Abstract
Purpose: The aim of this study is to examine whether or not myocilin (MYOC) genetic variations are associated with susceptibility to primary angle-closure glaucoma (PACG) in the Han Chinese population. Methods: Four single-nucleotide polymorphisms (SNPs)—rs235913, rs183532, rs12076134, and rs235875—in the MYOC gene were genotyped in 212 adult patients with PACG and 255 age-, sex-, and ethnic-matched healthy controls by using a polymerase chain reaction–restriction fragment length polymorphism assay. Data were analyzed by chi-square analysis. Results: The four SNPs in the MYOC gene were in the Hardy–Weinberg equilibrium in all the subjects. The frequencies of A allele rs183532 were significantly different between the PACG patients and the controls (0.238 vs. 0.169, p=0.008; OR=1.541; 95% CI: 1.117–2.127). The frequencies of the AA genotype and A allele of rs235913 were increased in PACG patients compared with controls, but the difference was not significant (p=0.037, p=0.017, respectively). A comparison of the distributions of the genotypes and alleles of rs12076134 and rs235875 showed no statistically significant differences between the PACG patients and the controls (p>0.05). Haplotype analysis indicated that the frequency of the AATG and AATA haplotypes was significantly higher for PACG patients than for control subjects (both p<0.001). However, the frequency of CGGA and CGTG haplotypes was lower for PACG patients than for control subjects (p<0.001). Conclusions: Our study suggests that rs183532 is associated with an increased risk of PACG in the Chinese Han population.
Introduction
Glaucoma is one of the largest causes of blindness worldwide. Globally, there are more than 70 million patients with glaucoma. It is estimated that there will be 79.6 million subjects suffering with glaucoma and about 11.2 million patients with bilateral blindness by 2020 (Quigley and Broman, 2006). In China, it is reported that primary angle-closure glaucoma (PACG) has relatively high visual morbidity rates and accounts for half of all blind glaucoma patients (Casson, 2008). According to the epidemiological survey, the majority of patients with PACG live in Asian regions, especially in China (Congdon et al., 1992; Foster et al., 1996). The characteristics of PACG consist of increased thickness of the lens (Foster et al., 1996), a shallow anterior chamber (Lin et al., 1997), and a short axial length (Abu-Amero et al., 2007), often accompanied by hypermetropic refraction error (Congdon et al., 1996; Dai et al., 2008). To date, the mechanism of PACG remains largely unknown. It is generally accepted that PACG is a multifactorial disorder resulting from the interaction between a genetic predisposition and environmental elements (Amerasinghe et al., 2011). It is demonstrated that the risk of developing PACG is much higher in families, in which first-degree relatives have the disease (Wang et al., 2002). Recently, genome-wide association studies have identified three susceptibility loci (rs110224102 in PLEKHA7, rs3753841 in COL11A1, and rs1015213 located between PCMTD1 and ST18 on chromosome 8q) for PACG (Vithana et al., 2012). Association studies also suggest the involvement of several single-nucleotide polymorphisms (SNPs) of the matrix metalloproteinase-9 (MMP-9) gene (Cong et al., 2009; Awadalla et al., 2011), optineurin (Rezaie et al., 2002), ABCC5 (Karla et al., 2009), and tumor necrosis factor-a (Wang et al., 2012). However, these genes only partly explain the genetic predisposition to PACG, and more research is needed to determine the causative genes of this disease.
MYOC is the first gene identified to be responsible for POAG (Sheffield et al., 1993; Stone et al., 1997). Mutations in MYOC account for over 8% of JOAG and 3–4% of adult-onset POAG (Adam et al., 1997; Mansergh et al., 1998). MYOC consists of three exons and encodes 504 amino acid residues. Myocilin is an acidic protein that contains an NH2-terminal myosin-like domain and a COOH-terminal olfactomedin-like domain (Waryah et al., 2013). Almost 80 mutations have been found in MYOC and about 90% of the mutations are located in the olfactomedin-like domain encoded by exon 3 (Fingert et al., 1999; Kanagavalli et al., 2003; Cheng et al., 2012; Waryah et al., 2013; Zhou et al., 2013). Although several studies indicated that the MYOC mutation was associated with POAG (Cai et al., 2012; Liu et al., 2012; Mendoza-Reinoso et al., 2012), the relationship between MYOC genetic polymorphism and PACG remains unclear. Although in the Quebec population, mutation in MYOC has been reported in isolated patients with PACG (Faucher et al., 2002), the association of MYOC mutation with PACG in the Chinese population is inconsistent. Dai et al. (2008) found that MYOC gene mutation was associated with PACG in a Chinese population. However, the study of Aung et al. (2005) did not support a role for MYOC mutations in the pathogenesis of PACG in the Chinese. Therefore, we performed this case–control study to clarify the relationship between MYOC and PACG in a Chinese population.
