Abstract
Veronaea botryosa is a rare agent of human phaeohyphomycosis. We describe the first case of subcutaneous disease occurring in the United States, alert clinicians to the second report of a transplant-associated mycosis in a heart transplant recipient, extend the previously defined area of endemicity, and review the literature.
CASE REPORT
A 62-year-old orthotopic cardiac transplant recipient presented for evaluation of an area of chronic induration and tenderness over the dorsum of the right hand. The patient, who was transplanted for ischemic cardiomyopathy in March 2002, developed acute pain in the hand after an attempted intravenous line insertion in June 2002. Discomfort and gradual swelling over the dorsum of the hand persisted. There had been no bouts of rejection or significant changes in the patient's immunosuppressive regimen. Past medical issues included need for a pacemaker insertion posttransplant, renolithiasis, cholelithiasis with cholecystectomy, and a 20-mm PPD at the time of cardiac transplant, for which a course of prophylactic isoniazid was administered. The patient worked as a truck driver prior to his transplantation. He was an ex-smoker and had no active gardening, water, or soil exposure. Medications included trimethoprim-sulfamethoxazole, rapamycin, tacrolimus, pantoprazole, isradipine, pravachol, glimepiride, and aspirin. An empirical course of cephalexin therapy did not alter the patient's symptoms of hand tenderness or swelling. The dorsal aspect of the right hand revealed an area of induration, mild erythema, and intense tenderness. Incision and drainage of the area were performed on 25 September 2002. A 2- by 1.1-cm area of skin was excised along with a separate 4- by 2- by 1-cm pink-gray cyst. Histopathology of the excised lesion revealed a dermal cystic nodule with extensive granulomatous inflammation and multiple neutrophils with microabscess formation. Skin excision likewise revealed acute inflammation and microabscess formation. Both lesions contained moniliform hyphal elements which were brown on the hematoxylin and eosin stain. Special fungal stains, the Gomori methenamine silver stain (Fig. 1A) and the Fontana-Masson stain for melanized hyphae (Fig. 1B), were also positive. Antifungal therapy was initiated with itraconazole at 200 mg twice a day (BID) but was changed 1 week later to voriconazole, 200 mg BID, owing to gastrointestinal complaints. The patient self-discontinued therapy after approximately 10 weeks. On follow-up in August 2003 the lesions were resolved and pain was markedly decreased.
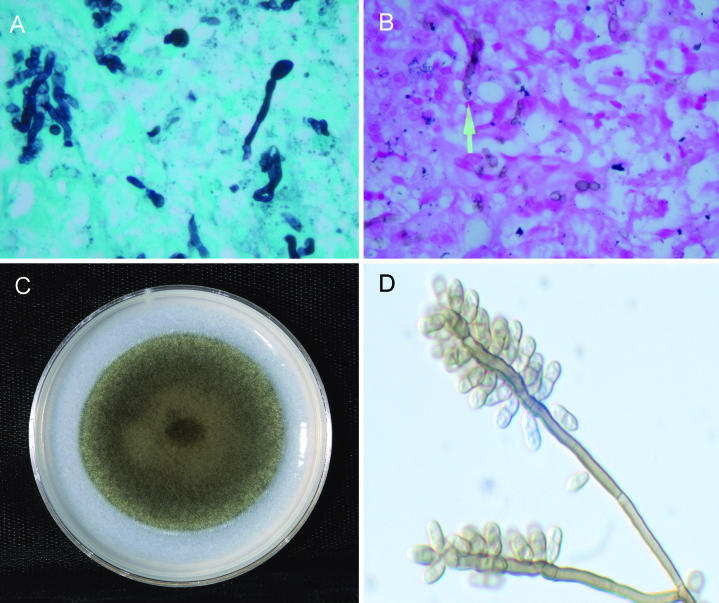
FIG. 1.
(A) Gomori methenamine silver stain of hyphae in tissue. (B) Fontana-Masson stain demonstrating melanized hyphae in tissue. (C) Colonial morphology on potato flake agar after 14 days of incubation at 25°C. (D) Branched conidiophores bearing numerous scars laterally and at the apex, producing predominately single-septate, brown, smooth-walled conidia with rounded apices and truncate bases.
