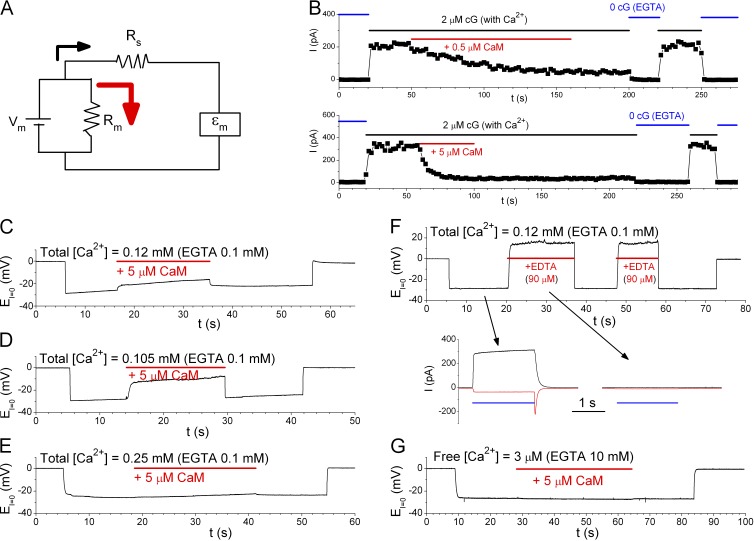
Jung and Lee have responded in this issue to our recent paper (Yu et al., 2014b) in which we concluded that calmodulin (CaM) does not alter anion permeability of the mouse ANO1/TMEM16A Ca2+-activated Cl− channel. In our paper, we suspected that the Ca2+-CaM (abbreviated as CaM) effect observed by Jung et al. (2013) may result from technical complications such as series resistance and/or ion accumulation problems. One important observation in Jung et al. (2013) that supported the CaM modulation of TMEM16A’s anion permeability was that the bi-ionic potentials in whole-cell patch-clamp recordings were different at low and high intracellular [Ca2+]. However, comparing bi-ionic potentials between currents of vastly different amplitudes could be problematic. For example, series resistance (Rs in the circuit of Fig. 1 A) in patch-clamp recordings should not be a significant problem if the membrane resistance (Rm) is relatively high. However, as Rm is reduced substantially (such as activating large numbers of channels or increasing the leak current), the battery power (and thus the membrane voltage Vm) is shunted away significantly (see Fig. 1 A), and a higher Rs exacerbates the effect. This voltage-shunting problem exists whether the measurement is made by the voltage-clamp or current-clamp method. Jung and Lee (2015) use Fig. 1 D in their Letter to the Editor (abbreviated as “Letter”) to argue that they did not have such a problem. However, the Vm at low conductance in that figure is already near 0 mV, which would preclude the Vm reduction from being observed.
Figure 1.
Effects of purified human brain CaM on the rat olfactory CNG channel (formed by subunit A2) and the mouse TMEM16A channel. All experiments were excised inside-out patch recordings. (A) Equivalent circuit showing that when membrane resistance (Rm) is reduced, the membrane voltage Vm is shunted away via a current flow through Rm (red arrow). Thus, the measured voltage (εm) is smaller than Vm in absolute values. (B) Effects of the purified human brain CaM on the olfactory CNG channel. Solutions were as those in Fig. 3 of Yu et al. (2014b), except that the total [Ca2+] in the 2-µM cGMP solution is 0.15 mM. Time constants of the CaM inhibition: 46 s (0.5 µM) and 6 s (5 µM). (C–E) Effects of the purified human brain CaM on TMEM16A’s anion permeability. Solutions contained 0.1 mM EGTA and total [Ca2+] of 0.12 mM (C), 0.105 mM (D), or 0.25 mM (E) throughout the recordings. The recordings started in symmetrical 140 mM NaCl (EI=0 = 0 mV). The intracellular solution was then changed to a solution containing 130 mM NaHCO3 plus 10 mM NaCl, during which 5 µM CaM was applied (red horizontal line). The values of EI=0 in CaM minus that without CaM (ΔV) were 0.2–5.9 mV (n = 6), 8.3–20.5 mV (n = 4), and −0.4 to 1.8 mV (n = 5) in C, D, and E, respectively. Notice that as total [Ca2+] is increased, the CaM effect is reduced. (F) Effects of the purified human brain CaM shown in C and D were likely caused by a reduction of the intracellular free [Ca2+]. Experimental solutions were the same as those in C except that 90 µM EDTA (instead of CaM) was added. ΔV = 16, 18, and 38 mV from three patches. Bottom panels: Voltage-clamp experiments (black, 40 mV; red, −40 mV) with the bath solutions being changed from the 0-Ca2+ solution (140 mM NaCl) to the HCO3− solutions (horizontal blue line) used in the current-clamp experiment as indicated by arrows. Notice that the solution containing an extra 90 µM EDTA did not activate detectable current. (G) The purified human brain CaM did not alter EI=0 in the solution used in Jung et al. (2013)—namely, 130 mM NaHCO3, 20 mM NaCl, 10 mM EGTA, and ∼9.8 mM total [Ca2+] (calculated free [Ca2+] = ∼3 µM).
