Abstract
Increasing rates of preterm births coupled with better survival of these infants have resulted in higher prevalence of systemic and ocular complications associated with prematurity. In addition to retinopathy of prematurity, infants who are born preterm may suffer from severe visual impairment as a result of hypoxic ischemic encephalopathy, hypoglycemia, and other metabolic imbalances. The effect of these processes on the anterior visual pathway may result in optic atrophy, optic nerve hypoplasia or optic disc cupping and affection of the posterior visual pathway leads to cortical visual impairment (CVI). Other ocular associations include strabismus, nystagmus, and ocular motor abnormalities such as tonic down gaze and defective saccades and pursuits. Cortical and subcortical involvement also manifests as defects in functional vision and these have not yet been completely understood. Children with CVI may have visual field defects, photophobia, defective visual processing, and deficient color vision. Since most of these children also suffer from additional systemic disabilities, evaluation, and management remains a challenge. However, early diagnosis and initiation of rehabilitation therapy can prove to be of significant benefit in these children.
Keywords: Cortical visual impairment, hypoxia, hypoglycemia, optic atrophy, prematurity, periventricular leukomalacia
India leads the world in the total number of preterm births. 23.6% of all preterm births in the world occur in India.[1] With recent advances in neonatal intensive care, there has been an increase in the survival rates of premature infants. These infants may however have significant morbidity as a result of multisystem damage. Previous studies from developed nations have shown that infants born prematurely, and those with low birth weight are at a risk of severe ophthalmic impairment.[2,3]
Retinopathy of prematurity (ROP) remains a significant cause of visual impairment in these children. However, children with or without ROP may have significant and sometimes more severe visual impairment due to other causes such as cortical visual impairment (CVI), optic atrophy, optic nerve hypoplasia, amblyopia, strabismus, visual field defects, and visual cognitive and perceptive defects.[4]
In India, only now are we realizing the burden of various neurological and neuro-ophthalmic sequelae in preterm babies.[5] This review aims to familiarize the reader with the various neuro-ophthalmic associations in premature infants who have additional neurological and ocular pathology as a result of hypoxia, hypoglycemia, or other damaging influences and to create awareness about the various causes and features of visual impairment in this group.
Pathogenesis and Mechanisms of Injury
The newborn brain is susceptible to various external influences. The most common damaging influences include hypoxia–anoxia, hypoglycemia, or a combination of both. Brodsky et al. have described two distinct subgroups of cortical visual loss in terms of the areas of damage and the time of injury.[6] They explain that the injury in full-term babies, predominantly involves the striate and peristriate cortex, whereas in preterm babies, injury involves the subcortical white matter, including the optic radiations.
At term, the vascular supply of the brain is derived primarily from the major cerebral arteries and its watershed areas lie at the interfaces between the major cerebral arterial distributions.[7,8,9] Hypoxic-ischemic injury in this period produces watershed infarctions in the parieto-occipital and parasagittal cortex, resulting in cortical visual loss.
In the developing brain, during the 27th and 34th week of gestation, the cortex and underlying white matter receive their blood supply from branches of the blood vessels on the surface of the hemispheres and the watershed zone lies within the periventricular white matter.[8] Hence, preterm injury to the brain results in injury to the subcortical white matter, resulting in periventricular leukomalacia (PVL).[8] Intraventricular hemorrhage is another common cause of neuronal injury in premature infants[10] and is, usually, caused by impaired autoregulation of the cerebral blood flow.
