Abstract
Oerskovia turbata is an unusual bacterial cause of endocarditis and septicemia in immunocompromised patients. In this study, we compared 12 isolates from a 1975 medical center cluster, 11 originally identified as O. turbata (four from the blood of a homograft aortic valve-associated endocarditis patient and seven from contaminated homograft valves) and one CDC group A-3 strain from the blood of a second endocarditis patient with fatal outcome, with eight control strains from unrelated locations. The control strains included type and reference strains of O. turbata, Cellulomonas hominis, and CDC group A-3. The four blood isolates from the first patient and six of the valve isolates shared identical biochemical, antimicrobial susceptibility, and BglI ribotype patterns that differed from the second patient's isolate and control strains. The blood isolate from the second patient and the remaining valve isolate shared a phenotypic and genotypic profile and were phenotypically identical to, but epidemiologically different from, the CDC group A-3 reference strain with the strain-specific enzyme. Also, these isolates differed from the type strain and the other reference strains of C. hominis and O. turbata. Our results indicate that the four blood isolates from the first patient and six of the homograft valve isolates represent a single clone of O. turbata associated with endocarditis. Additionally, our results indicate that the blood isolate from the second patient and one of the homograft valve isolates differ from O. turbata and C. hominis and represent a unique clone of CDC group A-3 associated with fatal endocarditis.
The genus Oerskovia, described by Prauser et al. and Sukapure et al. and amended by Lechevalier, consists of yellow-pigmented microorganisms with branched filaments that break up into motile bacilli (10, 16, 23). The bacterium is isolated from soil, aluminum hydroxide gel antacid, and dry grass cuttings (10, 16, 23). Oerskovia species are infrequently associated with human infections. In 1977, Sottnek et al. studied 35 clinical isolates of Oerskovia and similar organisms that were received from 1957 to 1977 in the Special Bacteriology Section and the Actinomycete Reference Laboratory at the Centers for Disease Control and Prevention (22). Although detailed clinical information was not available at the time, 18 of the isolates were from sites suggestive of invasive infection (blood, cerebrospinal fluid, heart valve and tissue, and liver biopsy tissue). Eight of these isolates were from the same medical center and were described in 1975 by Reller et al. in an investigation of an Oerskovia turbata endocarditis case resulting from the transplantation of a contaminated homograft valve (18). Also listed in the report of Sottnek et al. was a CDC group A-3 isolate that was later identified as a blood isolate from a second endocarditis patient at the same medical center (22).
Since these initial reports, to our knowledge, there have been only three additional reports of O. turbata infection (7, 11, 17). Two catheter-related bacteremias, one in a patient with acute myelogenous leukemia (11) and the other in a patient with AIDS and a coinfection with Comamonas acidovorans (7), have been described. The remaining report described an axillary abscess in a patient with AIDS (17). All these reports involved immunocompromised patients with chronic underlying conditions (7, 11, 17, 18); three of the infections were associated with implanted foreign bodies (homograft valves, catheters), whose removal was necessary for effective cure of the patients (7, 11, 18). There was a high frequency of relapse (75% in spite of treatment) among these patients. However, because of the paucity of infected patients, no reliable conclusions regarding the efficacy of specific antimicrobial therapy for O. turbata infections could be determined.
To test the hypothesis generated by the original investigation team that these patients' infections were linked to use of contaminated homograft valves in the medical center (18), we compared genetic and phenetic characteristics of 12 epidemiologically related isolates (four blood isolates from patient 1, one blood isolate from patient 2, and seven homograft isolates all from the same medical center) with the type strain of O. turbata, four unrelated clinical reference isolates of O. turbata, one reference isolate of CDC group A-3, and the type strain and a reference isolate of Cellulomonas hominis (6).
(This work was presented in part at the 96th General Meeting of the American Society for Microbiology, New Orleans, La., 19 to 23 May 1996.)
MATERIALS AND METHODS
Bacterial strains.
