Abstract
Aims:
The aim was to highlight recent advances in the treatment of thyroid eye disease.
Settings and Design:
Review article.
Materials and Methods:
Existing literature and the authors’ experience was reviewed.
Results:
Thyroid ophthalmopathy is a disfiguring and vision-threatening complication of autoimmune thyroid disease that may develop or persist even in the setting of well-controlled systemic thyroid status. Treatment response can be difficult to predict, and optimized algorithms for disease management do not exist. Thyroid ophthalmopathy should be graded for both severity and disease activity before choosing a treatment modality for each patient. The severity of the disease may not correlate directly with the activity; medical treatment is most effective in active disease, and surgery is usually reserved for quiescent disease with persistent proptosis and/or eyelid changes.
Conclusions:
Intravenous pulsed corticosteroids, orbital radiotherapy, and orbital surgical techniques form the mainstay of current management of thyroid ophthalmopathy. Immunosuppressive and biologic agents may have a role in treating active disease although additional safety and efficacy studies are needed.
Keywords: Chemosis, optic neuropathy, strabismus, thyroid eye disease
Clinical Management and Decision Making: Documentation of Disease Activity/Severity
Thyroid-related ophthalmopathy (TRO) usually follows Rundle's curve, with early inflammatory phase, followed by stability and often improvement with some residual, inactive disease. Signs and symptoms range from dry eye to dysthryoid optic neuropathy (DON). Both disease activity and severity must be determined for treatment decisions to be made. Werner proposed the first systematic classification under the acronym NOSPECS, using characteristic ocular signs and symptoms.[1] The classification of TRO has evolved over time to include more objective criteria and guidelines for therapeutic management, most notably the clinical activity score (CAS) used by the European Graves Orbitopathy Group.[2] More recently, Dolman and Rootman amalgamated these concepts under their VISA classification, which divides TRO into four disease end points: Vision, inflammation, strabismus, and appearance [Fig. 1].[3] Measurements obtained in each section allow monitoring of disease activity, grading of severity, and appropriate treatment selection.
Figure 1.
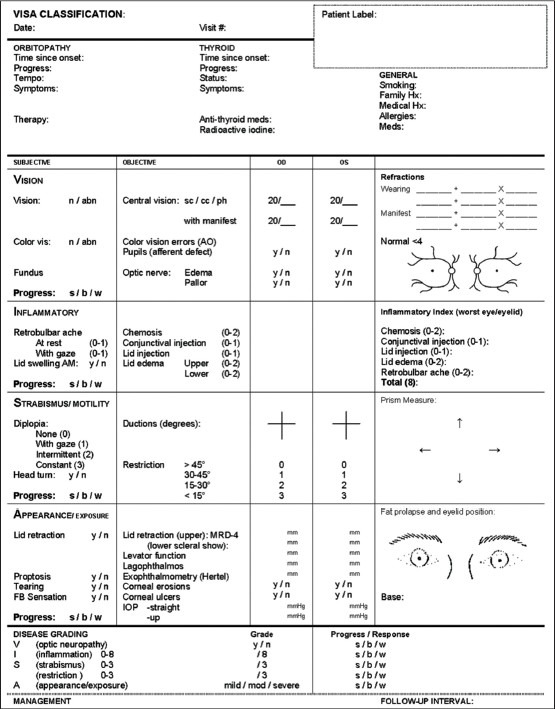
VISA classification: This classification system divides thyroid-related ophthalmopathy into four disease end points: Vision, inflammation, strabismus, and appearance
All patients with TRO should be referred to an internist and/or an endocrinologist for management of the systemic dysthyroid state. Systemic symptomatic control may be achieved with a beta-blocker, whereas hyperthyroidism may be treated by radioactive iodine ablation (RAI), surgical thyroidectomy, or with a thionamide such as propylthiouracil.[4] Patients with vision-threatening or severe TRO activity should avoid RAI if possible because of the risk worsening of TRO after therapy.[5] Thereafter, thyroid levels should be monitored every 4-8 weeks until normalized, then every 2-3 months. Smoking cessation should be recommended to all patients; it is among the most important risk factors for TRO progression, with the effect being proportional to the number of cigarettes smoked daily.[6] Orbital imaging should be obtained with ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI). Ultrasound provides fast in-office evaluation of the extraocular muscles but requires an experienced echographer. CT and MRI have the added benefit of visualizing the entire orbit, although CT has greater sensitivity in detecting extraocular muscle enlargement.[7] T2-weighted MRI sequences may show hyperintensity within the extraocular muscles as evidence of disease activity.
