Abstract
Introduction:
Stevia (S. rebaudiana) a herb which has medicinal value and was used in ancient times as a remedy for a great diversity of ailments and sweetener. Leaves of Stevia contain a high concentration of Stevioside and Rebaudioside which are supposed to be sweetening agents.
Aim:
To compare the efficacy of aqueous and alcoholic S. rebaudiana extract against Streptococcus mutans and Lactobacillus acidophilus in comparison to chlorhexidine.
Materials and Methods:
In the first part of the study, various concentrations of aqueous and ethanolic Stevia extract were prepared in the laboratory of Pharmacy College. It was then subjected to microbiological assay to determine its zone of inhibition using Agar disk diffusion test and minimum inhibitory concentration (MIC) using serial broth dilution method against Streptococcus mutans and Lactobacillus acidophilus. Chlorhexidine was used as a positive control. One way Analysis of Variance (ANOVA) test was used for multiple group comparisons followed by Tukey post hoc for group wise comparisons.
Results:
Minimum inhibitory concentration (MIC) of aqueous and ethnolic Stevia extract against Streptococcus mutans and Lactobacillus acidophilus were 25% and 12.5% respectively. Mean zone of inhibition of the aqueous and alcoholic Stevia extracts against Streptococcus mutans at 48 hours were 22.8 mm and 26.7 mm respectively. Mean zone of inhibition of the aqueous and alcoholic Stevia extracts against Lactobacillus acidophilus at 48 hours were 14.4 mm and 15.1 mm respectively. Mean zone of inhibition of the chlorhexidine against Streptococcus mutans and Lactobacillus acidophilus at 48 hours was 20.5 and 13.2 respectively.
Conclusion:
The inhibitory effect shown by alcoholic Stevia extract against Streptococcus mutans and Lactobacillus acidophilus was superior when compared with that of aqueous form and was inferior when compared with Chlorhexidine.
Keywords: Chlorhexidine, Stevia, Stevia rebaudiana
INTRODUCTION
Dental caries is a chronic, infectious, transmissible, biobehavioral disease that extends throughout the life span. The essential process of this disease involves bacterial adherence to tooth surfaces, dental plaque formation, and localized demineralization of tooth enamel by acids of bacterial origin produced from the fermentation of dietary carbohydrates.[1]
More than a century back, W. D. Miller had postulated the chemico-parasitic theory for the etiology of dental caries. Until today, the modern concepts of cariogram demonstrate microorganisms as one of the major etiological factors for dental caries. Mutans streptococci are shown to be highly associated with caries in humans. Considerable evidence exists implicating Streptococcus mutans as an important etiological agent in initiation of enamel caries, both in laboratory animals and in humans. The presence of ≥106 mutans streptococci/ml saliva may indicate a high caries risk or activity.[2]
For preventing this disease in an individual, the focus should be on increasing the ability of the host to respond to the insult, decreasing the cariogenicity of the bacterial agents, and altering the diet to be less caries promoting.