Characteristics of the strains used in this study are given in Table 1. For clarity, their designations from the Actinomycete Reference Laboratory, Special Bacteriology Section, and the University of Colorado Medical Center are listed. The medical center isolates studied included four blood culture isolates of one endocarditis patient (patient 1), one blood culture isolate W6929 of another endocarditis patient (patient 2), and seven isolates from homograft valves. Four unrelated clinical reference isolates of O. turbata (W2796 from lung, W4083 from blood [11], W6035 from blood, and W6123 from an unknown source [22]), one isolate of C. hominis (W6263 [DMMZ CE39]) from cerebrospinal fluid, and one isolate of CDC group A-3 (W6117) from blood were used as controls. The type strain, DSM 9581, and W6263 of C. hominis were received from G. Funke, and the type strain of O. turbata, ATCC 25835, was received from the American Type Culture Collection, Manassas, Va. The strains were stored as suspensions in defibrinated blood in liquid nitrogen or lyophilized in skimmed milk and stored in motility medium (24) until analysis.
TABLE 1.
Isolates used in this studya
| Name | ARL no. | SBS no. | University of Colorado Medical Center no. | Source | Geographic site (reference if applicable) |
|---|---|---|---|---|---|
| O. turbata (ATCC, 25835T) | W6114 | KC1355 | Soil | Unknown | |
| O. turbata | W6123 | C4239 | Unknown (human) | New York (22) | |
| O. turbata | W2796 | Lung | New York | ||
| O. turbata | W4083 | Blood | California (11) | ||
| O. turbata | W6035 | Blood | Wisconsin | ||
| O. turbata | W2622 | Blood (patient 1) | Colorado | ||
| O. turbata | W2728 | Blood (patient 1) | Colorado | ||
| O. turbata | W2729 | Blood (patient 1) | Colorado | ||
| O. turbata (ATCC BAA-886) | W6122 | C8821 | Blood (patient 1) | Colorado (22) | |
| O. turbata | W2732 | C6926 | 243 | Homograft valve | Colorado (22) |
| O. turbata | W2734 | C5919 | 199 | Homograft valve | Colorado (22) |
| O. turbata | W2736 | C5918 | 197 | Homograft valve | Colorado (22) |
| O. turbata | W2739 | C5520 | 145 | Homograft valve | Colorado (22) |
| O. turbata | W6120 (W2760, W2729) | C5614 | 128 | Homograft valve | Colorado (22) |
| O. turbata | W6121 (W2738) | C5519 | 139 | Homograft valve | Colorado (22) |
| CDC group A-3 (ATCC BAA-788) | W6929 (W2777) | C2508 | 610A | Blood (patient 2) | Colorado (22) |
| CDC group A-3b | W6124 (W2733) | C6927 | 246 | Homograft valve | Colorado (22) |
| C. hominis (DSM 9581T, DMMZ CE40) | W6264 | Cerebrospinal fluid | Switzerland (6) | ||
| C. hominis (DMMZ CE39) | W6263 | Cerebrospinal fluid | Switzerland (6) | ||
| CDC group A-3 | W6117 | A3936 | Blood | Ohio (22) |
ARL, Actinomycete Reference Laboratory; SBS, Special Bacteriology Section; ATCC; American Type Culture Collection (Manassas, Va.); DSM, Deutsche Sammlung von Mikroorganismen Zellkulturen (Braunschweig, Germany); DMMZ, Department of Medical Microbiology, University of Zurich, Switzerland.
Previously identified as O. turbata.
Phenotypic characteristics.
All cultures were incubated at 35°C aerobically unless otherwise noted. Gram-stain microscopic and colonial morphology were studied by using the methods described previously (22). Gram stains were made from 1-day heart infusion agar (HIA; Remel, Lenexa, Kans.) slant cultures. Colonial morphology was observed by a light microscope under low power (magnification, ×100) at 2 and 7 days of incubation for the presence of substrate and aerial hyphae. Biochemical characteristics were determined by the methods of Weyant et al. (24) and Berd (2). The optimal growth temperature was determined after 1 day of incubation. All other tests were read at 24 h, 48 h, and 7 days of incubation. Gelatin and casein decomposition tests were incubated an additional 7 days before final reading, and hypoxanthine and xanthine decomposition tests were incubated 21 days before final reading.