Subjects and Methods
Subjects
A total of 212 patients with PACG and 255 age-, sex-, and ethnic-matched healthy controls were recruited from General Hospital of Chinese People's Liberation Army, and No. 181 Hospital of Guilin from 2007 to 2013. Both the patients and healthy controls underwent a full ophthalmic examination, including a visual acuity test, a slit-lamp examination of the anterior chamber, a gonioscopy, the measurement of central corneal thickness and intraocular pressure (IOP), a fundus examination with special attention to optic disc parameters, and a visual field test. A total of 212 patients were selected for the PACG group according to the following diagnostic criteria: the presence of glaucomatous optic neuropathy with a cup:disc ratio ≥0.7, peripheral visual loss, an IOP of more than 21 mmHg, and the presence of at least 180 degrees of closed angle in which the trabecular meshwork (TM) was not visible on a gonioscopy. Patients with secondary angle-closure glaucoma, which was caused by uveitis, trauma, or lens subluxation, were excluded. The healthy individuals came from the same geographical regions as the PACG patients, and the controls were age-, sex-, and ethnic-matched with the PACG patients. The clinical characteristics of these subjects are shown in Table 1. The study was approved by the ethics committee of the General Hospital of Chinese People's Liberation Army and met the tenets of the Declaration of Helsinki. Informed consent was obtained from all of the subjects.
Table 1.
Clinical Characteristics of Study Participants
| Clinical features | PACG patients | Controls |
|---|---|---|
| Age (mean±SD) | 55.2±10.3 | 56.5±10.6 |
| Number | 212 | 255 |
| Sex (n, % female) | 115 (54.2) | 137 (53.7) |
| IOP in mmHg (mean±SD) | 25.2±3.4 | 12.2±4.3 |
| C/D (mean±SD) | 0.71±0.10 | 0.20±0.10 |
IOP, intraocular pressure; PACG, primary angle-closure glaucoma; SD, standard deviation.
SNP selection and genotyping
There are 541 SNPs for the human MYOC gene listed in the National Center for Biotechnology Information SNP database (www.ncbi.nlm.nih.gov/SNP). We also screened the data for the TagSNPs on the International HapMap Project website (www.hapmap.org/). According to the Xie's protocol (Xie et al., 2010, 2011), we utilized the Haploview 4.2 software and the HapMap phase II database and obtained four tagging SNPs (rs235913, rs183532, rs12076134, and rs235875) for Chinese Hans using minor allele frequency ≥0.10 and linkage disequilibrium (LD) patterns with r2≥0.8 as a cutoff.
Blood samples were collected from all participants, and genomic DNA was extracted from the peripheral blood leukocytes by phenol and chloroform extraction. Genotyping was confirmed by TaqMan methods according to the manufacturer's instructions. To ensure the results were verified, we used sequenced genomic DNAs as positive controls in our assays.
Quality control
To ensure the results were verified, of the genotyped samples, 10% were duplicated and there was at least one positive and one negative control per 96-well DNA plate in our assays. The accuracy of the genotyping was determined by the genotype concordance between duplicate samples. We obtained a 100% concordance between the genotyped duplicate samples for each of the SNPs. The genotyping success rate for each SNP was 100%.
Statistical analysis
The differences in age and gender between the cases and the controls were assessed by the t-test and χ2 test, respectively. The Hardy–Weinberg equilibrium was tested using the χ2 test. The significance of the differences in the allele and genotype distribution between the patients and the controls was evaluated with the use of the χ2 test performed by using SPSS (version 17.0; SPSS, Inc., Chicago, IL). When the number of genotypes or alleles was fewer than 5 counts, the Fisher's exact test was used. Based on the genotype data of the genetic variations, we performed the LD analysis and haplotype-based case–control analysis, using the expectation maximization algorithm and the software SNPAlyze version 3.2 (Dynacom Co, Ltd.,Yokohama, Japan). The pairwise LD analysis was performed using four SNP pairs. We used |D′| values of >0.5 to assign SNP locations to one haplotype block. SNPs with an r2 value of <0.5 were selected as tagged. In the haplotype-based case–control analysis, haplotypes with a frequency of <0.03 were excluded. The frequency distribution of the haplotypes was calculated by performing a permutation test using the SHEsis software. In addition, logistic regression analysis was performed to assess the contribution of the major risk factors. A Bonferroni corrected p-value of less than 0.0125 was considered statistically significant.