Preterm neonates and neonates with low birth weight are also at a risk for transient or persistent hypoglycemia. This is more common in neonates with additional risk factors such as hypoxia, sepsis, polycythemia, and shock.[11] Infants with hypoglycemia, show patterns of injury with increased severity in the occipital lobes.[12] Although the exact mechanism for this pattern is not understood, it is known that neonatal hypoglycemia can result in significant visual deficits.[12,13]
Structural Manifestations
Neuroimaging and structural changes in the brain
Imaging of the infant's brain is possible by ultrasound or computed tomography scanning (CT) or magnetic resonance imaging (MRI). While ultrasonography is useful in diagnosing large intraventricular hemorrhages, germinal hemorrhages, and cystic changes due to PVL, it has limited value in determining subtle damage due to hypoxia or early mild to moderate PVL changes.[14]
Computed tomography and MRI are of great use in the diagnosis of PVL. MRI is sensitive enough to pick up even subtle PVL changes. MRI evidence of PVL includes changes such as abnormal dilatation and irregularity of the lateral ventricles, high-intensity signals in the periventricular white matter on T2 weighted images and periventricular gliosis. Severe hypoxic injury may lead to encephalomalacia with surrounding gliosis.[15] Other abnormalities seen include thinning of the corpus callosum, altered signals from the thalamus and putamen and cerebellar atrophy[10,15] [Fig. 1].
Figure 1.
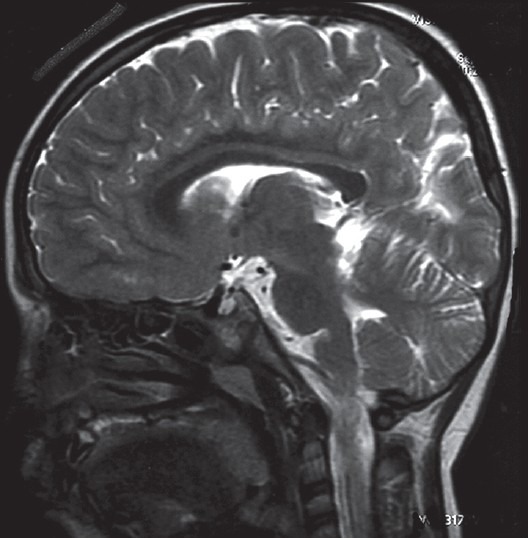
T1-weighted magnetic resonance imaging showing thinning of the corpus callosum in a child with hypoxic ischemic encephalopathy
Neuroimaging in infants with hypoglycemia has shown extensive white matter changes predominantly in the occipito-parietal regions, thalamic lesions, infarctions, and diffusion restriction in the occipital regions.[12,13] This has been correlated to the presence of CVI later in life.[13]
Mapping of the neural activity and corresponding cerebral blood flow changes with functional MRI (fMRI) techniques have further advanced our understanding of the mechanisms and pathways involved in PVL and CVI. In infants with PVL, fMRI studies have revealed decreased, slowed and aberrant cortical activation.[16]
Optic Disc Changes
In children with “pure” CVI, fundus examination may reveal normal optic discs with a healthy retina and macula. In these cases, the assumption is that the pathological processes affecting the posterior visual pathways and the cerebral cortex have not affected the anterior visual pathway. However, studies involving children with retrogeniculate vision loss, have described a variety of optic nerve abnormalities including optic atrophy, optic nerve hypoplasia, and pseudoglaucomatous cupping.[6,10,17]
Early prenatal damage may result in small optic nerves (optic nerve hypoplasia), whereas lesions occurring later than 28 weeks of gestation may result in normal sized optic discs with large cups as a result of reduction in the number of axons.[6,10] Transsynaptic degeneration, occurring after damage to the retrogeniculate pathways in the perinatal period, has been thought to be responsible for this pseudoglaucomatous cupping seen in children with hypoxic-ischemic encephalopathy (HIE).[10] Optic atrophy may be a direct result of partial or total damage to the anterior visual pathways by the disease process [Fig. 2].
Figure 2.