Antimicrobial susceptibility studies were performed by a previously described broth microdilution method with cation-supplemented Mueller-Hinton broth (12). The antimicrobial agents tested were amikacin, amoxicillin-clavulanic acid, ampicillin, cefotaxime, ceftriaxone, ciprofloxacin, doxycycline, erythromycin, imipenem, minocycline, sulfamethoxazole (SMX), trimethoprim-sulfamethoxazole (TMP-SMX), and vancomycin. Plates were incubated for 48 h at 35°C. The methods of testing and the breakpoints for resistance for this group of rare organisms have not been standardized or approved by the National Committee for Clinical Laboratory Standards (NCCLS). Therefore, the breakpoints for resistance used were those of the NCCLS for bacteria that grow aerobically (13). A direct beta-lactamase assay, the chromogenic cephalosporin nitrocefin method, was used on representative isolates (14).
Chemotaxonomic analyses.
The methods used for whole-cell analyses for diaminopimelic acid and monosaccharides are those described by Berd (2).
DNA purification and ribotyping.
Strains were subcultured from HIA into Trypticase soy broth (Becton Dickinson, Sparks, Md.) and grown for 1 to 2 days at 35°C before being harvested by centrifugation at 3,795 × g. DNA was purified from lysed protoplasts as described previously by Lasker et al. (9). Genomic DNA (1 to 2 mg/ml) was digested with 20 U of SmaI and BglI (Roche Molecular Biochemicals, Indianapolis, Ind.) for 8 h at 35°C in the buffer recommended by the manufacturer. DNA fragments were then transferred to a nylon membrane (Nytran; Schleicher & Schuell, Keene, N.H.) and hybridized with digoxigenin-labeled cDNA derived from 16S and 23S rRNA of Escherichia coli by reverse transcription as described previously (15). Ribosomal DNA-containing fragments were visualized according to the Genius kit (Roche Molecular Biochemicals) protocol.
16S rDNA sequencing.
Purified genomic DNA was amplified using an Expand high fidelity PCR system (Roche Diagnostics Corporation, Indianapolis, Ind.) proofreading enzyme Pfu (1) to increase fidelity of products. The protocol was modified with the addition of dimethyl sulfoxide (4) to increase the yield of high G+C templates. Briefly, each 50-μl portion contained approximately 10 ng of DNA, 2.5 U of polymerase, 1.5 mM MgCl2, 5% (vol/vol) dimethyl sulfoxide, 200 μM dNTPs, and 100 nM each primers fL1 and rL1 (21). Amplification was performed on an ABI 9700 (Applied Biosystems, Foster City, Calif.) thermocycler at 94°C for 5 min, 35 cycles of 94°C for 15 s, 50°C for 15 s, 72°C for 90 s, and finalized by a single extension of 72°C for 5 min followed by a 4°C hold.
Amplified products were confirmed by running 5 μl on a 1.2% agarose gel with a 500-bp ladder for 30 min at 85 V. Excess dNTPs and primers were inactivated with the ExoSAP method (United States Biochemical Corporation, Cleveland, Ohio). Cycle sequencing was performed with Big Dye v.2 dye terminator chemistry (Applied Biosystems) by using standard protocols with small subunit primers (5, 8, 20, 25). Excess dyes were removed with magnetic carboxylate beads (Agencourt Bioscience, Beverly, Mass.), and reactions were sequenced on an ABI 3100 analyzer (Applied Biosystems). Sequences were assembled in GCG's Seqmerge and trimmed to a minimum of two confirming reads. Unique sequences were aligned and compared with those of other bacterial 16S rRNA gene sequences available in GenBank. Related entries were aligned in Pileup and trimmed to consensus, and further analysis was performed in Bioedit and Treecon. In Bioedit, the sequences were realigned in Clustal W with 1,000 bootstraps, and a distance matrix was created. In Treecon, distances of aligned sequences were estimated with Jukes-Cantor and bootstrapped 1,000 times, and tree topology was determined by neighbor joining (3).
