Abstract
Envelope and membrane (E/M) and nonstructural protein NS1 serotype-specific capture Immunoglobulin M (IgM) enzyme-linked immunosorbent assays (ELISAs) were developed to differentiate four dengue virus serotypes. A total of 93 anti-dengue virus IgM-positive serum samples collected between days 5 and 45 of illness from 59 confirmed dengue patients were analyzed. The results showed that positive serotype specificity could be identified for 86.1 and 47.6% of serum samples tested for E/M-specific IgM antibodies versus 83.3 and 42.9% of serum samples tested for NS1-specific IgM antibodies from patients with primary and secondary dengue virus infections, respectively. Dual analyses with both E/M and NS1 serotype-specific capture IgM ELISAs showed that positive serotype specificity could be correctly identified for 98.6 and 61.9% of all of the primary and secondary serum samples tested, respectively. These findings suggested that E/M and NS1 serotype-specific capture IgM ELISAs have the potential to be of use in dengue virus serotyping.
Dengue is an endemic viral disease affecting tropical and subtropical regions around the world, predominantly in urban and semiurban areas. It is the most important arboviral disease in terms of morbidity and mortality. In recent years, dengue fever and its more serious forms, dengue hemorrhagic fever and dengue shock syndrome, have emerged as a major public health problem with expanded geographic distribution and increased epidemic activity (24).
Dengue virus is a mosquito-borne flavivirus and the most prevalent arbovirus in the world (3). There are four distinct serotypes, DEN-1, DEN-2, DEN-3, and DEN-4. Infection induces a life-long protective immunity to the homologous serotype but confers only partial and transient protection against subsequent infection by the other three serotypes. Therefore, multiple and sequential infections with the four dengue serotypes would be expected for people living in a region of hyperendemic dengue due to the lack of cross-protective neutralizing antibodies. Seroepidemiological studies have shown that secondary infection is a major risk factor for dengue hemorrhagic fever and dengue shock syndrome through antibody-dependent enhancement (5, 7). For epidemiological and pathological investigations, it is important to differentiate between primary and secondary dengue virus infection and to determine the dengue virus serotypes of past and current infections.
Although the hemagglutination inhibition test has traditionally been used for the differentiation of primary and secondary dengue virus infections, it is less popular now due to the inherent disadvantages of this test (8, 21). In contrast, capture immunoglobulin M (IgM) and IgG enzyme-linked immunosorbent assays (ELISAs) have become the most powerful assays for the detection and differentiation of primary and secondary dengue virus infections due to high sensitivity, high specificity, and simplicity (8, 9). We recently simplified the capture IgM and IgG ELISA originally developed by Innis et al. and used the modified method for the routine diagnosis of various flaviviruses (21).
For dengue virus serotyping, available methods include (i) virus isolation and subsequent identification with either type-specific monoclonal antibody immunofluorescence staining (23) or reverse transcription-PCR (RT-PCR) (10), (ii) RT-PCR and/or nucleotide sequencing, (iii) serotype-specific antigen capture ELISA (commercial kit, denTYPE RED from Globio Co, Beverly, Mass.), (iv) neutralization test (16), (v) envelope and membrane (E/M)-specific capture IgM ELISA (1, 15), (vi) NS1 serotype-specific IgG ELISA (19, 21), and (vii) recombinant antigens-based immunoblot strips dotted with the B domains of dengue virus serotypes 1 to 4 (11). The first three methods are used to identify serotype-specific antigenic determinants or nucleotide sequences in acute-phase serum samples, while the other four methods are used to analyze dengue virus serotype-specific IgM and/or IgG antibodies in acute- and convalescent-phase serum samples. Among these methods, virus isolation and characterization, RT-PCR, and the neutralization test were widely used and considered to be gold standards for dengue virus serotype analysis. However, only virus isolation and RT-PCR can be reliably used to detect the dengue virus serotypes of both primary and secondary dengue virus infections.
Burke first reported serotype specificity of IgM to dengue virus by IgM capture immunoassay with convalescent-phase serum and the four serotypes of dengue virus antigen in 1983 (1). He found that serotype-specific IgM responses corresponding to the virus type isolated for all 16 primary-infection patients but only 9 of 16 secondary-infection patients. Many laboratories, however, had difficulty in confirming this finding. Therefore, the reliability and usefulness of this serotype-specific IgM capture immunoassay remained uncertain. More recently, Nawa et al. analyzed serum samples from 14 confirmed dengue patients without knowledge of their immune status. They found that IgM responses were generally serotype cross-reactive but that in most cases IgM levels were highest against the infecting dengue virus serotype (15). However, the difficulty in reproducing these results in many laboratories suggests that dengue virus serotyping by capture IgM ELISA is not easy to perform.