Renewed interest in developing an antimicrobial approach for the management of dental caries has evolved as a result of: (1) Identifying certain members of the oral microflora as major cariogens and (2) increased understanding of the specific ecology of these cariogens. In conjunction with this concept, control and prevention of caries has been sought by reducing the number of colonizing bacteria. Reducing their level in the oral cavity will provide an additional rationale for the prevention of dental caries.[2]
Research in the field of caries prevention has been focusing on ways for reducing or totally eradicating cariogenic flora from the oral cavity. Studies have shown that caries can be prevented by regular tooth brushing and flossing. However, most of the studies have shown it difficult to eliminate S. mutans from the pits, fissures, and approximal surfaces by mechanical means alone. For effective caries control, these methods should be combined with the chemoprophylactic agents. These agents, e.g., chlorhexidine and antibiotics, act by lowering the number of microorganisms or inhibiting dental plaque formation. However, they have several undesirable side effects, including tooth staining and emergence of bacterial resistance. These side effects stimulate the search for alternative agents.[1]
Ayurveda is the traditional medicinal form prevalent in India since 2000 BC. The Ayurvedic treatment is entirely based on herbs, which have certain medicinal value or property. In the ancient times, the Indian sages believed that Ayurvedic herbs are one-stop solutions to cure a number of health-related problems and diseases. They studied about the herbs thoroughly and experimented with herbs to arrive at accurate conclusions about the efficacy of different plants and herbs that have medical value. Most of the Ayurvedic herbs, thus formulated, are free of side effects or reactions. This is the reason why Ayurveda is growing in popularity across the globe. The Ayurvedic herbs that have medicinal quality provide rational means for the treatment of many internal diseases which are otherwise considered incurable in other systems of medicine.[3] In 2003, according to the World Health Organization, the use of traditional herbal medicines has spread not only in developing countries but also in the industrialized ones, as a complementary way to treat and prevent illnesses.[1]
Plant extracts constitute rich sources of novel compounds with a variety of pharmacological activities. In many countries, plant extracts have been traditionally used for the treatment of oral mucosal lesions and periodontal diseases without any scientific validation.[1]
Herbal extracts have been successfully used in dentistry as tooth cleaning and antimicrobial plaque agents, as most of the oral diseases are due to bacterial infections and it has been well documented that medicinal plants confer considerable antibacterial activity against various microorganisms including the bacteria responsible for dental caries. The antibacterial activities of some plant species like Melia azadirachta, Calotropis gigantea, Leucas aspera, Vitex negundo, and others have been tested. In India, plant wealth is greatly exploited for its therapeutic potential and medicinal efficacy to cure dental caries. It includes M. azadirachta, Moringa pterygosperma, and Balsamodendron mukul. The stem, bark, root, and young fruits of M. azadirachta are used for their Bitter, tonic, antiseptic, astringent, and antibacterial properties. In several indigenous tooth powders, toothpastes, and toilet soaps, the extract from various parts of this tree is used. The use of neem twigs as tooth brush has been endorsed by the dentists to prevent caries. Azadirachta indica mouth wash is reported to inhibit the growth of S. mutans and carious lesions.[4]
The main aim of research into medicinal plants is to identify the plants that can potentially exhibit pharmacological activity and thus discover new substances or molecules having antimicrobial activity that could be modulated as various medications and, therefore, control or prevent infectious diseases.
One such shrub that has medicinal value is Stevia rebaudiana (Stevia). The leaves have been traditionally used for hundreds of years in both Brazil and Paraguay to sweeten local teas and medicines.[5] In addition to some flavonoids, the leaves of Stevia contain a high concentration of stevioside and rebaudioside, the concentration of which varies between 2.5 and 9% according to the source and work-up of the drug.
With its steviol glycoside extracts having up to 300 times the sweetness of sugar, Stevia has attracted attention with the rise in demand for low-carbohydrate, low-sugar sweeteners. Because Stevia has a negligible effect on blood glucose, it is attractive to people on carbohydrate-controlled diets.[5]
Studies have reported the antimicrobial effect of Stevia extracts on fungi and various bacteria. Hardly few studies have been reported on evaluation of the effect of Stevia extracts on S. mutans and Lactobacillus acidophilus and none of the studies have compared them with chlorhexidine.
The study was conducted with an aim to compare the efficacy of aqueous and alcoholic Stevia extracts against S. mutans and L. acidophilus, in comparison to chlorhexidine.
MATERIALS AND METHODS
A standard procedure was followed for finding the minimum inhibitory concen tration (MIC). Dried Stevia leaf extract powder was procured from M/s Amruth Kesari Depot, Bangalore, Karnataka.
The study was carried out for a period of 4 months from June to October 2013. Ethical permission was obtained from Ethical Clearance Committee, KVG Dental College, Sullia.
The materials used in this study were as follows.
-
Test materials used
- Stevia leaf powder
- Aqueous solution
- Ethanol
- Chlorhexidine (0.2%).
-
Microorganisms
- S. mutans ATCC 25175
- L. acidophilus ATCC 4356
The microbial strains selected for the present study were collected from the American Type Culture Collection (ATCC), USA.
Brain heart infusion agar
Vernier caliper.
