Abstract
The stability of cryptococcal antigen from Cryptococcus neoformans serotype A and D strains at different temperatures in serum and other solvents was studied. Samples stored at −20 or 4°C had equivalent stabilities as measured by the Premier Cryptococcal Antigen kit and the Cryptococcal Antigen Latex Agglutination System (CALAS) kit. However, using the Premier Cryptococcal Antigen kit, there was a 91% loss of reactivity in samples incubated in human serum for 4 weeks at 37°C. A loss of reactivity of more than 99% was observed after incubation at 45°C for 4 weeks. The capsular antigen was not detected by the Premier Cryptococcal Antigen kit after 16 weeks at elevated temperatures. Antigen titers were also reduced in a latex agglutination assay (CALAS) after 4 weeks at 37 and 45°C. The loss of antigen reactivity was a function of pH and temperature.
The incidence of cryptococcosis has decreased from 5 to 10% of human immunodeficiency virus-positive individuals to less than 1% in areas where highly active antiretroviral therapy is employed (4, 12). However, cryptococcosis remains a leading fungal disease in human immunodeficiency virus-positive individuals. Suspected cases of cryptococcosis are often confirmed through standardized diagnostic assays that detect cryptococcal capsular polysaccharide in samples of serum or cerebrospinal fluid (CSF) from patients. In fact, there have been recent proposals to discontinue culture analysis of CSF and instead to rely exclusively on cryptococcal antigen testing (1).
The measurement of cryptococcal antigen in serum and CSF can also provide a means to assess the response in patients to antifungal therapy. A decrease in titer with the standardized diagnostic kits is assumed to indicate a decrease in the antigen concentration and generally correlates with an improving clinical status of the patient (8). These tests repeatedly demonstrate high sensitivity and specificity (8-10, 13, 14). However, cases of culture-positive individuals whose CSF tested negative for cryptococcal antigen due to low antigen concentration are documented (7). Because the diagnostic assays rely on antibody-based methods, false-negative results could result from any condition that decreases the polysaccharide stability, thereby preventing antibody detection. In this study, we investigated the stability of cryptococcal capsular polysaccharide in human serum at different temperatures over extended periods of time.
Cryptococcus neoformans strains H99 (serotype A) and 24067 (serotype D) were grown in Sabouraud dextrose broth with gentle shaking at 30°C. The capsular polysaccharide antigen glucuronoxylomannan (GXM) was isolated from the medium of cultures grown for 14 days by selective precipitation with hexadecyltrimethylammonium bromide (6). One microgram of lyophilized GXM was dissolved in 1 ml of human serum (Cambrex, Walkersville, Md.), fetal bovine serum (FBS) (Gemini Bio-Products, Woodland, Calif.), phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 8.5 mM Na2HPO4), or ultrapure water and sealed in a 2-ml cryovial (Sarstadt). Samples were incubated at −20, 4, 37, and 45°C. The samples in human serum were tested every 2 weeks with the Premier Cryptococcal Antigen kit and the Cryptococcal Antigen Latex Agglutination System (CALAS) (Meridian Bioscience, Inc., Cincinnati, Ohio), according to the manufacturer's instructions. The samples in FBS, PBS, and water were tested every 4 weeks. A new vial from the −20°C condition was thawed and tested at each time point; the same vials from the other temperatures were repeatedly tested. Samples were diluted 1:10 and 1:30 in diluent buffer for the Premier Cryptococcal Antigen kit. This was particularly important for GXM incubated in water, since high concentrations of the solvent interfered with polysaccharide detection. The PBS samples were not tested with the CALAS kit, as the solvent interfered with the accurate detection of polysaccharide. A single lot of each of the commercially purchased reagents was used. The human serum samples did not contain antibodies reactive to GXM; this was confirmed by dot enzyme assay (2) using 20 μg of GXM and undiluted serum. Each diagnostic assay was performed by a different individual.