Recently, Ludolfs et al. reported the serological differentiation of infections with dengue virus serotypes 1 to 4 by using recombinant antigens (11). Immunoblot strips dotted with the B domains of dengue virus serotypes 1 to 4 expressed in Escherichia coli were used to detect serotype-specific antibodies in paired serum samples from 41 patients with primary and secondary dengue virus infections. Although some cross-reactivity with heterologous dengue virus antigens was observed, the results showed that correct serotyping was possible for 31 of 33 dengue cases with primary infection. However, serotyping is not reliable for secondary dengue virus infection by this method.
We have been interested in developing rapid and reliable assays for dengue virus serotyping. Successful dengue virus serotyping based on a real-time one-step RT-PCR and an NS1 serotype-specific IgG ELISA was reported recently (19, 20, 21). The results showed that NS1 serotype-specific IgG ELISA could be reliably used for dengue virus serotyping for convalescent-phase and postinfection serum from a primary infection but not for convalescent-phase serum from a secondary infection (21). The results also showed that NS1 serotype-specific IgG ELISA could replace the neutralization test for a seroepidemiologic study to differentiate Japanese encephalitis and dengue virus infections, primary and secondary dengue virus infections, and the dengue virus serotype of the primary infection (19). In this study, we established and compared E/M and NS1 serotype-specific capture IgM ELISAs in the differentiation of dengue virus serotypes with serum samples from primary and secondary infections.
MATERIALS AND METHODS
Human serum samples.
The serum samples used in this study were collected from patients with confirmed cases of dengue reported to the Center for Disease Control, Department of Health, Taipei, Taiwan, Republic of China, from 1998 to 2002. Dengue virus infections were defined as febrile illness associated with the isolation of dengue virus, a positive RT-PCR test (conventional nested RT-PCR or real-time one-step RT-PCR), or the detection of dengue virus-specific IgM and IgG antibodies (E/M-specific capture IgM and IgG ELISA and/or E/M-specific indirect IgG ELISA) (18, 20, 21). A total of 93 anti-dengue virus IgM-positive serum samples collected between days 5 and 45 of illness from 59 confirmed dengue patients were analyzed. Among these, 72 serum samples were from primary dengue virus infection and 21 serum samples were from secondary dengue virus infection, based on dual analyses of E/M-specific capture IgM/IgG optical density (OD) ratio and NS1 serotype-specific IgG ELISA (21). The dengue virus serotypes of the serum samples tested were confirmed by RT-PCR. Serum samples collected during days 1 to 7 after the onset of symptoms are referred to as acute-phase samples. Early- and late-convalescent-phase serum samples refer to specimens collected during days 8 to 13 and days 14 to 45, respectively.
Cell culture and antigen preparation.
Culture supernatants of Vero cells infected with one of the four dengue virus serotypes of local Taiwan strains, i.e., DEN-1 (strain 8700828), DEN-2 (454009), DEN-3 (8700829), and DEN-4 (8700544), or with a Japanese encephalitis virus vaccine strain (Beijing) were prepared and used as the source of viral E/M and NS1 antigens for ELISA as previously described (21). The control antigen was prepared by the same procedure from an uninfected Vero cell culture.
Monoclonal antibody production and purification.
D2/8-1 and D56-3 are flavivirus group-specific monoclonal antibodies directed against the NS1 and envelope antigens, respectively (2, 21). Monoclonal antibodies were purified from ascitic fluid by protein A-Sepharose 4B Fast Flow affinity chromatography (Pharmacia Biotech) as described previously (17).
ELISA. (i) NS1 serotype-specific IgG ELISA.
NS1 serotype-specific IgG ELISA was performed as previously described (17).
(ii) E/M-specific capture IgM and IgG ELISA.
The E/M-specific capture IgM and IgG ELISAs originally developed by Innis et al. were modified and performed as previously described (8, 21). The simplified ELISA has the advantage that primary and secondary dengue virus infection can be defined by the OD ratio of the IgM and IgG readings (≥1.2 and <1.2, respectively) directly without calculating the antibody units through the standard control.