Timing of Treatments and Urgent Interventions
Intervention is done to halt disease progression and reduce disease activity. Active disease is defined as progression of any of the VISA parameters or CAS of 4 or more (max score = 8), with the converse true of inactive disease. The level of activity can be further divided into mild (CAS 4 or less without deterioration), moderate to severe (CAS 5 or more, or progressive disease), or vision threatening.
Vision threatening TRO refers to DON or corneal ulceration and must be addressed immediately. Corneal ulceration requires emergent therapy because it can decompensate rapidly. Topical antibiotics and/or hourly preservative free lubrication are helpful but often inadequate alone, and the clinician should not hesitate to perform tarsorrhaphy or periorbital botulinum toxin for mechanical protection of the eye. Severe ulceration or perforation may require glue, amniotic membrane, and/or corneal grafting with the aid of an anterior segment specialist.
Dysthryoid optic neuropathy warrants either urgent intravenous (IV) steroid therapy given weekly at 0.25-0.5 g/week, as in moderate to severe disease below[8,9] or urgent posterior orbital decompression. In a prospective trial comparing surgical decompression with pulsed IV steroid therapy, there was no apparent advantage for either method, and some surgical patients still required subsequent IV steroids or radiotherapy.[10] The individual patient's medical co-morbidities and severity of optic neuropathy must be considered in deciding on surgical versus medical treatment. Consecutive day dosing of IV steroid may be considered for more severe DON or as a temporizing measure prior to decompression, administered for daily 3 days followed by another dose a week later. If the response to IV steroid is poor after one to 2 weeks, surgical decompression should be offered.[9] Additional IV steroid therapy with or without radiotherapy may be needed after surgery if the disease is still active. Superiority of a particular treatment algorithm has not been established.
Nonsurgical Management Options
Conservative measures
Standard therapy for mild TRO includes observation and supportive measures to alleviate dry eye symptoms (ocular lubrication, moisture goggles, sunglass wear, wind avoidance) and periocular swelling (elevation of the head of bed, humidification). A prospective study of oral selenium supplementation (sodium selenite 100 ug twice a day) in newly diagnosed patients with mild TRO showed a reduction in CAS activity and improved quality of life (GO-QOL), lid retraction, and soft tissue edema when compared to placebo after 6 or 12 months of treatment,[11] This study was conducted in a population that is often deficient in selenium, so generalization to a broader population should be made with caution. As the treatment carries little to no risk, it has become popular with physicians.
Steroids
Unless contraindicated, glucocorticoid (GC) therapy, either oral or IV, is usually the first choice for active and moderate to severe disease. Both modalities have side effects, with prolonged oral GC usage causing an increased risk of osteoporosis versus IV GCs, which carry a risk of worsening hepatic dysfunction or failure.[12] The advantages IV therapy has over oral are an overall reduction in total steroid burden and decreased chronic oral steroid intake, more rapid improvement in CAS, better tolerability, and lower risk of osteoporosis.[12,13,14] The optimal dosing schedule is not established; in our practice we prescribe 500 mg of IV methylprednisolone given weekly for 6 weeks followed by 250 mg of IV methylprednisolone weekly for 6 more weeks.[12,15] Hepatic function should be monitored, and the total dose of methylprednisolone should not exceed eight grams because of significantly increased risk of hepatic failure.[8]
Alternatively, oral GC therapy, in the form of prednisone, can be given at 1 mg/kg/day, followed by a prolonged taper of 10 mg/week until a dose of 20 mg/day is reached, then decreased 5 mg every week. During this time blood sugar, especially with concurrent diabetes mellitus, blood pressure, and monthly hepatic function should be followed. Bisphosphonates are recommended especially if duration of GC therapy exceeds 3 months or an average daily dose greater than 5 mg of prednisone is consumed. Due to data showing superior efficacy of IV GC, we avoid the use of oral therapy whenever possible.