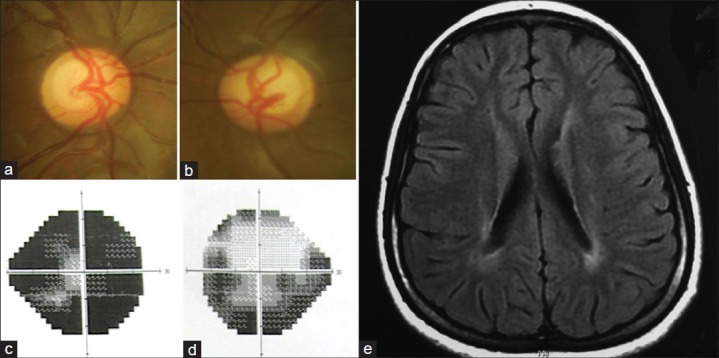
(a and b) Optic disc pictures of a 13-year-old girl referred with a diagnosis of juvenile glaucoma. The discs show large cups with mild to a moderate pallor. (c and d) Visual field defects not corresponding to the appearance of the optic discs. (e) T1-weighted magnetic resonance imaging showing periventricular changes due to hypoxic-ischemic encephalopathy
A large number of children with HIE related optic disc abnormalities are misdiagnosed and treated as juvenile or congenital glaucoma, and it may therefore, be prudent to consider neuroimaging in patients where visual acuity and field defects do not correspond to the appearance of the optic discs.
Nystagmus
Nystagmus is rare or absent in children with CVI. However, roving eye movements or occasional bursts of nystagmus may be seen. The presence of roving eye movements signifies severe visual impairment and an inability to fixate on objects.[6] The absence of nystagmus in CVI was explained by Fielder and Evans, who speculated that an intact geniculostriate pathway is a prerequisite for the development of congenital nystagmus. Hence, nystagmus is absent in extensive posterior pathway disease.[18] However, latent nystagmus and in some cases, manifest nystagmus is present in infants with PVL.[19] These children with CVI and nystagmus may have combined anterior and posterior visual pathway disease with “mixed” mechanism of visual loss.[10] Latent nystagmus is mediated subcortically by the nucleus of the optic tract within the midbrain, thus latent nystagmus in children with neurologic disease is generally associated with subcortical rather than cortical visual loss and can be considered a marker of PVL in children with other neurological deficits.[6]
Strabismus and Ocular Motor Deficits
Studies have shown that strabismus is more common in premature children when compared with children born full term[4,20] and maybe associated with neurological disease or ocular complications such as cicatricial ROP and refractive errors.[4] Esotropia is more common in children with PVL and may be associated with latent nystagmus, dissociated vertical deviation and “A” pattern.[15] Other studies have shown that cortical visual loss is associated with exotropia more commonly than esotropia, whereas in those with subcortical visual loss (PVL), a significant predominance of esotropia over exotropia was seen.[6] Furthermore, children with more extensive neurological damage may have a congenital exotropia.[10,15] Since congenital exotropia is much less common than esotropia, it is recommended to closely monitor these children for neurological and visual development.[6] Some children with PVL may also have “dyskinetic strabismus” in which the exotropia may alternate to esotropia with variable deviations[15] [Fig. 3].
Figure 3.
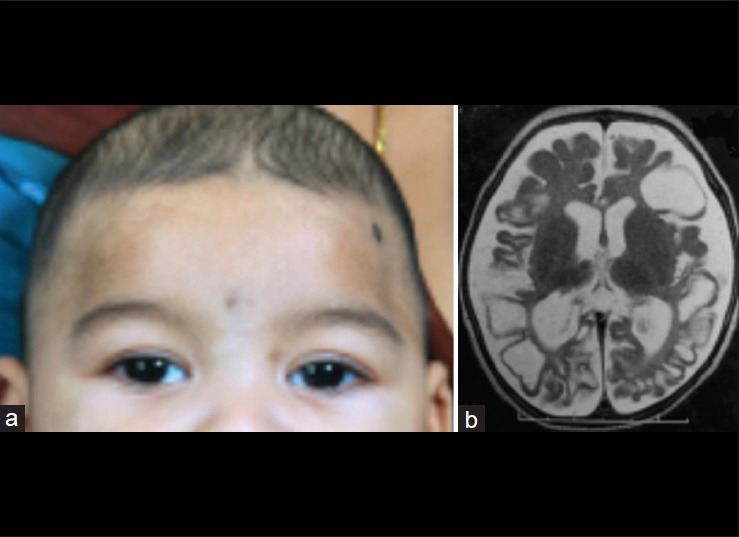
A child with microcephaly and congenital exotropia (a) with cystic encephalomalacia due to hypoxic-ischemic encephalopathy as seen on the T2-weighted magnetic resonance imaging (b)
Management of strabismus in children with neurological diseases is challenging and may have unpredictable or poor results due to the lack of a fusional capacity in these children.[21] However, these children do benefit, both functionally and cosmetically from strabismus surgery. Esotropia correction in such children can lead to overcorrections, and a lower surgical dose is recommended.[22] Surgery for exotropia in children with neurological diseases may also require decreasing the routine surgical dosage for large deviations.[23]
Ocular motor abnormalities may be present in children with or without strabismus and nystagmus. Children with PVL are known to have tonic downgaze. This may be related causally to the presence of intraventricular hemorrhage, hydrocephalus or thalamic infarcts.[6,15] Children with CVI have also been noted to have horizontal conjugate gaze deviation, in which both eyes are tonically deviated to one side, and the head is turned in the same direction.[15] This is postulated to be due to asymmetric damage to the cortical control centers for horizontal gaze. Defective smooth pursuits and saccadic eye movements have also been documented in children with PVL.[19] This may be due to damage to the dorsal pathway from the occipital cortex to the frontal and parietal cortices.[10]