(iii) E/M serotype-specific capture IgM ELISA.
For E/M serotype-specific capture IgM ELISA, microtiter wells were incubated with 100 μl of a cocktail containing 1 μg of monoclonal antibody D56-3 per ml and diluted culture supernatant of DEN-1-, DEN-2-, DEN-3-, DEN-4-, or Japanese encephalitis virus-infected Vero cells.
(iv) NS1 serotype-specific capture IgM ELISA.
The assay of NS1 serotype-specific capture IgM ELISA is similar to that for E/M serotype-specific capture IgM ELISA with two differences: monoclonal antibody D2/8-1 was used in the NS1 capture IgM ELISA, and patient serum samples were used at a 1:20 dilution.
Data analysis.
The ODs read from culture supernatants of Vero cells with and without dengue virus infection were designated the test absorbance and negative control value, respectively, for each sample in the ELISA. Positivity was determined by comparison to individual negative controls. A positive sample was defined as having a test absorbance/negative control ratio of ≥2.0, and a negative sample was defined as having a ratio of <2.0. For serum samples with positive E/M- or NS1-specific IgM antibody responses, E/M or NS1 serotyping was determined by the ratio of the highest OD value to the second highest OD value read for the four dengue virus serotypes. Positive serotype specificity was defined as an OD ratio of ≥1.2, and negative serotype specificity was defined as an OD ratio of <1.2. The serotype specificity of the E/M and NS1 serotype-specific capture IgM ELISAs was calculated as the total number of serum samples with positive serotype specificity divided by the total number of serum samples tested. The serotype specificity based on dual analyses of these two assays was calculated when either the E/M or NS1 ELISA ratio was ≥1.2.
RESULTS
Establishment of E/M and NS1 serotype-specific capture IgM ELISAs.
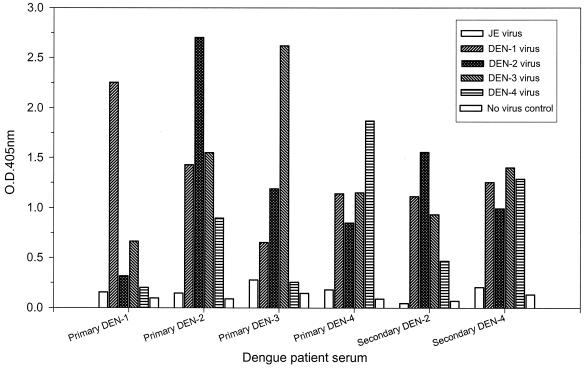
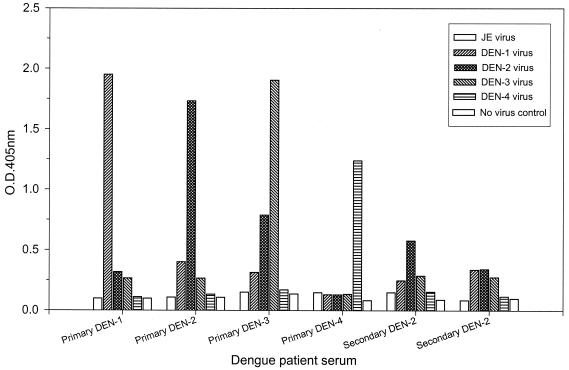
To address the question whether IgM capture immunoassay can be reliably used for dengue virus serotype analysis, we established E/M and NS1 serotype-specific capture IgM ELISAs with flavivirus group-specific monoclonal antibodies D56-3 (E specific) and D2/8-1 (NS1 specific), respectively. Culture supernatants harvested from DEN-1, DEN-2, DEN-3, and DEN-4 virus-infected Vero cells were carefully titrated and used as the antigens as previously described (17). It is important to emphasize that equivalent dengue virus E/M or NS1 antigens should be added to ensure reliable results. Figures 1 and 2 show representative data for the E/M and NS1 serotype-specific capture IgM ELISAs, respectively, with serum samples from patients with primary or secondary dengue virus infection. The results showed that (i) the E/M-specific IgM response was always earlier and higher than the NS1-specific IgM response, (ii) although the dengue virus-specific IgM responses were generally serotype cross-reactive, the IgM levels were always highest against the infecting dengue virus serotype in primary dengue virus infection, and (iii) the IgM levels were usually lower and the OD read from the infecting dengue virus serotype may not be the highest in secondary dengue virus infection.