Results
Study population characteristics
The patients in the PACG cohort numbered 212 and included 97 male and 115 female subjects. The average age of the PACG patients was 55.2±10.3 years. The healthy controls cohort included 255 subjects (90 males, 135 females) with a mean age of 56.5±10.6 years. The demographic characteristics between the PACG cases and the healthy controls were similar. There was no significant difference between the cases and the controls with respect to age and gender. The clinical features of the patients with PACG and the healthy controls are summarized in Table 1.
Association between MYOC polymorphisms and glaucoma
The four SNPs in the MYOC gene were in the Hardy–Weinberg equilibrium in all the subjects. The frequencies of A allele rs183532 were significantly different between the PACG patients and the controls (0.238 vs. 0.169, p=0.008; OR=1.541; 95% CI: 1.117–2.127, Table 2). The frequencies of the AA genotype and A allele of rs235913 were increased in PACG patients compared with controls, but the difference was not significant (p=0.037, p=0.017, respectively, Table 2). A comparison of the distributions of the genotypes and alleles of rs12076134 and rs235875 showed no statistically significant differences between the PACG patients and the controls (p>0.05, Table 2).
Table 2.
Distribution of Genotypes and Alleles
| SNP | Group | n | Genotypes | p | Alleles | p | OR [95% CI] | |||
|---|---|---|---|---|---|---|---|---|---|---|
| rs235913 | A/A | A/C | C/C | A | C | |||||
| PACG | 212 | 46 (21.7%) | 123 (58.0%) | 43 (20.3%) | 0.037 | 215 (0.507) | 209 (0.493) | 0.017 | 1.366 [1.055–1.776] | |
| Control | 255 | 39 (15.3%) | 141 (55.3%) | 75 (29.4%) | 219 (0.429) | 291 (0.571) | ||||
| rs183532 | A/A | A/G | G/G | A | G | |||||
| PACG | 212 | 13 (0.061) | 75 (0.354) | 124 (0.585) | 0.024 | 101 (0.238) | 323 (0.762) | 0.008 | 1.541 [1.117–2.127] | |
| Control | 255 | 6 (0.024) | 74 (0.290) | 175 (0.686) | 86 (0.169) | 424 (0.831) | ||||
| rs12076134 | G/G | G/T | T/T | G | T | |||||
| PACG | 212 | 10 (0.047) | 74 (0.349) | 128 (0.604) | 0.800 | 94 (0.222) | 330 (0.778) | 0.527 | 0.905 [0.666–1.230] | |
| Control | 255 | 15 (0.059) | 92 (0.361) | 148 (0.580) | 122 (0.239) | 388 (0.761) | ||||
| rs235875 | A/A | A/G | G/G | A | G | |||||
| PACG | 212 | 33 (0.156) | 97 (0.458) | 82 (0.387) | 0.989 | 163 (0.384) | 261 (0.616) | 0.905 | 0.984 [0.755–1.282] | |
| Control | 255 | 40 (0.157) | 118 (0.463) | 97 (0.380) | 198 (0.388) | 312 (0.612) | ||||
Bold value indicates the frequency of the AATG and AATA haplotypes was significantly higher for PACG patients than for control subjects (both p<0.001).
SNP, single-nucleotide polymorphism.
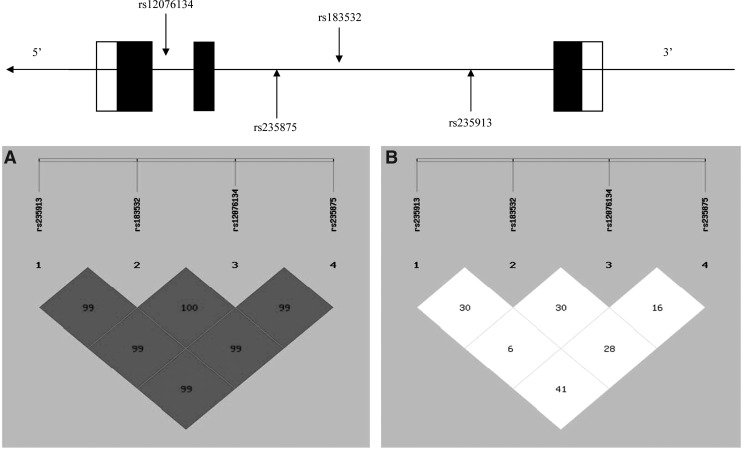
Figure 1 shows patterns of LD in the MYOC gene, with their |D′| and r2 values. All four SNPs are located in one haplotype block because all |D′| are beyond 0.5. All four SNPs were available for the performance of a haplotype-based case–control study because all of the r2 values were below 0.5.