Tissue from the surgical excision was placed on a Sabouraud dextrose agar plate, a Sabhi agar slant, and a Sabhi agar slant with chloramphenicol and gentamicin (BBL Microbiology Systems, Cockeysville, Md.). Colonies were visible on all media after 3 days of incubation and became dematiaceous. A potato dextrose agar slide culture (BBL Microbiology Systems) revealed a “Rhinocladiella-like” type of conidiation. The isolate was referred to the Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, where it was accessioned into their stock collection as UTHSC 02-2331. There it was subcultured onto potato flake agar, prepared in-house, for temperature studies, macroscopic morphology (Fig. 1C), and microscopic features (Fig. 1D). Physiologic testing included reactions on urease (Remel, Lenexa, Kans.) and nitrate agars (prepared in-house) and the organism's ability to grow on media containing cycloheximide (Mycobiotic; Remel) and 10 μg of benomyl (prepared in-house)/ml. In vitro antifungal susceptibility testing was performed in a macrobroth modification of the previously published National Committee for Clinical Laboratory Standards M38-A reference method for broth dilution antifungal susceptibility testing of filamentous fungi (12).
The organism was identified as Veronaea botryosa based upon the key features noted by Ellis (5) and de Hoog et al. (3), and the isolate has been deposited in the University of Alberta Microfungus Collection and Herbarium, Edmonton, Alberta, Canada, as UAMH 10374. Briefly, colonies were velvety to woolly and grayish- or blackish-brown centrally, with a dark green periphery. The reverse was olivaceous black. At 25°C on a 60-mm-diameter potato flake agar plate, the growth rate was moderately rapid, reaching 30 mm in 2 weeks (2.1 mm/day); slower growth occurred at 35°C, and no growth was visible at 40°C. Conidiophores were brown, septate, smooth, straight or flexuous, and branched or unbranched; measured up to 300 μm long by 2.5 to 4 μm wide; and appeared somewhat darker in the apical area. Smooth-walled, hyaline to pale brown, mostly two-celled conidia ranging from 2 to 4 by 5 to 12 μm, but predominately 3.5 by 8 μm with rounded apices and truncate bases, were borne from the geniculate apical conidia-bearing portion of the conidiogenous cell.
V. botryosa is a phaeoid mold first reported from olive slag in Italy. Among the numerous fungal species reported as agents of phaeohyphomycosis (2, 8), there is only a paucity of literature citations regarding this organism's ability to cause human disease. Four of the six previously published reports of subcutaneous mycoses have occurred in individuals with a history of soil or plant exposure, most have been seen in adults, and approximately half have clustered in China. One previous case has been reported in a transplant recipient (7). The most recent isolation occurred in a 12-year-old child displaying extensive granulomatous crusty lesions. The spread of these lesions was attributed to self-inoculation by scratching, and the prepubertal age of the patient may have been a predisposing factor. Similar lesions in children have been caused by Exophiala spinifera (4, 14).
This case is the second report of transplant-associated disease and the seventh case in the English literature (Table 1). Although the epidemiology of the organism remains elusive, a literature search revealed that, in addition to its being isolated in New Guinea and China (13), it has also been recovered from nature in the region of Botucata, State of São Paulo, Brazil, during research into the natural habitat of Paracoccidioides brasiliensis (11). Other reports show that it has been recorded in Poland from snow on gymnosperm trees and melting ice water (http://gateway2.ovid.como/ovidweb.cgi); from goat dung inRajasthan, India (http://wdcm.nig.ac.jp/database/MSDN/IMI/10979.html); from airborne spores in homes of asthmatics in Wellington, New Zealand (http://wdcm.nig.ac.jp/database/MSDN/IMI/10981.html); and from nesting material in alligator farms in Australia (http://www.rirdc.gov.au/reports/NAP/DAQ-188A.doc). Of the other eight species of Veronaea described by Ellis (5, 6), only V. apiculata has been previously isolated in the United States, from forest soil. Manifestation of disease occurred in our patient following an attempted intravenous line insertion. One can speculate, as was done for the liver transplant recipient, that the organism had been acquired sometime previously, remained quiescent, and was then reactivated due to immunosuppression. Alternatively, the organism may have been introduced at the time of the procedure, and the patient's immunosuppression facilitated tissue invasion. Either scenario is tenable, and the recovery of the organism from an individual in Houston, Tex., supports a more global distribution of the organism than has been previously recognized.