RESULTS
Gram stains from 1-day HIA slant cultures of all isolates showed gram-variable filaments with rudimentary branched forms. The colonial morphology of all patient 1's isolates and six homograft valve isolates on 2-day HIA plates varied from 1 to 2 mm in diameter; all had substrate hyphae, and all produced a yellow, nonsoluble pigment. No aerial hyphae were produced after 7 days of incubation. Except for variability in urease production (3 of 10 produced urease) and the inability of the type strain of O. turbata to reproduce the hydrolysis of casein, all strains had identical physiologic and biochemical profiles. All grew at 25 and 35°C, liquefied gelatin, were motile, and produced acid from d-glucose, d-xylose, lactose, sucrose, and maltose. All isolates were negative in the following tests: decomposition of adenine, hypoxanthine, tyrosine, and xanthine and the production of acid from d-mannitol and l-rhamnose. All isolates were resistant to amikacin, ampicillin, cefotaxime, ceftriaxone, ciprofloxacin, erythromycin, SMX, and TMP-SMX; they were susceptible to amoxicillin-clavulanic acid, doxycycline, imipenem, minocycline, and vancomycin. These isolates differed from the type strain and four clinical reference strains of O. turbata in their susceptibility to certain antimicrobial agents; one of the four clinical reference strains was susceptible to amikacin, all four clinical reference strains were susceptible to cefotaxime, the type strain and three clinical reference strains were susceptible to erythromycin, and the type strain and two clinical reference strains were susceptible to TMP-SMX.
Patient 2's blood isolate and one homograft valve isolate that was originally identified as O. turbata were identical to the clinical reference strain of CDC group A-3, W6117, and these isolates differed from all O. turbata isolates in their production of acid from l-rhamnose, their inability to produce substrate hyphae, and the lack of gelatinase. They differed from C. hominis in their resistance to cefotaxime, ciprofloxacin, and erythromycin. Characteristics that distinguished these isolates from the type strains of O. turbata and C. hominis and the reference clinical isolates are given in Table 2.
TABLE 2.
Comparison of patient 1's, patient 2's, and the homograft valve isolates with the type and clinical reference strains of O. turbata; C. hominis, and CDC group A-3
| Characteristic | Isolate
|
||||||
|---|---|---|---|---|---|---|---|
| O. turbata ATCC 25835T | O. turbata clinical reference isolates (n = 4) | Patient 1's isolates and six homograft valve isolates (n = 10) | C. hominis DSM 9581T | C. hominis clinical reference isolate (n = 1) | CDC group A-3 clinical reference isolate (n = 1) | Patient 2's isolate and one homograft isolate (n = 2) | |
| Pigment on HIA slants in 3 days | Yellow | Yellow | Yellow | Pale yellow | Pale yellow | Pale yellow | Pale yellow |
| Substrate hyphae | +a | 100b | 100 | − | − | − | 0 |
| Fermentative acid production from l-rhamnose | − | 0 | 0 | + | + | + | 100 |
| Urease production (Christensen agar slant) | − | 75 | 30 | + (−c) | + (−c) | − | 0 |
| Gelatin liquefaction (14 days) | + | 100 | 100 | − (+c) | − (+c) | − | 0 |
| Hydrolysis of casein | − | 100 | 100 | − | − | − | 0 |
| Resistanced (MIC) to: | |||||||
| Amikacin (≥ 64 μg/ml) | + | 75 | 100 | + | + | + | 100 |
| Ampicillin (≥4 μg/ml) | + | 100 | 100 | + | + | + | 100 |
| Cefotaxime (≥64 μg/ml) | + | 0 | 100 | − | − | + | 100 |
| Ciprofloxacin (≥4 μg/ml) | − | 100 | 100 | − | − | + | 100 |
| Erythromycin (≥8 μg/ml) | − | 25 | 100 | − | − | + | 100 |
| SMX (>32 μg/ml) | + | 100 | 100 | + | + | + | 100 |
| TMP-SMX (>2 to 38 μg/ml) | − | 50 | 100 | + | + | + | 100 |
Beta-lactamase production was not detected in any of the representative strains tested with the chromogenic cephalosporin nitrocefin method.
None of the isolates contained either the meso or the l isomer of diaminopimelic acid in the whole-cell analysis; however, all isolates contained galactose as the characteristic whole-cell carbohydrate.