FIG. 1.
Top: The structure of the human MYOC gene. This gene consists of three exons separated by two introns. Boxes indicate exons, and lines indicate introns and intergenic regions. Filled boxes indicate coding regions. Arrows mark the locations of polymorphisms. Below: The patterns of linkage disequilibrium in the MYOC gene, with their |D′| (A) and r2 values (B).
In the haplotype-based case–control analysis, haplotypes were established through the use of four SNPs (Table 2). The frequency of the AATG and AATA haplotypes was significantly higher for PACG patients than for control subjects (both p<0.001, Table 3). However, the frequency of the CGGA and CGTG haplotypes was lower for PACG patients than for control subjects (p<0.001, Table 3).
Table 3.
The Frequency of Haplotypes in Case and Control
| Haplotypes | Case (freq) | Control (freq) | χ2 | Pearson's p | OR [95% CI] |
|---|---|---|---|---|---|
| AAGA | 62.15 (0.147) | 85.94 (0.169) | 0.476 | 0.490 | 0.882 [0.618–1.259] |
| CGGA | 6.14 (0.014) | 36.00 (0.071) | 16.070 | <0.001 | 0.200 [0.084–0.476] |
| AGTA | 55.28 (0.130) | 75.00 (0.147) | 0.276 | 0.599 | 0.905 [0.622–1.316] |
| AATG | 52.59 (0.124) | 22.05 (0.043) | 22.076 | <0.001 | 3.259 [1.946–5.457] |
| AATA | 14.95 (0.035) | 7.00 (0.014) | 15.187 | <0.001 | 2.350 [1.595–7.145] |
| CGTG | 171.21 (0.404) | 289.94 (0.569) | 20.616 | <0.001 | 0.545 [0.419–0.709] |
Bold values indicate the frequency of the AATG and AATA haplotypes was significantly higher for PACG patients than for control subjects (both p<0.001).
Discussion
In this study, we found that the genetic polymorphisms and haplotypes of MYOC were associated with the risk of PACG in the Chinese Han population. At present, it is generally accepted that PACG is a complex multifactorial and polygenic disorder, in which multiple environmental and genetic factors are simultaneously involved. The foundation for human studies examining putative causative genes that may be involved in PACG is based on a candidate gene approach. This involves selecting a functionally relevant gene to study and subsequently investigating its association with the PACG phenotype.
MYOC encodes the protein myocilin, which is believed to have a role in the cytoskeletal function. MYOC is expressed in many ocular tissues, including the TM, and was revealed to be the TM glucocorticoid-inducible response protein (TIGR). The TM is a specialized eye tissue essential in regulating IOP, and mutations in MYOC have been identified as the cause of hereditary juvenile-onset open-angle glaucoma. The previous study indicated that MYOC mutation is associated with the risk for POAG (Kanagavalli et al., 2003; Cheng et al., 2012; Liu et al., 2012). However, the relationship between MYOC polymorphism and PACG remains unclear. MYOC is preferentially expressed in the anterior segment of the eye, where high amounts of myocilin mRNA have been detected in the TM, sclera, ciliary body, and iris. The finding that mutant MYOC proteins form aggregates that are not secreted suggests that mutant MYOC accumulates in TM cells and disturbs normal cellular function, resulting in impaired outflow of aqueous humor, elevated IOP, and glaucoma (Adam et al., 1997). However, as MYOC is expressed in the retina, it is also possible that MYOC causes glaucoma at the retinal ganglion cell level (Ortego et al., 1997). However, the role of the myocilin gene in PACG remains to be established. Worldwide, the most common form of PACG is the chronic asymptomatic type, in which affected individuals have painless progressive visual loss associated with increased IOP and optic disc cupping. The clinical phenotype has some similarities to POAG, the main differences being the configuration of the angle and a stronger association between IOP and severity of optic neuropathy (Tamm et al., 1999). Previous studies have reported the presence of MYOC mutations in individuals with PACG (Faucher et al., 2002; Aung et al., 2005; Dai et al., 2008). These reports provided preliminary evidence that PACG subjects may carry MYOC mutations, but were limited by a small sample size.