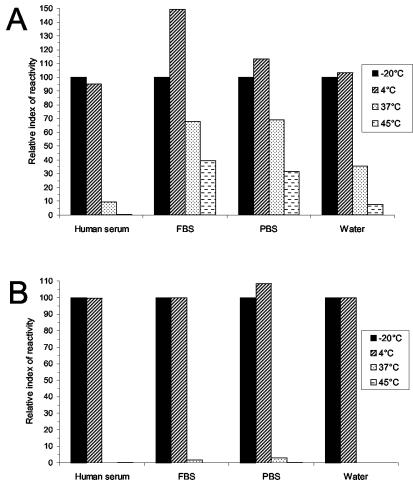
The amounts of capsular antigen GXM detected by both diagnostic kits for the samples incubated at −20 and 4°C were similar (Fig. 1). The effect of freezing on GXM stability is unclear and may explain the apparent increased reactivity in the 4°C FBS and PBS samples. Samples incubated at 37 and 45°C steadily lost reactivity when the Premier Cryptococcal Antigen kit was used, with a significant loss of detection occurring by 4 weeks (Fig. 1A). After 4 weeks of incubation, the human serum sample at 37°C had lost 91% of reactivity, and greater than 99% of reactivity was lost when the sample was incubated at 45°C. Significant loss was also detected in FBS, PBS, and water samples at 37 and 45°C. After 16 weeks, reactivity was not detected in any sample incubated at 37 or 45°C (Fig. 1B). The loss of reactivity was independent of the strain used to produce the antigen (data not shown). Similar results were obtained using the CALAS kit. After 4 weeks, agglutination was decreased in samples containing GXM from strain 24067 in water and incubated at 37 and 45°C. After 10 weeks, GXM from strain 24067 incubated at 37 and 45°C had fourfold-lower titers and GXM from strain H99 incubated at 45°C had at least a 32-fold-lower titer than those of the −20°C samples (data not shown).
FIG. 1.
The immunoreactivity of GXM in different solvents decreased at elevated temperatures. GXM from strain H99 was measured with the Premier Cryptococcal Antigen kit after 4 weeks (A) and 16 weeks (B) of incubation at −20, 4, 37, and 45°C. The samples in the different solvents (four bars for each solvent) were incubated at −20, 4, 37, and 45°C (shown left to right for each solvent). The absorbance of the −20°C samples was set at 100, and the remaining samples were normalized against this value.
After 8 weeks of incubation, the pHs of the samples were measured, since strong alkali treatment (pH 11) of GXM affects recognition by most antibodies (3). The pH values of human serum samples incubated at different temperatures were 8 to 8.5 at −20°C, 8.5 at 4°C, and 9 at 37 and 45°C. The pH values of FBS samples incubated at different temperatures were 7.5 at −20 and 4°C and 8 at 37 and 45°C. The pH was 7.5 for PBS samples incubated at −20, 4, 37, and 45°C. The pH was 5 for water samples incubated at −20, 4, 37, and 45°C. Further increases in the pH values were not detected after 16 weeks.
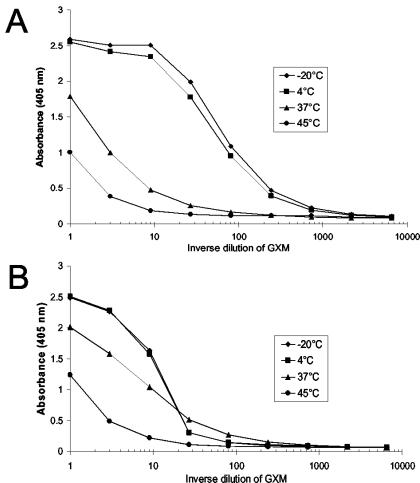
Given the elevated pH values in some samples, it was clinically important to determine whether the O acetylation state of GXM was affected by human serum. O acetylation is an important modification of GXM that is essential for the majority of antibodies to GXM to bind the polysaccharide (3). Enzyme immunoassays (EIAs) were performed using either a monoclonal antibody (MAb) that requires O acetylation (MAb 2D10, 2 μg/ml) (3) or a MAb that is inhibited by O acetylation (MAb 21D2) (11) to capture GXM (Fig. 2). GXM was serially diluted 3 times in each assay and then detected with MAb 18B7 (1 μg/ml). MAb 18B7 recognizes O-acetylated GXM and may bind weakly to de-O-acetylated GXM (3, 11).