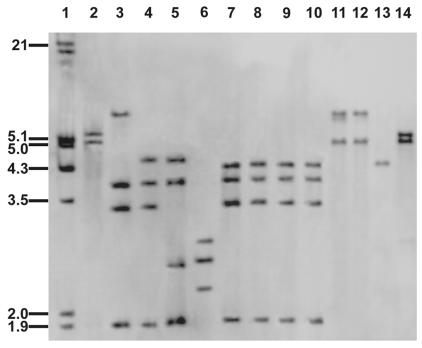
Representative ribotype patterns with BglI-digested DNA are given in Fig. 1. All of patient 1's isolates (n = 4) and six homograft valves isolates (lanes 7 to 10, only 4 of 10 isolates shown) were identical but differed from the patterns of the type strain (lane 2), the unrelated clinical reference isolates (lanes 3 to 6) of O. turbata, patient 2's isolate and one homograft valve isolate (lanes 11 and 12), the type strain of C. hominis (lane 13), and the unrelated clinical reference isolate of CDC group A-3 (lane 14). Patient 2's isolate and one homograft valve isolate (lanes 11 and 12) were identical and were different from the ribotype patterns of the type and clinical reference strains of O. turbata (lanes 2 to 6), patient 1's isolates (lanes 7 to 10), the type strain of C. hominis (lane 13), and the unrelated clinical isolate of CDC group A-3 (lane 14) (Fig. 1). When genomic DNA from patient 1's isolates and six homograft valve isolates was digested with SmaI, the patterns were identical to each other and to the clinical reference isolates of O. turbata (W2796 and W6123) but differed from those of the type strain, O. turbata ATCC 25835, and clinical reference strains W4083 and W6035. When genomic DNA from patient 2's isolate and one homograft valve isolate was digested with SmaI, the patterns were identical to each other and the clinical reference isolate W6117 of CDC group A-3, but these ribotype patterns differed from those of the type strain DSM 9581 of C. hominis and the type strain ATCC 25835 of O. turbata (data not shown).
FIG. 1.
Ribotype patterns from BglI-digested genomic DNAs of medical center isolates and type and clinical reference isolates of O. turbata, CDC group A-3, and C. hominis. Lane 1, bacteriophage 8 DNA molecular size marker digested with EcoRI and HindIII; lane 2, type strain O. turbata ATCC 25835; lanes 3 to 6, clinical reference isolates of O. turbata, W6123, W2796, W4083, and W6035, respectively; lanes 7 to 10, 4 of 10 medical center isolates of O. turbata, W2622, W2728, W2729, and W6122 (patient 1's blood isolates) (homograft valve isolates [n = 6] not shown); lanes 11 and 12, medical center isolates of CDC group A-3, W6124 (homograft valve) and W6929 (patient 2's blood isolate), respectively; lane 13, type strain C. hominis DSM 9581; lane 14, clinical reference isolate of CDC group A-3, W6117.
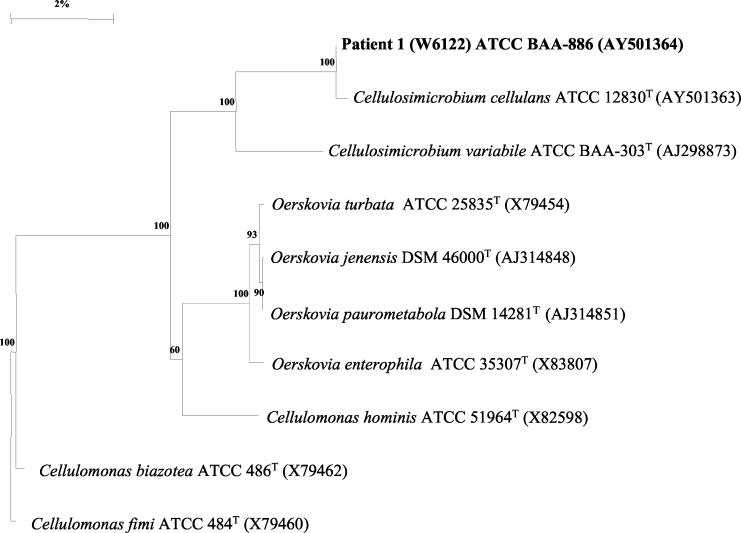
The phylogenetic interrelationship of patient 1's blood isolate W6122 (ATCC BAA-886) with Cellulosimicrobium species, Oerskovia species, and Cellulomonas species is shown in Fig. 2. Isolate W6122 was most closely related to the type strain of Cellulosimicrobium cellulans at 99.8% and related to the type strain of O. turbata at 95.3%.