Visual Field Defects
As a result of the wide spectrum of cortical injury in children with CVI, a variety of visual field defects may also be present in these children. It is extremely difficult to diagnose field defects in these infants and innovative methods such as moving brightly colored toys and objects in different areas of their visual field may be tried. Generalized restriction of the fields with a smaller size of the mean visual field has been found in studies on children with perinatal asphyxia.[10,24] Central scotomas may also be seen commonly due to bilateral occipital injury,[15] and the child may prefer to look at objects eccentrically in order to avoid the area of the scotoma. Altitudinal field defects may be present and may be difficult to diagnose. Congenital homonymous hemianopias have been reported in children, and although trauma and tumors are the most common causes of this defect, it may also be seen in children with porencephaly and PVL and is commonly associated with congenital hemiplegia.[15,25]
Functional Deficits
It must be remembered that visual function is a much larger concept and is not limited to visual acuity alone. Children with CVI have a plethora of cognitive and functional visual abnormalities independent of their visual acuity. In this regard, Brodsky et al. have introduced the concept of the four A's of visual loss: Acuity, assimilation, attention, and apraxia.[15] Despite relatively good acuity, children with CVI may have difficulty in simultaneous perception and have “crowding” phenomenon, wherein they are able to perceive objects better when seen individually against a plain background as compared to multiple objects or those seen against a patterned background.[15] They have an inconsistent visual performance that may vary at different times of the day and often use touch to identify objects.[26] They also visualize and function better in a familiar environment. It has been found that children with CVI have a stronger ability to identify colors than their perception of form.[26] Another peculiar feature is the tendency to stare at bright objects, such as fluorescent room lights or the sun. This is known as “light gazing” and is regarded as a sign of severe visual impairment.[27] In contrast, some children with CVI may exhibit photophobia. This may be caused by damage to the retina, thalamus or cortical structures.[28] It has also been noted that visual performance of some children may be better for objects in motion as compared to static objects; for example, they may see better while travelling in a car.[26] Other components of vision such as color vision and stereopsis are difficult to assess in these children. Though stereopsis is considered to be defective, color vision is largely felt to be normal.[10]
Conclusion
It is recognized that preterm infants who suffer from various complications of prematurity such as hypoxia, hypoglycemia, sepsis, etc., are at a risk not only of developing ROP but may also suffer from other visual deficits–both sensory and motor.
Clinical features may include severe visual impairment with a normal or near normal fundus appearance. These children are diagnosed to have CVI. In cases where there may be concomitant anterior visual pathway involvement, optic atrophy may be present. Other anomalies of the optic nerve associated with prematurity include optic nerve hypoplasia and pseudoglaucomatous cupping. These children may also have nystagmus, strabismus, and other ocular motor deficits. A variety of functional visual problems such as crowding and visual field defects may also affect visual behavior.