FIG. 1.
Dengue virus serotyping by E/M serotype-specific capture IgM ELISA. Serum samples collected between days 5 and 45 of illness after primary infection with DEN-1, DEN-2, DEN-3, or DEN-4 or secondary dengue virus infection were analyzed, and representative data are shown to illustrate the E/M serotype-specific IgM antibody responses. JE, Japanese encephalitis.
FIG. 2.
Dengue virus serotyping by NS1 serotype-specific capture IgM ELISA. Serum samples collected between days 5 and 45 of illness after primary infection with DEN-1, DEN-2, DEN-3, or DEN-4 or secondary dengue virus infection were analyzed, and representative data are shown to illustrate the NS1 serotype-specific IgM antibody responses. JE, Japanese encephalitis.
Comparisons of E/M and NS1 serotype-specific capture IgM ELISAs in the serotype analysis of primary and secondary dengue virus infection.
A total of 93 anti-dengue virus IgM-positive serum samples collected between days 5 and 45 of illness from 59 confirmed dengue patients were analyzed. Among these, 72 serum samples were from primary dengue virus infection and 21 serum samples were from secondary dengue virus infection, based on dual analyses of the E/M-specific capture IgM/IgG OD ratio and NS1 serotype-specific IgG ELISA (data not shown). Table 1 shows a summary of the overall analyses. Positive serotype specificity could be identified for 86.1 and 47.6% of serum samples tested for E/M-specific IgM antibodies, versus 83.3 and 42.9% of serum samples tested for NS1-specific IgM antibodies from patients with primary and secondary dengue virus infections, respectively. Dual analyses with both E/M and NS1 serotype-specific capture IgM ELISAs showed that positive serotype specificity could be correctly identified for 98.6 and 61.9% of all of the primary and secondary serum samples tested, respectively.
TABLE 1.
Comparison of dengue virus E/M and NS1 serotype-specific capture IgM ELISAs for patients with primary and secondary dengue virus infections
| Type of infection | Virus serotype | No. of serum samples testeda | % Serotype specificity (no. of samples positive/no. tested)
|
||
|---|---|---|---|---|---|
| E/M capture IgM ELISA | NS1 capture IgM ELISA | E/M or NS1 capture IgM ELISAb | |||
| Primary | DEN-1 | 14 | 64.3 (9/14) | 78.6 (11/14) | 92.9 (13/14) |
| DEN-2 | 42 | 90.5 (38/42) | 97.6 (41/42) | 100 (42/42) | |
| DEN-3 | 8 | 87.5 (7/8) | 75 (6/8) | 100 (8/8) | |
| DEN-4 | 8 | 100 (8/8) | 25 (2/8) | 100 (8/8) | |
| All four | 72 | 86.1 (62/72) | 83.3 (60/72) | 98.6 (71/72) | |
| Secondary | DEN-1 | 5 | 60 (3/5) | 60 (3/5) | 80 (4/5) |
| DEN-2 | 9 | 44.4 (4/9) | 55.6 (5/9) | 66.7 (6/9) | |
| DEN-3 | 5 | 60 (3/5) | 20 (1/5) | 60 (3/5) | |
| DEN-4 | 2 | 0 (0/2) | 0 (0/2) | 0 (0/2) | |
| All four | 21 | 47.6 (10/21) | 42.9 (9/21) | 61.9 (13/21) | |
Serum samples were collected between days 5 and 45 of illness.
Both anti-E/M and anti-NS1 IgM antibody-positive serum samples were included in the calculation of dengue virus serotype specificity.
Careful analyses showed the complementary results of these two assays and indicated that (i) positive serotype specificity could be obtained by both assays for the majority of serum samples tested, (ii) a few serum samples could only be correctly serotyped by one of these two assays, and (iii) the serotype specificity of the NS1-specific IgM ELISA is usually higher than that of the E/M-specific IgM ELISA. The above data also supported the previous two reports by Burke and Nawa et al. (1, 15) showing that dengue virus serotyping by E/M-specific IgM capture immunoassay is possible. Together with our previous study on dengue virus serotyping by NS1 serotype-specific IgG ELISA, there are now three different ELISA systems that can be reliably used for dengue virus serotype analysis. Figure 3 shows representative data for two patients with a primary DEN-1 and a primary DEN-2 infection analyzed by these three different assays. The results showed good correlation among the three assays.
