animals, wild deer, fallow deer, and wild boars from the same geographical area. This finding, coupled with the locations of the tuberculous lesions and specific ecological factors, indicates that wildlife species may act as maintenance hosts of M. bovis in Spain. This infection is a threat to livestock, wildlife, and endangered species such as the Iberian lynx. A better understanding of the transmission and distribution of disease in these populations will permit more precise targeting of control measures that would benefit both livestock and wildlife.
Acknowledgments
A. Aranaz has a fellowship from the Ramon y Cajal Program (Spanish Ministry of Science and Technology/U.C.M.). This research was funded by the Spanish Ministry of Agriculture, Fisheries and Food and the Comunidad of Madrid.
We thank national and regional animal health authorities, especially C. Escribano, M. C. Sánchez-Trujillano, J. L. Paramio, I. Carpio, A. de Miguel, and S. Moreno, for their continuous encouragement. We are grateful to N. Castro, V. Collado, J. L. Cumbreño, F. Moneo, P. J. Mora, J. M. Portas, J. L. Rodríguez, and J. M. Sebastián for samples and excellent collaboration. The kind willingness of the owners of the cattle and game properties that were sampled in this study is greatly appreciated. We also thank J. L. Garrido, Head of the Spanish Game School, for the data on hunted animals in Spain.
REFERENCES
- 1.Aranaz, A., E. Liébana, A. Mateos, L. Domínguez, D. Vidal, Domingo M., O. González, E. F. Rodríguez-Ferri, A. E. Bunschoten, J. D. A van Embden, and D. Cousins. 1996. Spacer oligonucleotide typing of Mycobacterium bovis strains from cattle and other animals: a tool for studying epidemiology of tuberculosis. J. Clin. Microbiol. 34:2734-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aranaz, A., E. Liébana, A. Mateos, L. Domínguez, and D. Cousins. 1998. Restriction fragment length polymorphism and spacer oligonucleotide typing: a comparative analysis of fingerprinting strategies for Mycobacterium bovis. Vet. Microbiol. 61:311-324. [DOI] [PubMed] [Google Scholar]
- 3.Aranaz, A., D. Cousins, A. Mateos, and L. Domínguez. 2003. Elevation of Mycobacterium tuberculosis subsp. caprae Aranaz et al. 1999 to species rank as Mycobacterium caprae comb. nov., sp. nov. Int. J. Syst. Evol. Microbiol. 53:1785-1789. [DOI] [PubMed] [Google Scholar]
- 4.Briones, V., L. de Juan, C. Sánchez, A. I. Vela, M. Galka, N. Montero, J. Goyache, A. Aranaz, A. Mateos, and L. Domínguez. 2000. Bovine tuberculosis and the endangered Iberian lynx. Emerg. Infect. Dis. 6:189-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruning-Fann, C. S., S. M. Schmitt, S. D. Fitzgerald, J. S. Fierke, P. D. Friedrich, J. B. Kaneene, K. A. Clarke, K. L. Butler, J. B. Payeur, D. L. Whipple, T. M. Cooley, J. M. Miller, and D. P. Muzo. 2001. Bovine tuberculosis in free-ranging carnivores from Michigan. J. Wildl. Dis. 37:58-64. [DOI] [PubMed] [Google Scholar]
- 6.Buddle, B. M., M. A. Skinner, and M. A. Chambers. 2000. Immunological approaches to the control of tuberculosis in wildlife reservoirs. Vet. Immunol. Immunopathol. 74:1-16. [DOI] [PubMed] [Google Scholar]
- 7.Clifton-Hadley, R. S., and J. W. Wilesmith. 1991. Tuberculosis in deer: a review. Vet. Rec. 129:5-12. [DOI] [PubMed] [Google Scholar]
