This month’s installment of Generally Physiological considers an odorant receptor evolutionarily linked to mosquito preference for humans, the evolution of voltage-gated Ca2+ and Na+ channels, and the first single-channel kinetic analysis of the bacterial pentameric ligand-gated ion channel ELIC.
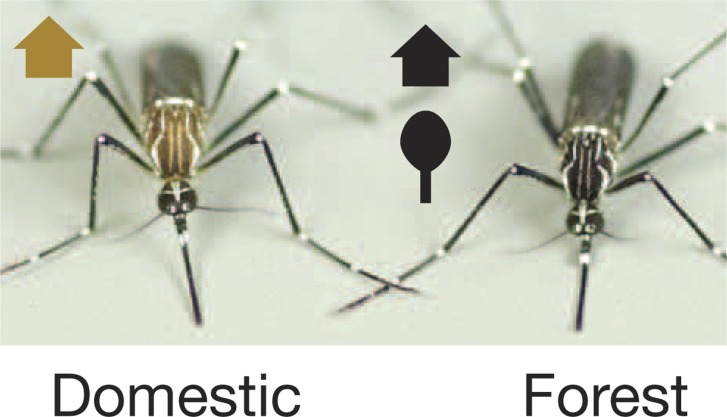
Individual representatives of the domestic and forest subspecies of female Aedes aegypti, showing the characteristic differences in body color. (Reprinted by permission from Macmillan Publishers, Ltd. C.S. McBride et al. Nature. http://dx.doi.org/10.1038/nature13964, copyright 2014.)
Evolving to prefer people
Remarkable as it might seem to anyone trying to enjoy a summer evening outdoors, only ∼100 of the 1 to 10 million insect species with which we share the earth preferentially feed on human blood. Among those insects that do, the mosquito Aedes aegypti is notorious as a vector for such diseases as dengue and yellow fever. In coastal regions of Kenya, an ancestral forest-dwelling subspecies, Ae. aegypti formosus, prefers to feed on nonhuman animals and coexists with a “domestic” form, Ae. aegypti aegypti, that prefers people and invades their homes. McBride et al. (2014) established laboratory colonies of either “domestic” or “forest” subspecies, which can be distinguished morphologically, and confirmed that these morphological differences correlated with preference for human or nonhuman hosts. Analysis of antennal genes identified 14 genes differentially expressed in the same direction in human-preferring versus guinea pig–preferring mosquito colonies and F2 hybrids; two of these differentially expressed genes, both up-regulated in human-preferring mosquitoes, encoded odorant receptors (Or4 and Or103). When heterologously expressed in Drosophila olfactory neurons, Or4 (the second most highly expressed ligand-selective OR in the antennae of domestic females) conferred sensitivity to sulcatone, a volatile component of human body odor that is less abundant in nonhuman species. Seven major alleles of Or4 were identified that encoded receptors with varying sensitivity to sulcatone, with preference for humans linked to both high Or4 expression and high ligand sensitivity, leading the authors to propose that increased behavioral sensitivity to sulcatone may help mosquitoes distinguish humans from other animals.
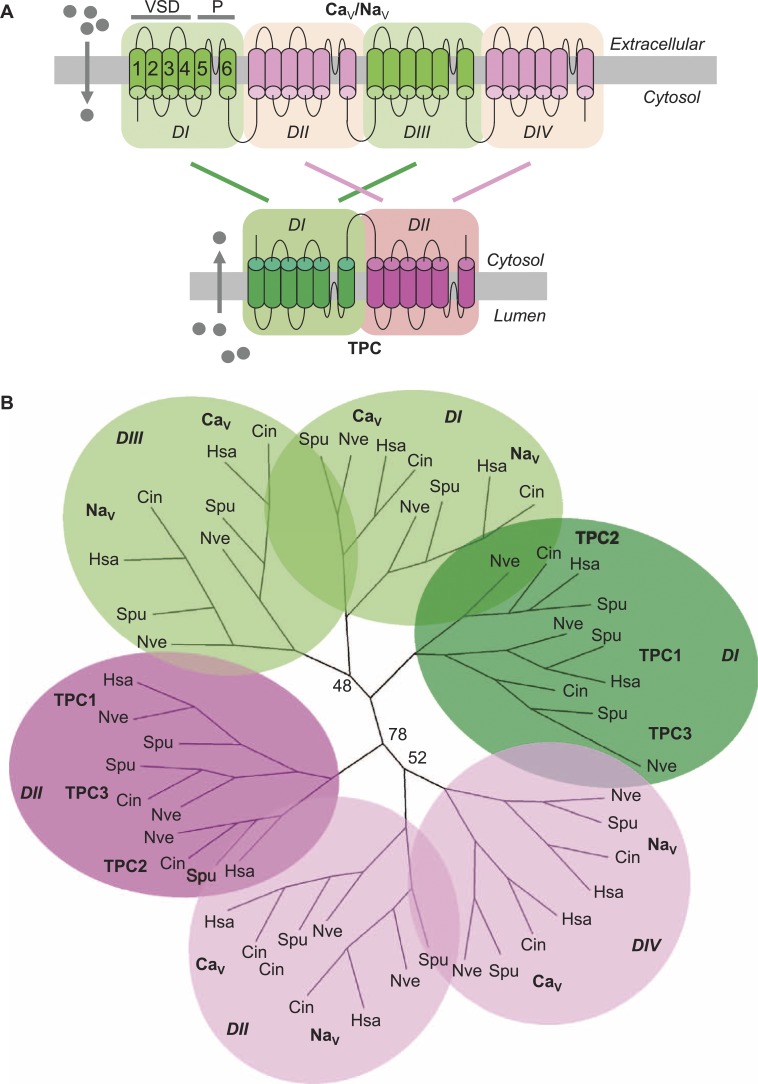
(A) Architecture of four-domain and two-domain ion channels, indicating DI–DIV of Navs and Cavs, and DI and DII of the TPCs. (B) Phylogenetic tree constructed using individual domains of TPCs, Cavs, and Navs. (From Rahman et al. 2014. Sci. Signaling. http://dx.doi.org/10.1126/scisignal.2005450. Reprinted with permission from AAAS.)
Which came first, the channel or its selectivity?