FIG. 3.
Dengue virus serotyping by NS1 serotype-specific IgG ELISA and E/M- and NS1 serotype-specific capture IgM ELISAs. Representative data for convalescent-phase serum samples from a primary DEN-1 patient and a primary DEN-2 patient were analyzed and are shown to illustrate the usefulness of the three different assays in serotyping of primary dengue virus infection. JE, Japanese encephalitis.
DISCUSSION
It is worth emphasizing that although the E/M-specific IgG antibodies elicited by viruses from different flavivirus serocomplexes were highly cross-reactive in secondary response, the IgM antibodies elicited by viruses from different serocomplexes were complex specific (8, 13, 14, 18, 22). Tardei et al. demonstrated that the capture IgM ELISA had acceptable sensitivity and specificity, showing little cross-reactivity with West Nile encephalitis virus-elicited IgM with members of the yellow fever, tick-borne encephalitis, and dengue virus serocomplexes of flaviviruses (22). More recently, Martin et al. showed significant cross-reactivity of IgM antibodies to various Japanese encephalitis serocomplex viruses, but the specificity of the capture IgM ELISA is sufficient to differentiate West Nile encephalitis virus infections from St. Louis encephalitis virus (and Japanese encephalitis virus) infections (13). We have found that the E/M-specific capture IgM ELISA can be used reliably for the differential diagnosis of Japanese encephalitis, dengue, yellow fever, and West Nile encephalitis infections by using a panel of virus-infected culture supernatants as the source of viral antigens and flavivirus-specific mouse monoclonal antibodies as detection antibodies (unpublished data).
Although dengue virus serotyping is important in epidemiological and pathological studies, it is not always easy to correctly identify the infecting serotype by serological methods. This is especially true for secondary dengue virus infection. The uncertainty is high due to (i) strong cross-reactivity of dengue virus-specific IgM antibodies raised against the four dengue virus serotypes and (ii) much stronger cross-reactivity between E/M-specific IgG antibodies raised against various flavivirus. Previous studies have shown that it is possible but difficult to identify dengue virus serotypes in serum samples of primary and/or secondary dengue virus infections by E/M-specific capture IgM ELISA (1, 4, 9, 15). The report from Nawa et al. is particularly interesting in that IgM responses were generally serotype cross-reactive but IgM levels were highest against the infecting dengue virus serotype in 9 of the 13 confirmed cases (15). It is tempting to speculate that most of the serum samples analyzed in that study were from primary dengue patients, since Japan is not an area where dengue is endemic. Therefore, most of the dengue patients could be imported cases with primary dengue virus infection (I. Kurane, personal communication).
For secondary dengue virus infection, dengue virus serotyping by serological methods is even more difficult. Although the traditional assay, the plaque reduction neutralization test, remains the gold standard and is highly specific in the identification of dengue virus serotypes in primary infections, this method is time-consuming and difficult to perform and not as amenable to testing large numbers of serum samples. Furthermore, although relatively specific neutralizing antibodies (most are IgG antibodies) are detected in early convalescence following primary infection, high-titer neutralizing antibodies against at least two and usually four dengue virus serotypes, as well as against other flaviviruses, are produced following secondary infections (6, 12). Therefore, the analysis of IgM antibody may be a better choice for secondary dengue virus infection because the IgM response has no immune memory. Interestingly, Burke reported serotype-specific IgM responses corresponding to the virus type isolated for all 16 primary patients but only 9 of 16 secondary patients (1). Similar results were observed in our study.
Theoretically, both IgM and IgG antibody responses based on serotype-specific epitopes can be used for dengue virus serotyping with either synthetic peptides or recombinant fragments. It has been very difficult if not impossible to perform serotype analysis based on synthetic peptides. Recently, Ludolfs et al. reported correct serotyping based on recombinant antigens with the B domains of dengue virus serotypes 1 to 4 expressed in Escherichia coli in primary dengue patients (11). However, dengue virus serotyping is not reliable for secondary dengue virus infection with this method, mainly because both IgM and IgG antibodies are measured in secondary dengue virus infection and the recombinant fragments contained many common epitopes in addition to the serotype-specific epitopes. Theoretically, this assay could be improved if only IgM antibodies are measured because the highly cross-reactive IgG antibodies would be eliminated and only IgM antibodies with a relatively low cross-reactivity would be detected with the recombinant fragments as antigens. Obviously, the sensitivity of this assay would be reduced significantly if only IgM antibodies were measured.