These children most often have multiple disabilities, and a multidisciplinary approach is required for effective rehabilitation. Awareness of these problems will aid in early detection and prompt institution of rehabilitative therapy. Parental counseling requires great sensitivity and patience on the part of the clinician. Parents need accurate and clear information about the condition, its associations and prognosis, techniques of handling such children and information about schooling. It is a part of the clinician's duty to ensure that CVI does not lead to poor general development of the child.
We hope that the current advances in neonatal care, especially in developing countries like India, and increasing awareness can help in the prevention of a significant percentage of visual impairment that may be caused by CVI.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. Lancet. 2012;379:2162–72. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 2.Cooke RW, Foulder-Hughes L, Newsham D, Clarke D. Ophthalmic impairment at 7 years of age in children born very preterm. Arch Dis Child Fetal Neonatal Ed. 2004;89:F249–53. doi: 10.1136/adc.2002.023374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robaei D, Kifley A, Gole GA, Mitchell P. The impact of modest prematurity on visual function at age 6 years: Findings from a population-based study. Arch Ophthalmol. 2006;124:871–7. doi: 10.1001/archopht.124.6.871. [DOI] [PubMed] [Google Scholar]
- 4.O’Connor AR, Wilson CM, Fielder AR. Ophthalmological problems associated with preterm birth. Eye (Lond) 2007;21:1254–60. doi: 10.1038/sj.eye.6702838. [DOI] [PubMed] [Google Scholar]
- 5.Swaminathan M. Cortical visual impairment in children – A new challenge for the future? Oman J Ophthalmol. 2011;4:1–2. doi: 10.4103/0974-620X.77654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brodsky MC, Fray KJ, Glasier CM. Perinatal cortical and subcortical visual loss: Mechanisms of injury and associated ophthalmologic signs. Ophthalmology. 2002;109:85–94. doi: 10.1016/s0161-6420(01)00849-1. [DOI] [PubMed] [Google Scholar]
- 7.Flodmark O, Jan JE, Wong PK. Computed tomography of the brains of children with cortical visual impairment. Dev Med Child Neurol. 1990;32:611–20. [PubMed] [Google Scholar]
- 8.Schwartz ES, Barkovich AJ. Brain and spine injures in infancy and childhood. In: Barkovich AJ, Raybaud C, editors. Pediatric Neuroimaging. 5th ed. Philadelphia: Lippincott Williams and Wilkins; 2000. p. 243. [Google Scholar]
- 9.Volpe J. 3rd ed. Philadelphia: W.B. Saunders; 1995. Neurology of the Newborn; pp. 403–63. [Google Scholar]
- 10.Jacobson LK, Dutton GN. Periventricular leukomalacia: An important cause of visual and ocular motility dysfunction in children. Surv Ophthalmol. 2000;45:1–13. doi: 10.1016/s0039-6257(00)00134-x. [DOI] [PubMed] [Google Scholar]
- 11.Jain A, Aggarwal R, Jeeva Sankar M, Agarwal R, Deorari AK, Paul VK. Hypoglycemia in the newborn. Indian J Pediatr. 2010;77:1137–42. doi: 10.1007/s12098-010-0175-1. [DOI] [PubMed] [Google Scholar]
- 12.Tam EW, Widjaja E, Blaser SI, Macgregor DL, Satodia P, Moore AM. Occipital lobe injury and cortical visual outcomes after neonatal hypoglycemia. Pediatrics. 2008;122:507–12. doi: 10.1542/peds.2007-2002. [DOI] [PubMed] [Google Scholar]
- 13.Burns CM, Rutherford MA, Boardman JP, Cowan FM. Patterns of cerebral injury and neurodevelopmental outcomes after symptomatic neonatal hypoglycemia. Pediatrics. 2008;122:65–74. doi: 10.1542/peds.2007-2822. [DOI] [PubMed] [Google Scholar]