FIG. 2.
Phylogenetic tree constructed by the neighbor-joining method showing the position of W6122 (patient 1's blood isolate) within the Cellulosimicrobium-Cellulomonas-Oerskovia lineage of the gram-positive bacilli with high G+C contents.
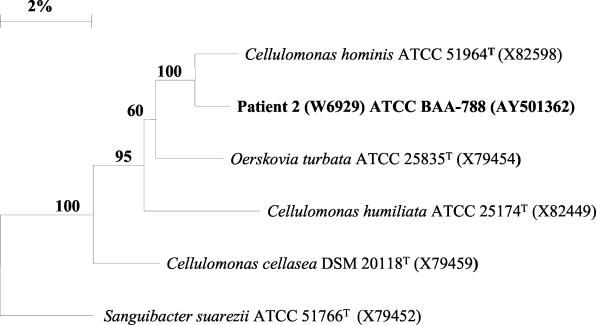
The phylogenetic interrelationship of patient 2's blood isolate W6929 (ATCC BAA-788) with Cellulomonas species, Oerskovia species, and Sanguibacter species is shown in Fig. 3. Isolate W6929 was most closely related to C. hominis at 98.5% and least related to Sanguibacter suarezii at 94.4%.
FIG. 3.
Phylogenetic tree constructed by the neighbor-joining method showing the position of W6929 (patient 2's blood isolate) within the Cellulomonas-Oerskovia-Sanguibacter lineage of the gram-positive bacilli with high G+C contents.
DISCUSSION
In this study, 4 of the 29 positive blood culture isolates and six of the seven homograft valve isolates were available from the initial study by Reller et al. (18). Unfortunately, little additional clinical information was available on the blood isolate from patient 2 other than a fatal outcome. There was also little clinical information available on three of the clinical reference isolates of O. turbata (one each from blood, lung, and an unknown human site). The fourth clinical reference isolate of O. turbata (blood, W4083) was reported by LeProwse et al. (11). These authors found O. turbata in their patient's initial peripheral blood culture and four of six blood cultures collected subsequently via the patient's intravascular catheter. The authors speculated that O. turbata W4083 gained entry into the patient's catheter as an environmental contaminant. In our investigation, we determined by molecular methods (Fig. 1) a clonal relationship between the four blood culture isolates of O. turbata from patient 1 and the six homograft valve isolates. In addition, we defined a clonal relationship between the CDC group A-3 blood isolate of patient 2 and one of the homograft valve isolates. It is not clear what mechanism may have been responsible for these epidemiologically related homograft valves becoming contaminated with the probable environmental strains of O. turbata and CDC group A-3. However, we know that cadaveric homograft valves at the medical center were not usually collected under sterile conditions and were exceedingly difficult to sterilize with antibiotics; despite 50 days of incubation in three antibacterial agents, these valves probably were not adequately sterilized before engraftment (18).
Patient 1's blood isolates and six homograft valve isolates differed from the type strain and the clinical reference isolates of O. turbata by resistance to antimicrobial agents: one clinical isolate was susceptible to amikacin, two clinical isolates were susceptible and two were intermediately susceptible to cefotaxime, the type strain and three clinical isolates were susceptible to erythromycin, and the type strain and two clinical isolates were susceptible to TMP-SMX (Table 2). These differences in antimicrobial susceptibility patterns emphasize the need for determining MIC patterns for clinically significant O. turbata isolates. The initial study occurred almost a quarter of a century ago; at the time, information on optimal treatment of O. turbata endocarditis was lacking. Currently, based on the in vitro results in this study and previous experience with related species, vancomycin would probably be considered the drug of choice (19). This antimicrobial agent is bactericidal and has good in vitro activity to all of the oerskoviae; however, the in vivo activity of vancomycin as effective therapy for endocarditis caused by O. turbata or CDC group A-3 remains unproven.
The development of molecular typing methods has given the clinical laboratory powerful tools to allow a detailed study of the epidemiology of bacterial infections. Our ribotyping data indicate that the problem of probable iatrogenic endocarditis in the medical center was more complicated than originally suspected. Two unique clusters of bacterial isolates were responsible: one cluster of 10 isolates of O. turbata and the other cluster of two isolates of CDC group A-3 were identified based on ribotype patterns, demonstrating the usefulness of ribotyping in furthering the understanding of this epidemiologic investigation. The identical ribotype bands between the CDC group A-3 endocarditis blood isolate and the reevaluated homograft valve isolate, previously identified as O. turbata, were unexpected.