Immunosuppressive agents
Glucocorticoid treatment is not effective in all patients with active disease, and side-effects may limit their use. Thus, the addition or substitution of immunosuppressive drugs has been studied with varying results. Cyclosporine monotherapy was found to be inferior to oral prednisone, but in combination appeared to be more effective than prednisone alone and potentially useful in those not responding to steroid therapy.[16] This treatment regimen has not had widespread use, and disappointing results have been seen with azathioprine and methotrexate.[17] Rituximab has shown promise in the treatment of severe steroid-refractory TRO, with several case reports and recent case series showing a substantial reduction in disease activity and severity.[18,19,20,21] In the United States, rituximab carries a “black box” warning, as patients have contracted progressive multifocal leukoencephalopathy with chronic therapy for other diseases. As a biologic agent, the drug also remains extremely expensive, and its role in the overall treatment algorithm remains very limited.
Radiotherapy
Determining the efficacy of orbital radiotherapy (OR) has been hampered by the lack of controlled studies and the frequent co-administration of GC during radiotherapy, which confounds retrospective data analysis. The available data do not support using OR in isolation or in the initial management of DON, and it appears beneficial only for moderate or worse active disease or with confirmed disease progression.[22,23,24] The standard dosing regimen is 20 Gy/orbit in ten fractions administered over two weeks, although 10 Gy may be as effective.[22] A temporary worsening of inflammation often occurs after OR and can be prevented with concurrent GC use. In fact, combination therapy with GC may be superior to either treatment alone, especially in improving diplopia.[25,26,27] OR carries an extremely low risk of causing either optic neuropathy or retinopathy, although the latter has been reported and may be related to concurrent diabetes and/or treatment error.[28] In general, OR is relatively contraindicated in diabetic patients and those with concurrent vascular disease due to the risk of worsening retinal microvascular function, and should be used with caution in patients under 35 years of age due to the theoretic risk of secondary malignancy.[29,30]
Surgical treatment
Aside from urgent treatment of DON (see above), surgical options usually are pursued when TRO is inactive, defined as no activity or progression over 6-12 months, and strabismus and/or disfiguring changes persist. A detailed description of surgical procedures is beyond the scope of this review. The sequence of surgery for inactive TRO, if necessary, is typically decompression, followed by strabismus surgery, and finally eyelid repair. Principles of orbital decompression include reducing the volume of the orbital contents by removing intraconal and extraconal fat; space is increased as well via bone removal while sparing the orbital rim as much as possible to preserve eyelid and canthal tendon positions. A number of approaches to orbital decompression have been described, with medial wall removal (especially posteriorly) most effective in relieving orbital apex crowding, and “balanced decompression” with medial and lateral wall removal and/or preservation of the inferomedial orbital strut most effective in minimizing postoperative diplopia.[31,32,33] Decompression alone rarely corrects strabismus when it already exists, and new strabismus may be seen in up to 30% of decompressions.[32] Fat removal alone has the lowest incidence of postoperative diplopia but requires greater surgical skill and experience to obtain an adequate result. Removal of the orbital roof is rarely if ever required and has limited utility. We typically perform either a balanced decompression (with endoscopic medial wall removal) or traditional 2- or 3-wall decompression through a lateral canthal and inferior transconjunctival approach.[34,35] Strabismus surgery is aimed at correcting diplopia in the primary position and downgaze by recessing the restricted muscles, frequently with adjustable sutures to improve postoperative alignment. Reoperation for late overcorrection is not uncommon. Eyelid surgery may involve recession of the upper eyelid retractors, recession of the lower eyelid retractors with or without a spacer graft, and conservative blepharoplasty.