Preparation of Stevia aqueous extract
Stevia powder was identified by a botanist and a pharmacognosist for its authenticity at the Department of Pharmacognosy, Bapuji College of Pharmacy, Davangere. The powder obtained thus was weighed up to 50 g and then mixed with 100 ml of sterile distilled water in a round bottom flask with occasional shaking. The extract was then filtered through a muslin cloth for coarse residue and finally through Whatman No. 1 filter paper and kept in an airtight amber-colored container.
Preparation of Stevia ethanolic extract
Stevia extract was prepared by macerating 50 g of dry powder with 100 ml of 70% (w/v) ethyl alcohol for a week in a round bottom flask with occasional shaking. The extract was then filtered through a muslin cloth for coarse residue and finally through Whatman No. 1 filter paper and was stored at 4°C for further use. Stock solutions of the crude extracts were prepared by mixing well appropriate amount of dried extracts with an inert solvent dimethyl sulfoxide to obtain the final concentrations.
Microbial analysis
Revival of the organisms
The bacterial strains from the stock were revived by plating on blood agar medium. After overnight incubation at 37°C, isolated colonies were selected and the identities of the organisms were confirmed. Isolated colonies were transferred to sterile Brain Heart Infusion (BHI) broth for bacteria and once again incubated overnight. The growth concentration was adjusted to 105 organisms/ml by using 0.5 McFarland's turbidity standard.
Agar ditch plate method for testing the antibacterial properties
Agar well diffusion assay was used to evaluate the antimicrobial potential of the extracts. Petri dishes containing 18 m1 of BHI agar for S. mutans and L. acidophilus were inoculated with approximately 100 μl of microbial strain using swab technique.
Wells of 8 mm diameter were cut into solidified agar media using a sterilized standard device. One hundred microliters of each extract was poured in the respective well and the plates were incubated at 37°C for 48 h. To ensure the consistency of all findings, the experiment was performed and repeated under strict aseptic conditions. The antibacterial activity of each extract was expressed in terms of the mean of diameter of zone of inhibition (in mm) produced by each extract at the end of incubation period.
MIC determination for the aqueous Stevia extract
Two hundred microliters of the BHI broth was added to each of 10 MIC tubes per bacterial strain. In the first MIC tube containing 200 μl broth, 200 μl of stock was added. After mixing well, 200 μl was transferred to the second MIC tube. This was continued till the last (10th) tube. From the last tube, 200 μl of final solution was discarded. By following this serial dilution, the concentration of the Stevia powder achieved was as follows: 50%, 25%, 12.5%, 6.25%, 3.1%, 1.56%, 0.78%, 0.39%, 0.19%, and 0.09%, respectively. To each of these 10 MIC tubes prepared with varying concentrations, 200 μl of the earlier prepared strain of S. mutans was added such that the final volume per tube was 400 μl.
After incubation, the MIC values were determined by visual inspection of the tubes. With each batch of tests, positive and negative controls were put up. Positive control containing broth plus bacterial strain showed turbidity and negative control containing the broth only appeared clear. In each series of tubes, the last tube with a clear supernatant was considered to be without any growth and taken as the MIC value. Turbidity in the MIC tube indicated growth of the bacterial strain, implying that the organisms were resistant to the aqueous Stevia extract.
IMC determination for the alcoholic Stevia extract
A solution of 50% concentration was prepared as the stock solution. The working concentration of the extract was as follows: 50%, 25%, 12.5%, 6.25%, 3.1%, 1.56%, 0.78%, 0.39%, 0.19%, and 0.09%, respectively. A similar procedure of serial dilution, as mentioned above, was followed to test the antimicrobial effect of the alcoholic Stevia extract.
All measurements of the zone of inhibition were carried out by a single examiner. Calibration of the examiner was done prior to and during the study by re-examining 5% of the samples, to minimize intra-examiner variability. Intra-examiner agreement was determined using kappa statistics (k) and the score thus obtained (k = 0.84) was almost perfect, according to Landis and Koch, thus meeting the scientific requirement for validity and reliability.