TABLE 1.
Mycoses caused by V. botryosaa
| Case | Yr | Reference(s) | Age (yr)/sex of patient | Geographic location | Underlying condition | Soil or plant exposure | Lesions | Susceptibility data (μg/ml) | Therapy | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1989 | 13 | —b | China | Unknown | Unknown | Skin | None | Unknown | Unknown |
| 2 | 1990 | 16 | 24/M | Henan Province, China | Unknown | Yes, farmer | Black, verrucous nodules and cysts on back of hands, cheeks, and forearm | None | Unknown | Unknown |
| 1991 | 15 | |||||||||
| 3 | 1995 | 1 | 28/F | Libyan woman, living in Tripoli | Unknown | None reported | Nodular-ulceronodular lesions on thumb and fifth finger, upper limb, nasal mucosa, and palate | None | Unknown | Unknown |
| 4 | 1998 | 10 | 34/M | Philippines | Unknown | Yes, medicine worker | Erythematous, pruritic papules, deltoid and skin | None | Unknown | Unknown |
| 5 | 1999 | 7 | 57/M | La Réunion Island, Indian Ocean, transplant in France | Liver transplant | Yes, sugarcane | Nodules on both feet, wrist, and forearm | None | ITRA, 300 mg q.d., 8 mo | Cured |
| 6 | 2003 | 9 | 12/M | Jiangsu Province, China | Prepubertal age | Yes, scratch to arm | Nodular crusted lesions coalesced to form plaques extensive on hands, face, legs, scrotum, and buttocks; spread by self-implantation due to scratching | None | Tablets of “lanmei su,” a Chinese herbal, for >1 yr; providone-iodine baths; local heat treatment; AMB facial injections; TERB at 125 mg/day for 6 mo; ITRA at 100 mg/day for 6 mo | Lesions on hands and legs better; face lesions worse; no therapy now; seeking treatment abroad |
| 7 | 2004 | This case report | 62/M | Houston, Tex. | Heart transplant | None known | At site of i.v. line insertion | FLU, > 64; ITRA, 0.25; VORI, 2 | ITRA, 200 mg; BID, 1 wk; VORI, 200 mg BID, 10 wk | Cured |
Abbreviations: M, male; F, female; ITRA, itraconazole; FLU, fluconazole; VORI, voriconazole; AMB, amphotericin B; TERB, terbinafine; i.v., intravenous; q.d., once a day.
—, no clinical information available.
V. botryosa is the type species described by Ciferri and Montemartini in 1957 (5). Identification of the remaining eight species is based mostly upon conidial size, septations, shape, roughness, and conidiophore features (6). Species excluded from consideration based upon conidial size, shape, septations, and roughness included V. indica, V. harunganae, V. apiculata, V. musae, V. parvispora, V. carlinae, and V. caricis. V. coprophila, isolated from goat dung in India, has conidial features closely resembling those of V. botryosa and is now considered synonymous with V. botryosa (9). In vitro antifungal susceptibility testing of this isolate revealed 120- and 144-h MICs of fluconazole, itraconazole, and voriconazole of >64, 0.25 and 0.25, and 2 and 2 μg/ml, respectively. Although no breakpoints have been established for these agents with V. botryosa, the in vitro MIC data, based upon achievable concentrations with standard dosing regimens, suggests resistance to both fluconazole and voriconazole and susceptibility to itraconazole. The patient's original regimen of itraconazole, 200 mg BID, although of short duration, may have inhibited the growth of the organism, as was previously shown with the administration of this agent in the liver transplant recipient (7). His subsequent 10-week course of voriconazole, also 200 mg BID, appears to have cleared the organism.
The ability of V. botryosa to grow at 35°C, albeit with reduced vigor, suggests its potential for invasive disease. Clinicians and laboratorians alike are alerted to the possible recovery of this organism in the setting of immune compromise. Surveillance cultures may provide a better understanding of the epidemiology of this organism and further delineate its ecology.