The importance of characterizing these archival O. turbata and CDC group A-3 isolates is evident. This molecular epidemiologic study supports the hypothesis that the homograft valve-related endocarditis was associated with the practices of obtaining and preserving the cadaveric homograft valves at this medical center. In addition, for strains that are difficult to analyze phenotypically, ribotyping has been shown to be useful in species identification, as has been demonstrated for other nocardioform bacteria.
When we analyzed the relatedness of the ribotype patterns for patient 1 and six homograft valve isolates digested with SmaI (the species-specific enzyme for this group of related organisms), we found they had bands that were identical to those of two clinical reference isolates of O. turbata but had only one of four bands in common with the type strain of O. turbata and clinical reference strain W6035 and one of three in common with clinical reference strain W4083. The molecular phylogenetic study revealed that patient 1's blood isolate is most closely related to the genus Cellulosimicrobium.
When we analyzed the ribotype patterns from the SmaI-digested DNAs of patient 2's isolate and one homograft valve isolate, we found they had one of three bands in common with the type strain of O. turbata, bands identical to those of the clinical reference isolate W6117 of CDC group A-3, and one of three bands in common with the type strain of C. hominis. This suggests that one homograft and patient 2 were infected with a new species within the heterogenous CDC group A-3 that was identical to W6117. The phylogenetically distinct position of patient 2's blood isolate (W6929) in the 16S rRNA gene phylogram suggests that this group represents a new species only distantly related to the genus Oerskovia but related to the genus Cellulomonas (Fig. 3).
Further studies of DNA-DNA hybridization will show whether 16S rRNA gene sequencing may assist in the taxonomic classification of these two groups.
With the extended survival of severely compromised patients and the increased use of long-term indwelling catheters and cadaveric homografts, it is highly probable that these microorganisms will be encountered more frequently. Knowledge of the reservoir of these species, their survival capabilities within the environment, their resistance to antimicrobial agents, and newer molecular techniques for strain comparison may assist in more efficient identification and control of O. turbata- and CDC group A-3-associated infections in the future.
REFERENCES
- 1.Barnes, W. M. 1994. PCR amplification of up to 35-kb DNA with high yield from lambda bacteriophage templates. Proc. Natl. Acad. Sci. USA 91:2216-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berd, D. 1973. Laboratory identification of clinically important aerobic actinomycetes. Appl. Microbiol. 25:665-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carvalho, M. G. S., A. G. Steigerwalt, R. E. Morey, P. L. Shewmaker, L. M. Teixeira, and R. R. Facklam. 2004. Characterization of three new enterococcal species, Enterococcus sp. nov. CDC PNS-E1, Enterococcus sp. nov. CDC PNS-E2, and Enterococcus sp. nov. CDC PNS-E3, isolated from human clinical specimens. J. Clin. Microbiol. 42:1192-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chester, N., and D. R. Marshak. 1993. Dimethyl sulfoxide-mediated primer Tm reduction: a method for analyzing the role of renaturation temperature in the polymerase chain reaction. Anal. Biochem. 209:284-290. [DOI] [PubMed] [Google Scholar]
- 5.Edwards, K. J., M. E. Kaufmann, and N. A. Saunders. 2001. Rapid and accurate identification of coagulase-negative staphylococci by real-time PCR. J. Clin. Microbiol. 39:3047-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Funke, G., C. P. Ramos, and M. D. Collins. 1995. Identification of some clinical strains of CDC coryneform group A-3 and A-4 bacteria as Cellulomonas species and proposal of Cellulomonas hominis sp. nov. for some group A-3 strains. J. Clin. Microbiol. 33:2091-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lair, M. I., S. Bentolila, D. Grenet, P. Cahen, and P. Honderlick. 1996. Oerskovia turbata and Comamonas acidovorans bacteremia in a patient with AIDS. Eur. J. Clin. Microbiol. Infect. Dis. 15:424-426. [DOI] [PubMed] [Google Scholar]
- 8.Lane, D. J. 1991. 16S/23S rRNA sequencing, p. 115-175. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley & Sons, Chichester, United Kingdom.