In this study, we evaluated the E/M and NS1 serotype-specific capture IgM ELISAs for the differentiation of dengue virus serotypes with serum samples from primary and secondary dengue patients. The results demonstrated the usefulness of these two assays in dengue virus serotyping. Although the dengue virus-specific IgM response was found to be serotype cross-reactive, the highest response was always directed against the infecting serotype in primary dengue virus infection. Therefore, correct dengue virus serotyping is possible in almost all (98.6%) and in about 62% of serum samples from primary and secondary dengue virus infections, respectively.
Compared to NS1 serotype-specific IgG ELISA, the serotype-specific capture IgM ELISA has the advantage that dengue virus serotypes from more than half of the patients with secondary infections can be correctly identified. In contrast, the NS1 serotype-specific IgG ELISA has the advantages of differentiating primary and secondary dengue virus infections and determining the dengue virus serotypes of the primary dengue virus infections. Most important, the NS1 serotype-specific IgG ELISA can be reliably used for seroepidemiologic studies to analyze the seroprevalence of dengue virus infection among various age groups (19). In summary, our results demonstrate that the E/M and NS1 serotype-specific capture IgM ELISAs have the potential to be of use in dengue virus serotyping.
Acknowledgments
We thank Hsiu-Ling Pan, Chih-Heng Chen, and Tong-Huei Chen for expert technical assistance.
This work was in part supported by grants DOH91-DC-2016 and DOH92-DC-2005 from the Center for Disease Control, Department of Health, Taipei, Taiwan, Republic of China.
REFERENCES
- 1.Burke, D. S. 1983. Rapid methods in the laboratory diagnosis of dengue virus infections, p. 72-84. In T. Pang and T. Pathmananathan (ed.), Proceedings of the International Conference on Dengue/Dengue Hemorrhagic Fever. University of Malaya, Kuala Lumpur, Malaysia.
- 2.Chen, L. K., C. L. Liao, C. G. Lin, S. C. Lai, C. I. Liu, S. H. Ma, Y. Y. Huang, and Y. L. Lin. 1996. Persistence of Japanese encephalitis virus is associated with abnormal expression of nonstructural protein NS1 in host cells. Virology 217:220-229. [DOI] [PubMed] [Google Scholar]
- 3.Gubler, D. J. 1997. Dengue and dengue hemorrhagic fever: its history and resurgence as a global public health problem, p. 1-22. In D. J. Gubler and G. Kuno (ed.), Dengue and dengue hemorrhagic fever. CAB International, New York, N.Y.
- 4.Gubler, D. J. 1998. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 11:480-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halstead, S. B., H. Shotwell, and J. Casals. 1973. Studies on the pathogenesis of dengue infection in monkeys II. Clinical laboratory responses to heterologous infection. J. Infect. Dis. 128:15-22. [DOI] [PubMed] [Google Scholar]
- 6.Halstead, S. B., S. Rojanasuphot, and N. Sangkawibha. 1983. Original antigenic sin in dengue. Am. J. Trop. Med. Hyg. 32:154-156. [DOI] [PubMed] [Google Scholar]
- 7.Halstead, S. B. 1988. Pathogenesis of dengue: challenge to molecular biology. Science 239:476-481. [DOI] [PubMed] [Google Scholar]
- 8.Innis, B. L., A. Nisalak, S. Nimmannitya, S. Kusalerdchariya, V. Chongswasdi, S. Suntayakorn, P. Puttisri, and C. H. Hoke. 1989. An enzyme-linked immunosorbent assay to characterize dengue infections where dengue and Japanese encephalitis co-circulate. Am. J. Trop. Med. Hyg. 40:418-427. [DOI] [PubMed] [Google Scholar]
- 9.Innis, B. L. 1997. Antibody responses to dengue virus infection, p. 221-243. In D. J. Gubler and G. Kuno (ed.), Dengue and dengue hemorrhagic fever. CAB International, New York, N.Y.