FIG. 2.
The loss of O acetylation on GXM incubated in human serum is detected by EIA. GXM from strain H99 that was incubated for 4 weeks in human serum was measured in EIAs using MAbs that can distinguish the acetylation state of the polysaccharide. (A) EIA using MAb 2D10 as the capture MAb and MAb 18B7 as the detecting MAb. (B) EIA using MAb 21D2 as the capture MAb and MAb 18B7 as the detecting MAb.
Binding curves were obtained following spectrophotometric detection at 405 nm of p-nitrophenyl phosphate hydrolysis by alkaline phosphatase-coupled goat anti-mouse immunoglobulin G1 MAb. The binding curves for GXM incubated at 4°C were similar to the −20°C samples in both EIAs. Eighty to 90% of the binding was lost for the 45°C samples in both EIAs. Differences between the EIAs were detected when the 37°C samples were tested. When MAb 2D10 was the capture MAb, the 37°C sample had 27-fold less binding than the −20°C sample. In contrast, an approximately twofold reduction in binding was detected in the 37°C sample when MAb 21D2 was the capture MAb. The increased binding of the 37°C sample detected by MAb 21D2 compared to the binding detected by MAb 2D10 suggests that O acetylation of GXM was lost in human serum at 37°C. More extensive loss of O acetylation in the GXM incubated at 45°C probably occurred but could not be distinguished by these EIAs, since both assays utilized a detecting MAb that recognizes O-acetylated GXM.
The failure to detect GXM by the Premier Cryptococcal Antigen kit and the decrease in titer by the CALAS kit demonstrated that the immunogenicity of the polysaccharide is not stable after prolonged incubation at 37 and 45°C. The increase in antigen detection by MAb 21D2, which is inhibited by O acetylation on the antigen, and the higher pH values of human serum and FBS samples incubated at 37 and 45°C suggest that storage under prolonged mild alkaline conditions causes the loss of acetylation and may lead to a false-negative result. However, storage temperature is also critical. Both diagnostic kits detected losses in reactivity in the samples in PBS and water at 37 and 45°C, despite the fact that the samples had the same pH as the samples stored at −20 and 4°C. These results are not influenced by proteins or other serum components, because pronase processing is part of the CALAS procedure and similar results were obtained in the nonserum samples.
In the clinical setting where rapid diagnosis is essential, the majority of tests for cryptococcal antigen are conducted shortly after the sample is obtained and therefore should retain optimal detection. In the rare instances where samples were not refrigerated or were stored under conditions that are suspected of increasing the pH, such as the use of an improper diluent, our data suggest that the results obtained from the Premier Cryptococcal Antigen or CALAS kit may underestimate the amount of antigen present.
Our findings may be particularly relevant to clinical studies of cryptococcosis where serum is banked for extended periods of time. The loss of reactivity observed at elevated temperatures may reflect a process that occurs at a significantly lower rate in frozen samples, as stated by the Arrhenius equation, and would be detected only after longer storage times than those tested in our study. Furthermore, the instability of the cryptococcal antigen at 37°C in human serum suggests that the immunoreactivity of the antigen decreases over time in patients and that clinical strains could vary in their stability because of differences in O-acetylation content (5). This would imply that diagnostic kits may be underestimating the quantity of antigen present and are biased towards more recently synthesized antigen. The instability of GXM at the elevated temperatures in all solvents tested would suggest that GXM may also be unstable in CSF samples. If this is confirmed, then antigen detection in CSF would also not be accurate. Hence, we urge caution in the interpretation of negative results, particularly when measurements involve samples that have been stored for some time.