- 14.Carson SC, Hertzberg BS, Bowie JD, Burger PC. Value of sonography in the diagnosis of intracranial hemorrhage and periventricular leukomalacia: A postmortem study of 35 cases. AJR Am J Roentgenol. 1990;155:595–601. doi: 10.2214/ajr.155.3.2117361. [DOI] [PubMed] [Google Scholar]
- 15.Brodsky M. Pediatric Neuro-Ophthalmology. 2nd ed. New York: Springer; 2010. The apparently blind infant; pp. 1–58. [Google Scholar]
- 16.Yu B, Guo Q, Fan G, Liu N. Assessment of cortical visual impairment in infants with periventricular leukomalacia: A pilot event-related fMRI study. Korean J Radiol. 2011;12:463–72. doi: 10.3348/kjr.2011.12.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobson L, Hellström A, Flodmark O. Large cups in normal-sized optic discs: A variant of optic nerve hypoplasia in children with periventricular leukomalacia. Arch Ophthalmol. 1997;115:1263–9. doi: 10.1001/archopht.1997.01100160433007. [DOI] [PubMed] [Google Scholar]
- 18.Fielder AR, Evans NM. Is the geniculostriate system a prerequisite for nystagmus? Eye (Lond) 1988;2(Pt 6):628–35. doi: 10.1038/eye.1988.116. [DOI] [PubMed] [Google Scholar]
- 19.Jacobson L, Ygge J, Flodmark O. Nystagmus in periventricular leucomalacia. Br J Ophthalmol. 1998;82:1026–32. doi: 10.1136/bjo.82.9.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holmström G, Rydberg A, Larsson E. Prevalence and development of strabismus in 10-year-old premature children: A population-based study. J Pediatr Ophthalmol Strabismus. 2006;43:346–52. doi: 10.3928/01913913-20061101-04. [DOI] [PubMed] [Google Scholar]
- 21.Ghasia F, Brunstrom-Hernandez J, Tychsen L. Repair of strabismus and binocular fusion in children with cerebral palsy: Gross motor function classification scale. Invest Ophthalmol Vis Sci. 2011;52:7664–71. doi: 10.1167/iovs.10-6906. [DOI] [PubMed] [Google Scholar]
- 22.Pickering JD, Simon JW, Lininger LL, Melsopp KB, Pinto GL. Exaggerated effect of bilateral medial rectus recession in developmentally delayed children. J Pediatr Ophthalmol Strabismus. 1994;31:374–7. doi: 10.3928/0191-3913-19941101-06. [DOI] [PubMed] [Google Scholar]
- 23.Bang GM, Brodsky MC. Neurological exotropia: Do we need to decrease surgical dosing? Br J Ophthalmol. 2013;97:241–3. doi: 10.1136/bjophthalmol-2012-302279. [DOI] [PubMed] [Google Scholar]
- 24.Luna B, Dobson V, Scher MS, Guthrie RD. Grating acuity and visual field development in infants following perinatal asphyxia. Dev Med Child Neurol. 1995;37:330–44. doi: 10.1111/j.1469-8749.1995.tb12011.x. [DOI] [PubMed] [Google Scholar]
- 25.Guzzetta A, Fazzi B, Mercuri E, Bertuccelli B, Canapicchi R, van Hof-van Duin J, et al. Visual function in children with hemiplegia in the first years of life. Dev Med Child Neurol. 2001;43:321–9. doi: 10.1017/s0012162201000603. [DOI] [PubMed] [Google Scholar]
- 26.Jan JE, Groenveld M, Sykanda AM, Hoyt CS. Behavioural characteristics of children with permanent cortical visual impairment. Dev Med Child Neurol. 1987;29:571–6. doi: 10.1111/j.1469-8749.1987.tb08498.x. [DOI] [PubMed] [Google Scholar]
- 27.Jan JE, Groenveld M, Sykanda AM. Light-gazing by visually impaired children. Dev Med Child Neurol. 1990;32:755–9. doi: 10.1111/j.1469-8749.1990.tb08478.x. [DOI] [PubMed] [Google Scholar]
- 28.Jan JE, Groenveld M, Anderson DP. Photophobia and cortical visual impairment. Dev Med Child Neurol. 1993;35:473–7. doi: 10.1111/j.1469-8749.1993.tb11677.x. [DOI] [PubMed] [Google Scholar]