- 10.Lanciotti, R. S., C. H. Calisher, D. J. Gubler, G. J. Chang, and A. V. Vorndam. 1992. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J. Clin. Microbiol. 30:545-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ludolfs, D., S. Schilling, J. Altenschmidt, and H. Schmitz. 2002. Serological differentiation of infections with dengue virus serotypes 1 to 4 by using recombinant antigens. J. Clin. Microbiol. 40:4317-4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makino, Y., M. Tadano, M. Saito, N. Maneekarn, N. Sittisombut, V. Sirisanthana, B. Poneprasert, and T. Fukunaga. 1994. Studies on serological cross-reaction in sequential flavivirus infections. Microbiol. Immunol. 38:951-955. [DOI] [PubMed] [Google Scholar]
- 13.Martin, D. A., B. J. Biggerstaff, B. Allen, A. J. Johnson, R. S. Lanciotti, and J. T. Roehrig. 2002. Use of immunoglobulin M cross-reactions in differential diagnosis of human flaviviral encephalitis infections in the United States. Clin. Diagn. Lab. Immunol. 9:544-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monath, T. P., R. R. Nystrom, R. E. Bailey, C. H. Calisher, and D. J. Muth. 1984. Immunoglobulin M antibody capture enzyme-linked immunosorbent assay for diagnosis of St. Louis encephalitis. J. Clin. Microbiol. 20:784-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nawa, M., K. I. Yamada, T. Takasaki, T. Akatsuka, and I. Kurane. 2000. Serotype-cross-reactive immunoglobulin M responses in dengue virus infections determined by enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 7:774-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russell, P. K., A. Nisalak, P. Sukhavachana, and S. Vivona. 1967. A plaque reduction test for dengue virus neutralizing antibodies. J. Immunol. 99:285-290. [PubMed] [Google Scholar]
- 17.Shu, P. Y., L. K. Chen, S. F. Chang, Y. Y. Yueh, L. Chow, L. J. Chien, C. Chin, T. H. Lin, and J. H. Huang. 2000. Dengue NS1-specific antibody responses: isotype distribution and serotyping in patients with dengue fever and dengue hemorrhagic fever. J. Med. Virol. 62:224-232. [DOI] [PubMed] [Google Scholar]
- 18.Shu, P. Y., L. K. Chen, S. F. Chang, Y. Y. Yueh, L. Chow, L. J. Chien, C. Chin, T. H. Lin, and J. H. Huang. 2001. Antibody to the nonstructural protein NS1 of Japanese encephalitis virus: potential application of mAb-based indirect ELISA to differentiate infection from vaccination. Vaccine 19:1753-1763. [DOI] [PubMed] [Google Scholar]
- 19.Shu, P. Y., L. K. Chen, S. F. Chang, Y. Y. Yueh, L. Chow, L. J. Chien, C. Chin, H. H. Yang, T. H. Lin, and J. H. Huang. 2002. Potential application of nonstructural protein NS1 serotype-specific immunoglobulin G enzyme-linked immunosorbent assay in the seroepidemiologic study of dengue virus infection: correlation of results with those of the plaque reduction neutralization test. J. Clin. Microbiol. 40:1840-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shu, P. Y., S. F. Chang, Y. C. Kuo, Y. Y. Yueh, L. J. Chien, C. L. Sue, T. H. Lin, and J. H. Huang. 2003. Development of group- and serotype-specific one-step SYBR Green I-based real-time reverse transcription-PCR assay for dengue virus. J. Clin. Microbiol. 41:2408-2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shu, P. Y., L. K. Chen, S. F. Chang, Y. Y. Yueh, L. Chow, L. J. Chien, C. Chin, T. H. Lin, and J. H. Huang. 2003. Comparison of capture immunoglobulin M (IgM) and IgG enzyme-linked immunosorbent assay (ELISA) and nonstructural protein NS1 serotype-specific IgG ELISA for differentiation of primary and secondary dengue virus infections. Clin. Diagn. Lab. Immunol. 10:622-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tardei, G., S. Ruta, V. Chitu, C. Rossi, T. F. Tsai, and C. Cernescu. 2000. Evaluation of immunoglobulin M (IgM) and IgG enzyme immunoassay in serologic diagnosis of West Nile virus infections. J. Clin. Microbiol. 38:2232-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. 1997. Dengue haemorrhagic fever: diagnosis, treatment, prevention and control, 2nd ed. World Health Organization, Geneva, Switzerland.
- 24.World Health Organization. 1999. Strengthening implementation of the global strategy for dengue fever and dengue haemorrhagic fever, prevention and control. Report of the informal consultation, 18-20 October. World Health Organization, Geneva, Switzerland.