Conclusions
A standardized treatment algorithm for thyroid ophthalmopathy does not exist, in part because the natural history of the disease is often favorable and thus allows observation of many patients. When treatment is indicated because of vision-threatening disease or documented disease progression, corticosteroids are first-line therapy for most cases and are effective. In refractory cases, other drugs may be needed, and surgery in the quiescent phase is directed at correcting the sequelae of the active disease phase.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Werner SC. Classification of the eye changes of Graves’ disease. Am J Ophthalmol. 1969;68:646–8. doi: 10.1016/0002-9394(69)91246-x. [DOI] [PubMed] [Google Scholar]
- 2.Mourits MP, Koornneef L, Wiersinga WM, Prummel MF, Berghout A, van der Gaag R. Clinical criteria for the assessment of disease activity in Graves’ ophthalmopathy: A novel approach. Br J Ophthalmol. 1989;73:639–44. doi: 10.1136/bjo.73.8.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dolman PJ, Rootman J. VISA classification for graves orbitopathy. Ophthal Plast Reconstr Surg. 2006;22:319–24. doi: 10.1097/01.iop.0000235499.34867.85. [DOI] [PubMed] [Google Scholar]
- 4.Bahn RS, Burch HB, Cooper DS, Garber JR, Greenlee MC, Klein I, et al. Hyperthyroidism and other causes of thyrotoxicosis: Management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Endocr Pract. 2011;17:456–520. doi: 10.4158/ep.17.3.456. [DOI] [PubMed] [Google Scholar]
- 5.Bartalena L, Marcocci C, Bogazzi F, Manetti L, Tanda ML, Dell’Unto E, et al. Relation between therapy for hyperthyroidism and the course of Graves’ ophthalmopathy. N Engl J Med. 1998;338:73–8. doi: 10.1056/NEJM199801083380201. [DOI] [PubMed] [Google Scholar]
- 6.Pfeilschifter J, Ziegler R. Smoking and endocrine ophthalmopathy: Impact of smoking severity and current vs lifetime cigarette consumption. Clin Endocrinol (Oxf) 1996;45:477–81. doi: 10.1046/j.1365-2265.1996.8220832.x. [DOI] [PubMed] [Google Scholar]
- 7.Polito E, Leccisotti A. MRI in Graves orbitopathy: Recognition of enlarged muscles and prediction of steroid response. Ophthalmologica. 1995;209:182–6. doi: 10.1159/000310609. [DOI] [PubMed] [Google Scholar]
- 8.Kahaly GJ, Pitz S, Hommel G, Dittmar M. Randomized, single blind trial of intravenous versus oral steroid monotherapy in Graves’ orbitopathy. J Clin Endocrinol Metab. 2005;90:5234–40. doi: 10.1210/jc.2005-0148. [DOI] [PubMed] [Google Scholar]
- 9.Bartalena L, Baldeschi L, Dickinson AJ, Eckstein A, Kendall-Taylor P, Marcocci C, et al. Consensus statement of the European group on Graves’ orbitopathy (EUGOGO) on management of Graves’ orbitopathy. Thyroid. 2008;18:333–46. doi: 10.1089/thy.2007.0315. [DOI] [PubMed] [Google Scholar]
- 10.Wakelkamp IM, Baldeschi L, Saeed P, Mourits MP, Prummel MF, Wiersinga WM. Surgical or medical decompression as a first-line treatment of optic neuropathy in Graves’ ophthalmopathy? A randomized controlled trial. Clin Endocrinol (Oxf) 2005;63:323–8. doi: 10.1111/j.1365-2265.2005.02345.x. [DOI] [PubMed] [Google Scholar]
- 11.Marcocci C, Kahaly GJ, Krassas GE, Bartalena L, Prummel M, Stahl M, et al. Selenium and the course of mild Graves’ orbitopathy. N Engl J Med. 2011;364:1920–31. doi: 10.1056/NEJMoa1012985. [DOI] [PubMed] [Google Scholar]