The pore-forming subunits of eukaryotic voltage-gated Ca2+ (Cav) and Na+ (Nav) channels comprise four homologous domains (DI to DIV) that function as a pseudotetramer. Individual Cav and Nav channel domains resemble the single domains of voltage-gated K+ channels, which function as tetramers, suggesting that four-domain channels may have evolved from single-domain channels through consecutive rounds of intragenic duplication. Noting that two-pore channels (TPCs) are two-domain channels thought to function as pseudotetrameric dimers, making them potential descendants of a two-domain evolutionary intermediate, Rahman et al. (2014) explored their phylogenetic relationship to Cav and Nav channels. Domain-based phylogenetic analysis indicated that the DI domains of TPCs grouped with the DI and DIII domains of Cav and Nav channels, whereas their DII domains grouped with the Cav and Nav channel DII and DIV domains, consistent with such a duplication event. Molecular docking analyses using homology models based on a single-domain bacterial Nav channel indicated that Cav and Nav antagonists bound to the TPC pore region, likely through a common site. Moreover, such antagonists blocked NAADP-dependent Ca2+ signals in sea urchin egg homogenates (thought to be mediated by TPCs) and in cells heterologously expressing sea urchin TPC1. Modeling data indicated that the TPC selectivity filter was noncanonical compared with those of Navs and Cavs. Intriguingly, however, phylogenetic analysis identified a TPC clade in unicellular organisms, which the authors named TPCR (for TPC-related), in which the putative selectivity filter had features reminiscent of those of Cavs. The authors thus postulate that acquisition of a “blueprint” pharmacological profile and changes in ion selectivity may have occurred before the duplication event that gave rise to four-domain voltage-gated ion channels.
Mosquito preference for people, the evolution of Cav and Nav channels, and ELIC activation
Analyzing ELIC activation
Decades of biophysical analysis have provided substantial insight into the mechanisms whereby members of the Cys-loop family of pentameric ligand-gated ion channels, which includes fast neurotransmitter receptors such as the nicotinic aceltylcholine receptor and the glycine receptor, gate. The structural correlates of intermediate states identified through single-channel analysis, however, have remained unclear, as is the exact nature and sequence of the conformational changes these channels undergo upon activation. Noting that full-length structural information is available for the bacterial homologue ELIC (Erwinia chrysanthemi ligand-gated ion channel), in this issue Marabelli et al. expressed ELIC in HEK293 cells and recorded single-channel and macroscopic currents to perform a kinetic analysis of this model channel and model its activation. Showing that it is possible to develop a mechanistic scheme for this prokaryotic channel—an important step toward the goal of mapping structure to function for members of this superfamily—their analyses indicate that, when maximally activated by binding of at least two molecules of agonist, ELIC opens to two open states (Marabelli et al., 2015).
References
- Marabelli, A., et al. 2015. J. Gen. Physiol. 145:23–45. 10.1085/jgp.201411234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride, C.S., et al. 2014. Nature. 515:222–227. 10.1038/nature13964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman, T., et al. 2014. Sci. Signal. 7:ra109. 10.1126/scisignal.2005450 [DOI] [PMC free article] [PubMed] [Google Scholar]