Statistical analysis
The collected data were classified and tabulated in Microsoft Office Excel. SPSS for Windows version 17 software (Chicago, IL, USA) was employed for statistical analysis. Frequency distributions of responses to the questions were produced. Since the data were of continuous type, parametric tests were used for analysis. Mean (X) and Standard Deviation (SD) were calculated. One-way analysis of variance (ANOVA) test was used for multiple group comparisons, followed by Tukey post-hoc for group-wise comparisons, and P < 0.05 was considered statistically significant.
RESULTS
At the end of 48 h, statistically significant antimicrobial activity was demonstrated by all the test specimens used in this study (P = 0.002).
Table 1 shows the antimicrobial activity of the extracts against S. mutans at 48 h. Chlorhexidine showed the highest inhibition rate against S. mutans, compared with alcoholic and aqueous forms of Stevia. However, the alcoholic Stevia extract was found to be better than the aqueous form and the results were found to be statistically significant.
Table 1.
Antimicrobial activity of the extracts against Streptococcus mutans at 48 h
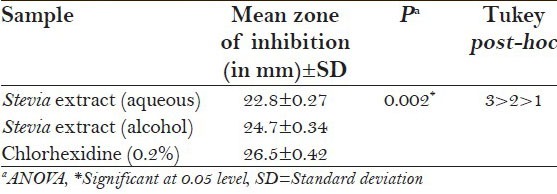
Table 2 shows the antimicrobial activity of the extracts against L. acidophilus at 48 h. Again chlorhexidine was found to be superior in inhibiting L. acidophilus, compared with alcoholic and aqueous forms of Stevia and the results were found to be statistically significant (P < 0.003). However, alcoholic Stevia extract was found to be closely competent enough with chlorhexidine but was much superior than the aqueous extract.
Table 2.
Antimicrobial activity of the extracts against Lactobacillus acidophilus at 48 h
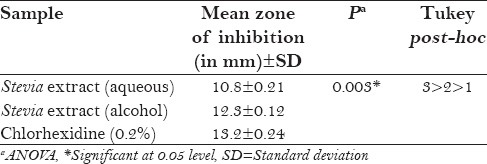
Table 3 shows the MIC of aqueous Stevia extract against S. mutans and L. acidophilus. S. mutans showed sensitivity to aqueous Stevia extract at concentrations of 50% and 25% and demonstrated resistance to concentrations of 12.5%, 6.25%, 3.1%, 1.56%, 0.78%, 0.39%, 0.19%, and 0.09%. L. acidophilus showed sensitivity to aqueous Stevia extract only at the concentration of 50% and demonstrated resistance to concentrations of 25%, 12.5%, 6.25%, 3.1%, 1.56%, 0.78%, 0.39%, 0.19%, and 0.09%. Hence, the MIC of aqueous Stevia extract for S. mutans was 25% and for L. acidophilus was 50%.
Table 3.
Minimum inhibitory concentration of aqueous Stevia extract against Streptococcus mutans
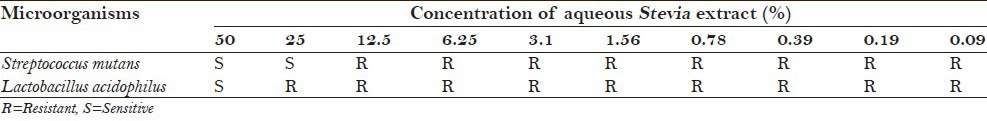
Table 4 shows the MIC of alcoholic Stevia extract against S. mutans and L. acidophilus. S. mutans showed sensitivity to aqueous Stevia extract at concentrations of 50%, 25%, and 12.5% and demonstrated resistance to concentrations of 6.25%, 3.1%, 1.56%, 0.78%, 0.39%, 0.19%, and 0.09%. L. acidophilus showed sensitivity to aqueous Stevia extract at concentrations of 50%, 25%, 12.5%, and 6.25% and demonstrated resistance to concentrations of 3.1%, 1.56%, 0.78%, 0.39%, 0.19%, and 0.09%. Hence, the MIC of alcoholic Stevia extract for S. mutans was 12.5% and for L. acidophilus was 6.25%.