Ion accumulation may also be a problem in bi-ionic potential measurements. A significant change of ion concentrations adjacent to the membrane can occur in 10 s with merely 1 nA of current in whole-cell recordings (Vocke et al., 2013). Jung and Lee (2015) argue that accumulation of intracellular HCO3− in their whole-cell recordings cannot explain the increase of PHCO3/PCl because an increase of intracellular [HCO3−] should have decreased the PHCO3/PCl ratio. However, in their recording conditions (with low extracellular [Cl−]), a large TMEM16A conductance would not only accumulate intracellular HCO3− but also deplete intracellular Cl−. As both HCO3− and Cl− gradients across the membrane were reduced, the measured reversal potential would approach 0 mV (or the calculated PHCO3/PCl approached 1). Because the normal PHCO3/PCl ratio is ∼0.3, a depletion of the ionic gradients would increase the calculated PHCO3/PCl ratio!
Jung and Lee also show in their Letter that different sources of CaM may differentially alter the HCO3− permeability of TMEM16A. We previously found no effect of recombinant bovine CaM (Sigma-Aldrich) in altering the anion permeability of TMEM16A (Yu et al., 2014b). The new results in Fig. 1 C of Jung and Lee’s Letter agree with our conclusion that the recombinant bovine CaM has little effect. However, they show that purified human brain CaM (EMD Millipore) significantly alters the bi-ionic potential (Fig. 1 A of their Letter). They suspect that the His-tag attached to the recombinant CaM may affect its properties, thus explaining the negative effect of the recombinant bovine CaM. It should be noted that inconsistent results were obtained from Jung and Lee’s experiments using recombinant CaM. In Fig. 4 D of Jung et al. (2013), it was shown that the recorded bi-ionic potential quickly approached 0 mV upon adding recombinant CaM. In Fig. 1 C of their Letter, such a robust effect of recombinant CaM is not observed.
To address the effect of the purified human brain CaM from EMD Millipore shown in Fig. 1 A of Jung and Lee’s Letter, we first confirmed the effectiveness of CaM by showing that this CaM readily inhibits olfactory cyclic nucleotide–gated (CNG) channels (Fig. 1 B). One feature of the CaM effect on the olfactory CNG channel (regardless of the source of CaM) is that after the channel is inhibited by CaM, if the inside-out patch is perfused with a solution containing saturating [Ca2+] without CaM, the current cannot be recovered (Fig. 1 B; also see Fig. 3 of Yu et al., 2014b). The current recovery is only observed after exposing the patch to a 0-Ca2+ solution containing Ca2+ chelators. In contrast, for all of Jung and Lee’s recordings that show a positive CaM effect on TMEM16A, the CaM effect disappears in the Ca2+-containing washout solution.
To examine the effect of the purified human brain CaM on TMEM16A, we first prepared a 5-µM CaM solution using our standard saturated [Ca2+] solution containing 0.12 mM of total [Ca2+] and 0.1 mM EGTA. The estimated free [Ca2+] would be ∼20 µM if no other Ca2+ chelator (including CaM) is in the solution. However, this purified human brain CaM was lyophilized from a solution containing 2 mM EDTA, resulting in ∼90 µM [EDTA] in the 5-µM CaM solution (information from the vendor). Part of the EDTA molecules probably have been bound with Ca2+ in the CaM-preparation process, in which EDTA was likely used for eluting substrate-bound CaM. When we delivered this 5-µM CaM solution to the cytoplasmic side of the patch using the SF-77 fast-solution exchanger (Warner Instruments), a small change of the recorded voltage in I = 0 current-clamp recording mode (EI=0) was immediately observed (Fig. 1 C). Most strikingly, this “CaM effect” disappeared immediately after CaM was removed by switching the intracellular solution back to the control HCO3− solution containing ∼20 µM of free [Ca2+]!