Acknowledgments
We thank Diane Ward in the microbiology laboratory of St. Luke's Episcopal Hospital for providing details of the original isolation and initial growth characteristics of the organism and Dora McCarthy of the Fungus Testing Laboratory for performing antifungal susceptibility testing.
REFERENCES
- 1.Ayadi, A., M. R. Huerre, and C. de Bievre. 1995. Phaeohyphomycosis caused by Veronaea bothryosa. Lancet 346:1703-1704. [DOI] [PubMed] [Google Scholar]
- 2.Chabasse, D. 2002. Les phaeohyphomycetes agents de phaeohyphomycosis: des champignons emergents. J. Mycol. Med. 12:65-85. [Google Scholar]
- 3.de Hoog, G. S., J. Guarro, J. Gené, and M. J. Figueras. 2000. Atlas of clinical fungi, 2nd edition. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
- 4.de Hoog, G. S., N. Poonwan, and A. H. G. Gerrits van den Ande. 1999. Taxonomy of Exophiala spinifera and its relationship to E. jeanselmei. Stud. Mycol. 43:133-142. [Google Scholar]
- 5.Ellis, M. B. 1971. Dematiaceous hyphomycetes. Commonwealth Agricultural Bureaux, Farnham Royal, Slough, United Kingdom.
- 6.Ellis, M. B. 1976. More dematiaceous hyphomycetes. Commonwealth Agricultural Bureaux, Farnham Royal, Slough, United Kingdom.
- 7.Foulet, V., C. Duvoux, C. de Bievre, C. Hezode, and S. Bretagne. 1999. Cutaneous phaeohyphomycosis caused by Veronaea bothryosa in a liver transplant recipient successfully treated with itraconazole. Clin. Infect. Dis. 29:689-690. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto, T., and L. Ajello. 1998. Agents of phaeohyphomycosis, p. 503-524. In L. Collier, A. Balows, and M. Sussman (ed.), Topley and Wilson's microbiology and microbial infections, vol. 4. Oxford University Press, New York, N.Y.
- 9.Matsushita, A., L. Jilong, M. Hiruma, M. Kobayashi, T. Matsumoto, H. Ogawa, and A. A. Padhye. 2003. Subcutaneous phaeohyphomycosis caused by Veronaea botryosa in the People's Republic of China. J. Clin. Microbiol. 41:2219-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medina, A. L., J. A. D. Redondo, and L. M. Nebrida. 1998. Two unusual cases of mycoses in the Philippines, p. 88. In Proceedings of the 4th China Japan International Congress on Mycology.
- 11.Montenegro, M. R., M. Miyaji, M. Granco, K. Nishimura, K. I. Coelho, Y. Horie, R. P. Mendes, A. Sano, K. Fukushima, and D. Fecchio. 1996. Isolation of fungi from nature in the region of Botucata, State of São Paulo, Brazil, an endemic area of paracoccidioidomycosis. Mem. Inst. Oswaldo Cruz 91:665-670. [DOI] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard M38-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 13.Nishimura, K., M. Miyaji, H. Taguchi, D. L. Wang, R. Y. Li, and Z. H. Meng. 1989. An ecological study on pathogenic dematiaceous fungi in China, p. 17-20. In Current problems of opportunistic fungal infections. Proceedings of the 4th International Symposium of the Research Center for Pathogenic Fungi and Microbial Toxicosis. Chiba University, Chiba, Japan.
- 14.Padhye, A. A., L. Ajello, F. W. Chandler, J. E. Banos, E. Hernandez-Perez, J. Llerena, and L. M. Linares. 1983. Phaeohyphomycosis in El Salvador caused by Exophiala spinifera. Am. J. Trop. Med. Hyg. 32:799-803. [DOI] [PubMed] [Google Scholar]
- 15.Wang, D. L., R. Y. Li, X. H. Wang, and H. E. Zhang. 1991. Studies on Veronaea botryosa agent of the first human case. Acta Mycol. Sin. 10:159-165. [Google Scholar]
- 16.Zhang, H. E., D. L. Wang, and R. Y. Li. 1990. Report of the case of phaeohyphomycosis caused by Veronaea botryosa. Chin. J. Dermatol. 23:96-98. [Google Scholar]