- 12.Zang S, Ponto KA, Kahaly GJ. Clinical review: Intravenous glucocorticoids for Graves’ orbitopathy: Efficacy and morbidity. J Clin Endocrinol Metab. 2011;96:320–32. doi: 10.1210/jc.2010-1962. [DOI] [PubMed] [Google Scholar]
- 13.Aktaran S, Akarsu E, Erbagci I, Araz M, Okumus S, Kartal M. Comparison of intravenous methylprednisolone therapy vs. oral methylprednisolone therapy in patients with Graves’ ophthalmopathy. Int J Clin Pract. 2007;61:45–51. doi: 10.1111/j.1742-1241.2006.01004.x. [DOI] [PubMed] [Google Scholar]
- 14.Kauppinen-Mäkelin R, Karma A, Leinonen E, Löyttyniemi E, Salonen O, Sane T, et al. High dose intravenous methylprednisolone pulse therapy versus oral prednisone for thyroid-associated ophthalmopathy. Acta Ophthalmol Scand. 2002;80:316–21. doi: 10.1034/j.1600-0420.2002.800316.x. [DOI] [PubMed] [Google Scholar]
- 15.Sánchez-Ortiga R, Moreno-Pérez O, González Sánchez V, Arias Mendoza N, Mauri Dot M, Alfayate Guerra R, et al. Treatment of Graves’ ophthalmopathy with high-dose intravenous methylprednisolone: A comparison of two dosing regimens. Endocrinol Nutr. 2009;56:118–22. doi: 10.1016/S1575-0922(09)70841-1. [DOI] [PubMed] [Google Scholar]
- 16.Prummel MF, Mourits MP, Berghout A, Krenning EP, van der Gaag R, Koornneef L, et al. Prednisone and cyclosporine in the treatment of severe Graves’ ophthalmopathy. N Engl J Med. 1989;321:1353–9. doi: 10.1056/NEJM198911163212002. [DOI] [PubMed] [Google Scholar]
- 17.Stiebel-Kalish H, Robenshtok E, Hasanreisoglu M, Ezrachi D, Shimon I, Leibovici L. Treatment modalities for Graves’ ophthalmopathy: Systematic review and metaanalysis. J Clin Endocrinol Metab. 2009;94:2708–16. doi: 10.1210/jc.2009-0376. [DOI] [PubMed] [Google Scholar]
- 18.Silkiss RZ, Reier A, Coleman M, Lauer SA. Rituximab for thyroid eye disease. Ophthal Plast Reconstr Surg. 2010;26:310–4. doi: 10.1097/IOP.0b013e3181c4dfde. [DOI] [PubMed] [Google Scholar]
- 19.Salvi M, Vannucchi G, Campi I, Beck-Peccoz P. Rituximab in the treatment of thyroid eye disease: Science fiction? Orbit. 2009;28:251–5. [PubMed] [Google Scholar]
- 20.Khanna D, Chong KK, Afifiyan NF, Hwang CJ, Lee DK, Garneau HC, et al. Rituximab treatment of patients with severe, corticosteroid-resistant thyroid-associated ophthalmopathy. Ophthalmology. 2010;117:133–139.e2. doi: 10.1016/j.ophtha.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salvi M, Vannucchi G, Campi I, Rossi S, Bonara P, Sbrozzi F, et al. Efficacy of rituximab treatment for thyroid-associated ophthalmopathy as a result of intraorbital B-cell depletion in one patient unresponsive to steroid immunosuppression. Eur J Endocrinol. 2006;154:511–7. doi: 10.1530/eje.1.02119. [DOI] [PubMed] [Google Scholar]
- 22.Bartalena L, Marcocci C, Tanda ML, Rocchi R, Mazzi B, Barbesino G, et al. Orbital radiotherapy for Graves’ ophthalmopathy. Thyroid. 2002;12:245–50. doi: 10.1089/105072502753600223. [DOI] [PubMed] [Google Scholar]
- 23.Gorman CA, Garrity JA, Fatourechi V, Bahn RS, Petersen IA, Stafford SL, et al. A prospective, randomized, double-blind, placebo-controlled study of orbital radiotherapy for Graves’ ophthalmopathy. Ophthalmology. 2001;108:1523–34. doi: 10.1016/s0161-6420(01)00632-7. [DOI] [PubMed] [Google Scholar]