- 9.Lasker, B. A., J. M. Brown, and M. M. McNeil. 1992. Identification and epidemiological typing of clinical and environmental isolates of the genus Rhodococcus with use of a digoxigenin-labeled rDNA gene probe. Clin. Infect. Dis. 15:223-233. [DOI] [PubMed] [Google Scholar]
- 10.Lechevalier, M. P. 1972. Description of a new species, Oerskovia xanthineolytica and emendation of Oerskovia Prauser et al. Int. J. Syst. Bacteriol. 22:260-264. [Google Scholar]
- 11.LeProwse, C. R., M. M. McNeil, and J. M. McCarty. 1989. Catheter-related bacteremia caused by Oerskovia turbata. J. Clin. Microbiol. 27:571-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNeil, M. M., J. M. Brown, W. R. Jarvis, and L. Ajello. 1990. Comparison of species distribution and antimicrobial susceptibility of aerobic actinomycetes from clinical specimens. Rev. Infect. Dis. 12:778-783. [DOI] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 14.O'Callaghan, C. H., A. Morris, S. M. Kirby, and A. H. Shingler. 1972. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob. Agents Chemother. 1:283-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Popovic, T. C., C. A. Bopp, O. Olsvik, and J. Kiehlbauch. 1993. Ribotyping in molecular epidemiology, p. 573-583. In D. H. Persing, T. F. Smith, F. C. Tenover, and T. J. White (ed.), Diagnostic molecular microbiology. American Society for Microbiology, Washington, D.C.
- 16.Prauser, H., M. P. Lechevalier, and H. Lechevalier. 1970. Description of Oerskovia gen. n. to harbor Orskov's motile Nocardia. Appl. Microbiol. 19:534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reina, J., I. Llompart, and J. Altes. 1991. An axillary abscess produced by Oerskovia turbata in an AIDS patient. Rev. Clin. Esp. 188:485-486. [PubMed] [Google Scholar]
- 18.Reller, L. B., G. L. Maddoux, M. R. Eckman, and G. Pappas. 1975. Bacterial endocarditis caused by Oerskovia turbata. Ann. Intern. Med. 83:664-666. [DOI] [PubMed] [Google Scholar]
- 19.Rils, J. D. 2002. Oerskovia species, p. 501-503. In U. L. Yu, R. Weber, and D. Raoult (ed.), Antimicrobial therapy and vaccines, 2nd ed. Apple Tree Productions, LLC, New York, N.Y.
- 20.Sacchi, C. T., A. M. Whitney, L. W. Mayer, R. Morey, A. Steigerwalt, A. Boras, R. S. Weyant, and T. Popovic. 2002. Sequencing of 16S rRNA gene: a rapid tool for identification of Bacillus anthracis. Emerg. Infect. Dis. 8:1117-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Segonds, C., T. Heulin, N. Marty, and G. Chabanon. 1999. Differentiation of Burkholderia species by PCR-restriction fragment length polymorphism analysis of the 16S rRNA gene and application to cystic fibrosis isolates. J. Clin. Microbiol. 37:2201-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sottnek, F. O., J. M. Brown, R. E. Weaver, and G. F. Carroll. 1977. Recognition of Oerskovia species in the clinical laboratory: characterization of 35 isolates. Int. J. Syst. Bacteriol. 27:263-270. [Google Scholar]
- 23.Sukapure, R. S., M. P. Lechevalier, H. Reber, M. L. Higgins, H. A. Lechevalier, and H. Prauser. 1970. Motile nocardoid Actinomycetales. Appl. Microbiol. 19:527-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weyant, R. S., C. W. Moss, R. E. Weaver, et al. 1996. Identification of unusual pathogenic gram-negative aerobic and facultatively anaerobic bacteria, 2nd ed. Williams & Wilkins, Baltimore, Md.
- 25.Wuyts, J., Y. Van de Peer, T. Winkelmans, and R. De Wachter. 2002. The European database on small subunit ribosomal RNA. Nucleic Acids Res. 30:183-185. [DOI] [PMC free article] [PubMed] [Google Scholar]