- 8.Coleman J. D., and M. M. Cooke. 2001. Mycobacterium bovis infection in wildlife in New Zealand. Tuberculosis (Edinburgh) 81:191-202. [DOI] [PubMed] [Google Scholar]
- 9.Corner, L. A., R. H. Barrett, A. W. D. Lepper, V. Lewis, and C. W. Pearson. 1981. A survey of mycobacteriosis of feral pigs in the Northern Territory. Aust. Vet. J. 57:537-542. [DOI] [PubMed] [Google Scholar]
- 10.Corner, L. A., and A. C. Trajstman. 1988. An evaluation of 1-hexadecyl-pyridinium chloride as a decontaminant in the primary isolation of Mycobacterium bovis from bovine lesions. Vet. Microbiol. 18:127-134. [DOI] [PubMed] [Google Scholar]
- 11.Corner, L. A., B. M. Buddle, D. U. Pfeiffer, and R. S. Morris. 2001. Aerosol vaccination of the brushtail possum (Trichosurus vulpecula) with the bacille Calmette-Guérin: the duration of protection. Vet. Microbiol. 81:181-191. [DOI] [PubMed] [Google Scholar]
- 12.Cosivi, O., J. M. Grange, C. J. Daborn, M. C. Raviglione, T. Fujikura, D. Cousins, R. A. Robinson, H. F. Huchzermeyer, I. de Kantor, and F. X. Meslin. 1998. Zoonotic tuberculosis due to Mycobacterium bovis in developing countries. Emerg. Infect. Dis. 4:59-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costello, E., D. O'Grady, O. Flynn, R. O'Brien, M. Rogers, F. Quigley, J. Egan, and J. Griffin. 1999. Study of restriction fragment length polymorphism analysis and spoligotyping for epidemiological investigation of Mycobacterium bovis infection. J. Clin. Microbiol. 37:3217-3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cousins, D., S. Williams, E. Liébana, A. Aranaz, A. Bunschoten, J. D. A. van Embden, and T. Ellis. 1998. Evaluation of four DNA typing techniques in epidemiological investigations of bovine tuberculosis. J. Clin. Microbiol. 36:168-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delibes, M. 1980. Feeding ecology of the Spanish lynx in the Coto Doñana. Acta Theriol. 25:309-324. [Google Scholar]
- 16.De Lisle, G. W., R. G. Bengis, S. M. Schmitt, and D. J. O'Brien. 2002. Tuberculosis in free-ranging wildlife: detection, diagnosis and management. Rev. Sci. Tech. 21:317-334. [DOI] [PubMed] [Google Scholar]
- 17.Duffield, B. J., and D. A. Young. 1985. Survival of Mycobacterium bovis in defined environmental conditions. Vet. Microbiol. 10:193-197. [DOI] [PubMed] [Google Scholar]
- 18.Fanning A., and S. Edwards. 1991. Mycobacterium bovis infection in human beings in contact with elk (Cervus elaphus) in Alberta, Canada. Lancet 338:1253-1255. [DOI] [PubMed] [Google Scholar]
- 19.Gallagher, J., and R. S. Clifton-Hadley. 2000. Tuberculosis in badgers; a review of the disease and its significance for other animals. Res. Vet. Sci. 69:203-217. [DOI] [PubMed] [Google Scholar]
- 20.Georghiou P., A. M. Patel, and A. Konstantinos. 1989. Mycobacterium bovis as an occupational hazard in abattoir workers. Aust. N. Z. J. Med. 19:409-410. [DOI] [PubMed] [Google Scholar]
- 21.Gormley, E., and J. D. Collins. 2000. The development of wildlife control strategies for eradication of tuberculosis in cattle in Ireland. Tuberc. Lung Dis. 80:229-236. [DOI] [PubMed] [Google Scholar]
- 22.Grange, J. M., and C. H. Collins. 1987. Bovine tubercle bacilli and disease in animals and man. Epidemiol. Infect. 99:221-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffin, J. F., C. G. Mackintosh, L. Slobbe, A. J. Thomson, and G. S. Buchan. 1999. Vaccine protocols to optimise the protective efficacy of BCG. Tuberc. Lung Dis. 79:135-143. [DOI] [PubMed] [Google Scholar]