In the present study, we find that rs183532 of the MYOC gene was significantly associated with the risk of glaucoma. The AA or AG genotype of rs183532 significantly differed between PACG patients and control subjects, indicating that the risk of PACG is increased in subjects with the A allele of rs183532. In addition, we successfully established haplotypes for the MYOC gene from the different combinations of the four SNPs. The AATG and AATA haplotypes were significantly higher for PACG patients than for control subjects. However, the frequency of CGGA and CGTG haplotypes was lower for PACG patients than for control subjects.
In conclusion, the present results indicate that PACG is associated with the polymorphism rs183532 of the MYOC gene in the Chinese population.
Acknowledgment
The authors are grateful to all the patients and healthy controls for their participation in this study.
Author Disclosure Statement
No competing financial interests exist.
References
- Abu-Amero KK, Morales J, Osman MN, Bosley TM. (2007) Nuclear and mitochondrial analysis of patients with primary angle-closure glaucoma. Invest Ophthalmol Vis Sci 48:5591–5596 [DOI] [PubMed] [Google Scholar]
- Adam MF, Belmouden A, Binisti P, et al. (1997) Recurrent mutations in a single exon encoding the evolutionarily conserved olfactomedin-homology domain of TIGR in familial open-angle glaucoma. Hum Mol Genet 12:2091–2097 [DOI] [PubMed] [Google Scholar]
- Amerasinghe N, Zhang J, Thalamuthu A, et al. (2011) The heritability and sibling risk of angle closure in Asians. Ophthalmology 118:480–485 [DOI] [PubMed] [Google Scholar]
- Aung T, Yong VH, Chew PT, et al. (2005) Molecular analysis of the myocilin gene in Chinese subjects with chronic primary-angle closure glaucoma. Invest Ophthalmol Vis Sci 46:1303–1306 [DOI] [PubMed] [Google Scholar]
- Awadalla MS, Burdon KP, Kuot A, et al. (2011) Matrix metalloproteinase-9 genetic variation and primary angle closure glaucoma in a Caucasian population. Mol Vis 17:1420–1424 [PMC free article] [PubMed] [Google Scholar]
- Cai SP, Muhemaiti P, Yin Y, et al. (2012) A novel MYOC heterozygous mutation identified in a Chinese Uygur pedigree with primary open-angle glaucoma. Mol Vis 18:1944–1951 [PMC free article] [PubMed] [Google Scholar]
- Casson RJ. (2008) Anterior chamber depth and primary angle-closure glaucoma: an evolutionary perspective. Clin Exp Ophthalmol 36:70–77 [DOI] [PubMed] [Google Scholar]
- Cheng JW, Cheng SW, Ma XY, et al. (2012) Myocilin polymorphisms and primary open-angle glaucoma: a systematic review and meta-analysis. PLoS One 7:e46632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong Y, Guo X, Liu X, et al. (2009) Association of the single nucleotide polymorphisms in the extracellular matrix metalloprotease-9 gene with PACG in southern China. Mol Vis 15:1412–1417 [PMC free article] [PubMed] [Google Scholar]
- Congdon N, Wang F, Tielsch JM. (1992) Issues in the epidemiology and population-based screening of primary angle-closure glaucoma. Surv Ophthalmol 36:411–423 [DOI] [PubMed] [Google Scholar]
- Congdon NG, Quigley HA, Hung PT, et al. (1996) Screening techniques for angle-closure glaucoma in rural Taiwan. Acta Ophthalmol Scand 74:113–119 [DOI] [PubMed] [Google Scholar]
- Dai X, Nie S, Ke T, et al. (2008) Two variants in MYOC and CYP1B1 genes in a Chinese family with primary angle-closure glaucoma. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 25:493–496 [PubMed] [Google Scholar]
- Faucher M, Anctil JL, Rodrigue MA, et al. (2002) Founder TGIR/myocilin mutations for glaucoma in Quebec population. Hum Mol Genet 11:2077–2090 [DOI] [PubMed] [Google Scholar]
- Fingert JH, Héon E, Liebmann JM, et al. (1999) Analysis of myocilin mutations in 1703 glaucoma patients from five different populations. Hum Mol Genet 8:899–905 [DOI] [PubMed] [Google Scholar]