Acknowledgments
This work was supported in part by Public Health Service grants AI33774, AI3342, and HL59842-01 from the National Institutes of Health. D.C.M. is a Burroughs Wellcome Fund fellow of the Life Science Research Foundation.
REFERENCES
- 1.Barenfanger, J., J. Lawhorn, and C. Drake. 2004. Nonvalue of culturing cerebrospinal fluid for fungi. J. Clin. Microbiol. 42:236-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belay, T., R. Cherniak, E. B. O'Neill, and T. R. Kozel. 1996. Serotyping of Cryptococcus neoformans by dot enzyme assay. J. Clin. Microbiol. 34:466-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casadevall, A., J. Mukherjee, S. J. Devi, R. Schneerson, J. B. Robbins, and M. D. Scharff. 1992. Antibodies elicited by a Cryptococcus neoformans-tetanus toxoid conjugate vaccine have the same specificity as those elicited in infection. J. Infect. Dis. 165:1086-1093. [DOI] [PubMed] [Google Scholar]
- 4.Casadevall, A., and J. Perfect. 1998. Cryptococcus neoformans. ASM Press, Washington, D.C.
- 5.Cherniak, R., L. C. Morris, T. Belay, E. D. Spitzer, and A. Casadevall. 1995. Variation in the structure of glucuronoxylomannan in isolates from patients with recurrent cryptococcal meningitis. Infect. Immun. 63:1899-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cherniak, R., H. Valafar, L. C. Morris, and F. Valafar. 1998. Cryptococcus neoformans chemotyping by quantitative analysis of 1H nuclear magnetic resonance spectra of glucuronoxylomannans with a computer-simulated artificial neural network. Clin. Diagn. Lab. Immunol. 5:146-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Currie, B. P., L. F. Freundlich, M. A. Soto, and A. Casadevall. 1993. False-negative cerebrospinal fluid cryptococcal latex agglutination tests for patients with culture-positive cryptococcal meningitis. J. Clin. Microbiol. 31:2519-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gade, W., S. W. Hinnefeld, L. S. Babcock, P. Gilligan, W. Kelly, K. Wait, D. Greer, M. Pinilla, and R. L. Kaplan. 1991. Comparison of the PREMIER cryptococcal antigen enzyme immunoassay and the latex agglutination assay for detection of cryptococcal antigens. J. Clin. Microbiol. 29:1616-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray, L. D., and G. D. Roberts. 1988. Experience with the use of pronase to eliminate interference factors in the latex agglutination test for cryptococcal antigen. J. Clin. Microbiol. 26:2450-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamilton, J. R., A. Noble, D. W. Denning, and D. A. Stevens. 1991. Performance of cryptococcus antigen latex agglutination kits on serum and cerebrospinal fluid specimens of AIDS patients before and after pronase treatment. J. Clin. Microbiol. 29:333-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McFadden, D. C., and A. Casadevall. 2004. Unexpected diversity in the fine specificity of monoclonal antibodies that use the same variable region gene to glucuronoxylomannan of Cryptococcus neoformans. J. Immunol. 172:3670-3677. [DOI] [PubMed]
- 12.Perfect, J. R., and A. Casadevall. 2002. Cryptococcosis. Infect. Dis. Clin. N. Am. 16:837-874, v-vi. [DOI] [PubMed] [Google Scholar]
- 13.Temstet, A., P. Roux, J. L. Poirot, O. Ronin, and F. Dromer. 1992. Evaluation of a monoclonal antibody-based latex agglutination test for diagnosis of cryptococcosis: comparison with two tests using polyclonal antibodies. J. Clin. Microbiol. 30:2544-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu, T. C., and S. Y. Koo. 1983. Comparison of three commercial cryptococcal latex kits for detection of cryptococcal antigen. J. Clin. Microbiol. 18:1127-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]