Table 4.
Minimum inhibitory concentration of alcoholic Stevia extract against Lactobacillus acidophilus
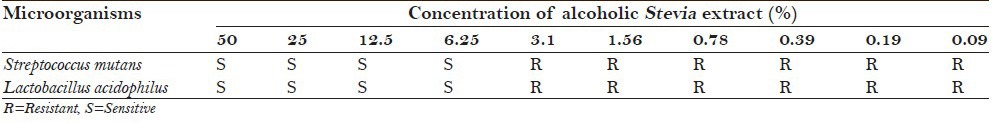
DISCUSSION
The current antimicrobial strategies used to treat dental caries consist primarily of mechanical removal of dental plaque or generalized killing of oral bacteria with antibacterial compounds. These remove-all, kill-all approaches have shown limited efficacy, since a cleaned tooth surface provides an equal opportunity for commensal and pathogenic bacteria to re-colonize in the non-sterile environment of the oral cavity. Cariogenic bacteria usually re-dominate the dental plaque after the treatment and start another cycle of cariogenesis. This study proposes to develop a targeted antimicrobial therapy against S. mutans. By selectively killing or inhibiting the cariogenic bacteria within a pathogenic dental plaque, a non-pathologic, commensal microbial community could be established. This healthy plaque would then serve as an effective barrier to prevent the subsequent colonization of cariogenic bacteria on the tooth surface, leading to a sustained anti-caries therapeutic effect.
Stevia is a green leafy plant that is native to South America. Whole or crushed Stevia leaves, or extract (either liquid or powder), or a refined version of the plant's isolated compounds can be used as a sweetener and they have medicinal value too. The two major sweet compounds that are isolated from the Stevia leaves, called as stevioside and rebaudioside, are hundred times sweeter than sugar.
A study was carried out to find the antibacterial activity of chloroform and methanol extracts of S. rebaudiana leaves against S. mutans and reported that the chloroform and methanol extracts of S. rebaudiana leaves exhibited a concentration-dependent antibacterial and antifungal activity. Acetone and ethanol extracts of S. rebaudiana leaves presented a better inhibitory effect on S. mutans.[6]
Another study evaluated the antibacterial activity of S. rebaudiana leaves extracted using various solvents against Escherichia coli, Bacillus subtilis, Staphylococcus aureus, Salmonella typhi, and Vibrio cholera, and it was found in the study that the acetone extract showed greater activity against Gram-positive bacteria than Gram-negative bacteria.[7]
In spite its use in medicinal field, less light has been shed for the use of Stevia in Dentistry. Therefore, this study was aimed to assess the antimicrobial effect of Stevia extracts on caries causative microorganisms, i.e. S. mutans and L. acidophilus, in comparison to chlorhexidine.
Chlorhexidine was considered as the positive control in this study. The bis-biguanide chlorhexidine (CHX), which has been studied extensively for over 25 years, is currently the most potent antimicrobial agent against mutans streptococci and dental caries. Its method of action has been comprehensively reviewed by Hugo, whose classical studies demonstrated that CHX concentrations is a potent membrane active against both Gram-positive and Gram -negative bacteria, including the release of K+, 260 nm-absorbing material, and pentose. It is also an inhibitor of adenosine triphosphatase activity. At higher bactericidal concentrations, CHX induces precipitation of cytoplasmic protein and nucleic acids. It blocks the activity of the phosphoenolpyruvate-phosphotransferase sugar transport system, and thereby markedly inhibits acid production in oral streptococci cariogenic bacteria in subjects with a high risk of developing caries.[2]
It was unanticipated that chlorhexidine would yield greater inhibition rates than Stevia extracts. Particularly, alcoholic Stevia extract was superior in inhibiting S. mutans and Lactobacillus, compared with the aqueous form.