- Foster PJ, Baasanhu J, Alsbirk PH, et al. (1996) Glaucoma in Mongolia. A population-based survey in Hövsgöl province, northern Mongolia. Arch Ophthalmol 114:1235–1241 [DOI] [PubMed] [Google Scholar]
- Kanagavalli J, Krishnadas SR, Pandaranayaka E, et al. (2003) Evaluation and understanding of myocilin mutations in Indian primary open angle glaucoma patients. Mol Vis 9:606–614 [PubMed] [Google Scholar]
- Karla PK, Quinn TL, Herndon BL, et al. (2009) Expression of multidrug resistance associated protein 5 (MRP5) on cornea and its role in drug efflux. J Ocul Pharmacol Ther 25:121–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YW, Wang TH, Hung PT. (1997) Biometric study of acute primary angleclosure glaucoma. J Formos Med Assoc 96:908–912 [PubMed] [Google Scholar]
- Liu W, Liu Y, Challa P, et al. (2012) Low prevalence of myocilin mutations in an African American population with primary open-angle glaucoma. Mol Vis 18:2241–2246 [PMC free article] [PubMed] [Google Scholar]
- Mansergh FC, Kenna PF, Ayuso C, et al. (1998) Novel mutations in the TIGR gene in early and late onset open angle glaucoma. Hum Mutat 11:244–251 [DOI] [PubMed] [Google Scholar]
- Mendoza-Reinoso V, Patil TS, Guevara-Fujita ML, et al. (2012) Novel and known MYOC exon 3 mutations in an admixed Peruvian primary open-angle glaucoma population. Mol Vis 18:2067–2075 [PMC free article] [PubMed] [Google Scholar]
- Ortego J, Escribano J, Coca-Prados M. (1997) Cloning and characterization of subtracted cDNAs from human ciliary body library encoding TIGR, a protein involved in juvenile open angle glaucoma with homology to myosin and olfactomedin. FEBS Lett 413:349–353 [DOI] [PubMed] [Google Scholar]
- Quigley HA, Broman AT. (2006) The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 90:262–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaie T, Child A, Hitchings R, et al. (2002) Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science 295:1077–1079 [DOI] [PubMed] [Google Scholar]
- Sheffield VC, Stone EM, Alward WL, et al. (1993) Genetic linkage of familial open angle glaucoma to chromosome 1q21-q31. Nat Genet 4:47–50 [DOI] [PubMed] [Google Scholar]
- Stone EM, Fingert JH, Alward WL, et al. (1997) Identification of a gene that causes primary open angle glaucoma. Science 275:668–670 [DOI] [PubMed] [Google Scholar]
- Tamm E, Russell P, Epstein DL, et al. (1999) Modulation of myocilin/TIGR expression in human trabecular meshwork. Invest Ophthalmol Vis Sci 40:2577–2582 [PubMed] [Google Scholar]
- Vithana EN, Khor CC, Qiao C, et al. (2012) Genome-wide association analyses identify three new susceptibility loci for primary angle closure glaucoma. Nat Genet 44:1142–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CY, Shen YC, Wei LC, et al. (2012) Polymorphism in the TNF-alpha(-863) locus associated with reduced risk of primary open angle glaucoma. Mol Vis 18:779–785 [PMC free article] [PubMed] [Google Scholar]
- Wang N, Wu H, Fan Z. (2002) Primary angle closure glaucoma in Chinese and Western populations. Chin Med J (Engl) 115:1706–1715 [PubMed] [Google Scholar]
- Waryah AM, Narsani AK, Sheikh SA, et al. (2013) The novel heterozygous Thr377Arg MYOC mutation causes severe Juvenile Open Angle Glaucoma in a large Pakistani family. Gene 528:356–359 [DOI] [PubMed] [Google Scholar]
- Xie X, Ma YT, Yang YN, et al. (2010) Polymorphisms in the SAA1/2 gene are associated with carotid intima media thickness in healthy Han Chinese subjects: the Cardiovascular Risk Survey. PLoS One 5:e13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Ma YT, Yang YN, et al. (2011) Polymorphisms in the SAA1 gene are associated with ankle-to-brachial index in Han Chinese healthy subjects. Blood Press 20:232–238 [DOI] [PubMed] [Google Scholar]
- Zhou XM, Yin Y, Fan N, et al. (2013) Single nucleotide polymorphism of MYOC affected the severity of primary open angle glaucoma. Int J Ophthalmol 6:264–268 [DOI] [PMC free article] [PubMed] [Google Scholar]