- 24.Prummel MF, Terwee CB, Gerding MN, Baldeschi L, Mourits MP, Blank L, et al. A randomized controlled trial of orbital radiotherapy versus sham irradiation in patients with mild Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2004;89:15–20. doi: 10.1210/jc.2003-030809. [DOI] [PubMed] [Google Scholar]
- 25.Marcocci C, Bartalena L, Bogazzi F, Bruno-Bossio G, Lepri A, Pinchera A. Orbital radiotherapy combined with high dose systemic glucocorticoids for Graves’ ophthalmopathy is more effective than radiotherapy alone: Results of a prospective randomized study. J Endocrinol Invest. 1991;14:853–60. doi: 10.1007/BF03347943. [DOI] [PubMed] [Google Scholar]
- 26.Ng CM, Yuen HK, Choi KL, Chan MK, Yuen KT, Ng YW, et al. Combined orbital irradiation and systemic steroids compared with systemic steroids alone in the management of moderate-to-severe Graves’ ophthalmopathy: A preliminary study. Hong Kong Med J. 2005;11:322–30. [PubMed] [Google Scholar]
- 27.Mourits MP, van Kempen-Harteveld ML, García MB, Koppeschaar HP, Tick L, Terwee CB. Radiotherapy for Graves’ orbitopathy: Randomised placebo-controlled study. Lancet. 2000;355:1505–9. doi: 10.1016/S0140-6736(00)02165-6. [DOI] [PubMed] [Google Scholar]
- 28.Wakelkamp IM, Tan H, Saeed P, Schlingemann RO, Verbraak FD, Blank LE, et al. Orbital irradiation for Graves’ ophthalmopathy: Is it safe? A long-term follow-up study. Ophthalmology. 2004;111:1557–62. doi: 10.1016/j.ophtha.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 29.Viebahn M, Barricks ME, Osterloh MD. Synergism between diabetic and radiation retinopathy: Case report and review. Br J Ophthalmol. 1991;75:629–32. doi: 10.1136/bjo.75.10.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robertson DM, Buettner H, Gorman CA, Garrity JA, Fatourechi V, Bahn RS, et al. Retinal microvascular abnormalities in patients treated with external radiation for graves ophthalmopathy. Arch Ophthalmol. 2003;121:652–7. doi: 10.1001/archopht.121.5.652. [DOI] [PubMed] [Google Scholar]
- 31.Goldberg RA, Perry JD, Hortaleza V, Tong JT. Strabismus after balanced medial plus lateral wall versus lateral wall only orbital decompression for dysthyroid orbitopathy. Ophthal Plast Reconstr Surg. 2000;16:271–7. doi: 10.1097/00002341-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Guo S, Wagner R, Gewirtz M, Maxwell D, Pokorny K, Tutela A, et al. Diplopia and strabismus following ocular surgeries. Surv Ophthalmol. 2010;55:335–58. doi: 10.1016/j.survophthal.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Fatemi N, Dusick JR, de Paiva Neto MA, Malkasian D, Kelly DF. Endonasal versus supraorbital keyhole removal of craniopharyngiomas and tuberculum sellae meningiomas. Neurosurgery. 2009;64(5 Suppl 2):269–84. doi: 10.1227/01.NEU.0000327857.22221.53. [DOI] [PubMed] [Google Scholar]
- 34.Unal M, Leri F, Konuk O, Hasanreisoglu B. Balanced orbital decompression combined with fat removal in Graves ophthalmopathy: Do we really need to remove the third wall? Ophthal Plast Reconstr Surg. 2003;19:112–8. doi: 10.1097/01.IOP.0000056145.71641.F5. [DOI] [PubMed] [Google Scholar]
- 35.O’Malley MR, Meyer DR. Transconjunctival fat removal combined with conservative medial wall/floor orbital decompression for Graves orbitopathy. Ophthal Plast Reconstr Surg. 2009;25:206–10. doi: 10.1097/IOP.0b013e3181a424cc. [DOI] [PubMed] [Google Scholar]