- 24.Haddad, N., A. Ostyn, C. Karoui, M. Masselot, M. F. Thorel, S. L. Hughes, J. Inwald, R. G. Hewinson, and B. Durand. 2001. Spoligotype diversity of Mycobacterium bovis strains isolated in France from 1979 to 2000. J. Clin. Microbiol. 39:3623-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hermans, P. W. M., D. van Soolingen, J. W. Dale, A. R. J. Schuitema, R. A. McAdam, D. Catty, and J. D. A. van Embden. 1990. Insertion element IS986 from Mycobacterium tuberculosis: a useful tool for the diagnosis and epidemiology of tuberculosis. J. Clin. Microbiol. 28:2051-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamerbeek, J., L. Schouls, A. Kolk, M. Van Agterveld, D. van Soolingen, S. Kuijper, A. Bunschoten, H. Molhuizen, R. Shaw, M. Goyal, and J. D. A. van Embden. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35:907-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keet, D. F., N. P. Kriek, M. L. Penrith, A. Michel, and H. Huchzermeyer. 1996. Tuberculosis in buffaloes (Syncerus caffer) in the Kruger National Park: spread of the disease to other species. Onderstepoort J. Vet. Res. 63:239-244. [PubMed] [Google Scholar]
- 28.Krebs J. R., R. Anderson, T. Clutton-Brock, I. Morrison, D. Young, C. A. Donnelly, S. Frost, and R. Woodroffe. 1997. Bovine tuberculosis in cattle and badgers. Report to Rt. Hon. Dr. Cunningham, MP. Her Majesty's Stationery Office, London, England.
- 29.Little, T. W. A., P. F. Naylor, and J. W. Wilesmith. 1982. Laboratory study of Mycobacterium bovis infection in badgers and calves. Vet. Rec. 111:550-557. [PubMed] [Google Scholar]
- 30.Livingstone P. G. 2000. Avances en materia de diagnóstico, control y erradicación de la tuberculosis bovina (Mycobacterium bovis) en animales domésticos y salvajes. 68th Sesión General, Comité International. Office International des Epizooties, Paris, France.
- 31.McInerney, J., K. J. Small, and P. Caley. 1995. Prevalence of Mycobacterium bovis infection in feral pigs in the Northern Territory. Aust. Vet. J. 72:448-451. [DOI] [PubMed] [Google Scholar]
- 32.Morris, R. S., D. U. Pfeiffer, and R. Jackson. 1994. The epidemiology of Mycobacterium bovis infections. Vet. Microbiol. 40:153-157. [DOI] [PubMed] [Google Scholar]
- 33.Morrison, I., J. Bourne, D. R. Cox, C. A. Donnelly, G. Gettinby, J. P. McInerney, and R. Woodroffe. 2000. Pathogenesis and diagnosis of infections with Mycobacterium bovis in cattle. Vet. Rec. 146:236-242. [PubMed] [Google Scholar]
- 34.Niemann, S., E. Richter, and S. Rüsch-Gerdes. 2002. Biochemical and genetic evidence for the transfer of Mycobacterial tuberculosis subsp. caprae Aranaz et al. 1999 to the species Mycobacterium bovis Karlson and Lessel 1970 (Approved Lists 1980) as Mycobacterium bovis subsp. caprae comb. nov. Int. J. Syst. E vol. Microbiol. 52:433-436. [DOI] [PubMed] [Google Scholar]
- 35.Nolan, A., and J. W. Wilesmith. 1994. Tuberculosis in badgers (Meles meles). Vet. Microbiol. 40:179-191. [DOI] [PubMed] [Google Scholar]
- 36.Nowell, K., and P. Jackson (ed.). 1996. Wild cats: status survey and conservation action plan, p. 382. IUCN Publications. The Burlington Press, Cambridge, Mass.