The reason why the mean inhibition rates were more with alcoholic Stevia extract than the aqueous form is unknown. However, the reasons may be better solubility of the Stevia compound in alcohol or the very presence of alcohol. This finding is in agreement with the findings of other studies.[6],[8]
With respect to the MIC of Stevia extract, inhibition of S. mutans and L. acidophilus by alcoholic Stevia extract at lower concentration was superior when compared with the aqueous form. This may be due to better dissolving capacity in alcohol, better bioavailability (thus enhancing bioactivity), and polarity of the antibacterial compounds which makes the compounds to be more readily extracted by organic solvents.
However, a limitation of the study is that the study could have been conducted with the other group of 70% ethyl alcohol to clearly state that it is the effect of Stevia alone which has led to the inhibition of S. mutans and L. acidophilus and not that of alcohol.
CONCLUSION
The inhibitory effect shown by chlorhexidine against S. mutans and L. acidophilus was superior when compared with that of the aqueous extract and chlorhexidine. However, it was also observed that alcoholic Stevia extract was competent enough with chlorhexidine and was many times better than the aqueous form in inhibiting S. mutans and L. acidophilus.
Recommendations
Stevia is a popular alternative sweetener used by those who cannot use cane sugar
Leaves of Stevia plants have functional and sensory properties superior to those of many other high-potency sweeteners. Stevia is likely to become a major source of high-potency sweetener for the growing natural food market in the future. Although Stevia is useful to all, there are certain groups who are more likely to benefit from its remarkable sweetening potential.[9] Also, because of its antimicrobial property, it appears to offer protection against dental caries. For these reasons, cultivation and marketing of Stevia should be increased, wherein inclusion of liquorice can be considered in various food stuffs. Health concerns due to sucrose usage, obesity, and diabetes may also increase the demand of such sweeteners. A few developed countries are widely marketing confectionery incorporating this compound and other countries should follow suit.
If further studies show promise, the Stevia compounds could eventually be used as cavity-fighting components in mouthwash and toothpaste. Drug industries can also incorporate such extracts, which can be delivered as syrups or in other products. Stevioside can serve as an efficient vehicle for topical oral medications because of its agreeable sweet taste, excellent dispersing qualities, and its capability to form stable aqueous gels
Animal studies, in vivo studies, and large clinical trials have to be carried out to ascertain the effect of Stevia extract on microorganisms. Research assessing the action of Stevia extract on periodontal pathogens, other caries-causing microorganisms, and fungal species is recommended.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Mittal S, Hiregoudar M, Subramaniam R, Muralikrishna KS, Sakeenabi B, Prashant GM, et al. Dental effect of three herbal extracts against Streptococcus mutans and Lactobacillus acidophilus in comparison to chlorhexidine. J Indian Assoc Public Health Dent. 2011:336–40. [Google Scholar]
- 2.Pallavi SK. Effect of chlorhexidine on Mutans Streptococci and dental caries. J Indian Assoc Public Health Dentd. 2011:678–83. [Google Scholar]
- 3. [Last accessed on 2011 Oct 09]. Available from: http://ayurveda.iloveindia.com/herbology/medicinal-value-of-herbs.html .
- 4.Saini R, Sharma S, Saini S. Ayurveda and herbs in dental health. Ayu. 2011;32:285–6. doi: 10.4103/0974-8520.92542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. [Last accessed on 2014 Jul 12]. Available from: http://en.wikipedia.org/wiki/Stevia .
- 6.Debnath M. Clonal propagation and antimicrobial activity of an endemic medicinal plant Stevia rebaudiana. J Med Plants Res. 2008;2:45–51. [Google Scholar]
- 7.Sathishkumar J, Muthu Saravanan M, Seethalakshmi I. In-vitro antimicrobial and antitumor activities of stevia rebaudiana (asteraceae) leaf extracts. Trop J Pharm Res. 2008;7:1143–9. [Google Scholar]
- 8.Thadani MB, Subhash R. In vitro antimicrobial activity of Stevia rebaudiana bertoni leaves. Trop J Pharm Res. 2006;5:557–60. [Google Scholar]
- 9.Goyal SK, Samsher, Goyal RK. Stevia (Stevia rebaudiana) a bio-sweetener: A review. Int J Food Sci Nutr. 2010;61:1–10. doi: 10.3109/09637480903193049. [DOI] [PubMed] [Google Scholar]