- 37.O'Mairtin, D., D. H. Williams, J. M. Griffin, and J. A. Eves. 1998. The effect of badger removal programme on the incidence of tuberculosis in an Irish cattle population. Prev. Vet. Med. 34:47-56. [DOI] [PubMed] [Google Scholar]
- 38.O'Reilly L. M., and C. J. Daborn. 1995. The epidemiology of Mycobacterium bovis infections in animals and man: a review. Tuberc. Lung Dis. 76(Suppl. 1):1-46. [DOI] [PubMed] [Google Scholar]
- 39.Pérez, J., J. Calzada, L. León-Vizcaíno, M. J. Cubero, and E. Mozos. 2001. Tuberculosis in an Iberian lynx (Lynx pardina). Vet. Rec. 148:414-415. [DOI] [PubMed] [Google Scholar]
- 40.Robinson P., D. Morris, and R. Antic. 1988. Mycobacterium bovis as an occupational hazard in abattoir workers. Aust. N. Z. J. Med. 18:701-703. [DOI] [PubMed] [Google Scholar]
- 41.Rodríguez, A., and M. Delibes. 1992. Current range and status of the Iberian lynx Felis pardina Temminck 1824 in Spain. Conserv. Biol. 61:189-196. [Google Scholar]
- 42.Roring, S., D. Brittain, A. E. Bunschoten, M. S. Hughes, R. A. Skuce, J. D. A. van Embden, and S. D. Neill. 1998. Spacer oligotyping of Mycobacterium bovis isolates compared to typing by restriction fragment length polymorphism using PGRS, DR and IS6110 probes. Vet. Microbiol. 61:111-120. [DOI] [PubMed] [Google Scholar]
- 43.Serraino, A., G. Marchetti, V. Sanguinetti, M. C. Rossi, R. G. Zanoni, L. Catozzi, A. Bandera, W. Dini, W. Mignone, F. Franzetti, and A. Gori. 1999. Monitoring of transmission of tuberculosis between wild boars and cattle: genotypical analysis of strains by molecular epidemiology techniques. J. Clin. Microbiol. 37:2766-2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmitt, S. M., S. D. Fitzgerald, T. M. Cooley, C. S. Bruning-Fann, L. Sullivan, D. Berry, T. Carlson, R. B. Minnis, J. B. Payeur, and J. Sikarskie. 1997. Bovine tuberculosis in free-ranging white-tailed deer from Michigan. J. Wildl. Dis. 33:749-758. [DOI] [PubMed] [Google Scholar]
- 45.Southey, A., D. P. S. Sleeman, K. Lloyd, D. Dalley, M. A. Chambers, R. G. Hewinson, and E. Gormley. 2001. Immunological responses of Eurasian badgers (Meles meles) vaccinated with Mycobacterium bovis BCG (bacillus Calmette-Guérin). Vet. Immunol. Immunopathol. 79:197-207. [DOI] [PubMed] [Google Scholar]
- 46.Tanner, M., and A. L. Michel. 1999. Investigation of the viability of M. bovis under different environmental conditions in the Kruger National Park. Onderstepoort J. Vet. Res. 66:185-190. [PubMed] [Google Scholar]
- 47.Tweddle, N. E., and P. Livingstone. 1994. Bovine tuberculosis control and eradication programs in Australia and New Zealand. Vet. Microbiol. 40:23-39. [DOI] [PubMed] [Google Scholar]
- 48.Wakelin, C. A., and O. T. Churchman. 1991. Prevalence of bovine tuberculosis in feral pigs in central Otago. Surveillance 18:19-20. [Google Scholar]
- 49.Wilton, S., and D. Cousins. 1992. Detection and identification of multiple mycobacterial pathogens by DNA amplification in a single tube. PCR Methods Appl. 1:209-273. [DOI] [PubMed] [Google Scholar]