We suspected that the contaminating EDTA in the CaM solution may be the culprit of altering EI=0 because the membrane potential (Vm), namely EI=0, is a weighted sum of the reversal potential of TMEM16A current (EC) and that of the background current (EB) according to the equation:
where gC and gB are the TMEM16A conductance and the background conductance, respectively. Therefore, a contaminating EDTA could chelate free Ca2+, reduce the gC/gB ratio, and therefore render Vm approaching EB. This problem can be demonstrated by changing the total [Ca2+] in the HCO3− solution to 0.105 mM (Fig. 1 D) and 0.25 mM (Fig. 1 E). With a lower [Ca2+] (Fig. 1 D), the CaM effect is stronger. When the total [Ca2+] is 0.25 mM (Fig. 1 E), the CaM effect is negligible. We also conducted similar experiments by adding 90 µM Na-EDTA without CaM (Fig. 1 F). In this solution (free [Ca2+] estimated to be ∼75 nM), no detectable TMEM16A current was observed (comparing the two voltage-clamp experiments in the bottom panels of Fig. 1 F), and the EI=0 values, which can be considered as the reversal potential of the background conductance (or EB), were in the positive range. Finally, if the CaM effect is caused by a low gC as a result of insufficient free [Ca2+], we expect that this source of CaM should not generate an effect in the solution used in Jung et al. (2013) because 10 mM EGTA provides a large Ca2+-buffering power. This is indeed observed as shown in Fig. 1 G (n = 3).
We thus conclude that the effect of the purified human brain CaM from EMD Millipore in our experiments is not a genuine CaM effect. (If it is a CaM effect, why is the effect weaker when more Ca2+ ions are present in the solution?) The effect we observed can be explained by a reduction of gC caused by the extra Ca2+-chelating power from the contaminating EDTA molecules. Although the CaM effect in our experiments is similar to that of Jung and Lee in that the effect disappears upon removing “CaM” in the presence Ca2+, we do not know if the result in Fig. 1 A of Jung and Lee’s Letter can be explained by the extra Ca2+-chelating power because the information of the total [Ca2+] and Ca2+-buffering power in that experiment is not available to us.
Various laboratories have provided evidence arguing that CaM may or may not modulate the functions of TMEM16A (Tian et al., 2011; Jung et al., 2013; Terashima et al., 2013; Vocke et al., 2013; Tien et al., 2014; Yu et al., 2014a,b). In our experiments, whether CaM is a recombinant bovine CaM or the purified human brain CaM, we have not yet observed any genuine CaM effect in altering the anion permeability of the TMEM16A Ca2+-activated Cl− channel, although both types of CaM are effective in inhibiting the olfactory CNG channel.
Acknowledgments
Angus C. Nairn served as editor.
References
- Jung J., and Lee M.G.. 2015. Does calmodulin regulate the bicarbonate permeability of ANO1/TEME16A or not? J. Gen. Physiol. 145:75–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J., Nam J.H., Park H.W., Oh U., Yoon J.H., and Lee M.G.. 2013. Dynamic modulation of ANO1/TMEM16A HCO3− permeability by Ca2+/calmodulin. Proc. Natl. Acad. Sci. USA. 110:360–365 10.1073/pnas.1211594110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima H., Picollo A., and Accardi A.. 2013. Purified TMEM16A is sufficient to form Ca2+-activated Cl− channels. Proc. Natl. Acad. Sci. USA. 110:19354–19359 10.1073/pnas.1312014110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y., Kongsuphol P., Hug M., Ousingsawat J., Witzgall R., Schreiber R., and Kunzelmann K.. 2011. Calmodulin-dependent activation of the epithelial calcium-dependent chloride channel TMEM16A. FASEB J. 25:1058–1068 10.1096/fj.10-166884 [DOI] [PubMed] [Google Scholar]
- Tien J., Peters C.J., Wong X.M., Cheng T., Jan Y.N., Jan L.Y., and Yang H.. 2014. A comprehensive search for calcium binding sites critical for TMEM16A calcium-activated chloride channel activity. eLife. 3 10.7554/eLife.02772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocke K., Dauner K., Hahn A., Ulbrich A., Broecker J., Keller S., Frings S., and Möhrlen F.. 2013. Calmodulin-dependent activation and inactivation of anoctamin calcium-gated chloride channels. J. Gen. Physiol. 142:381–404 10.1085/jgp.201311015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K., Zhu J., Qu Z., Cui Y.Y., and Hartzell H.C.. 2014a. Activation of the Ano1 (TMEM16A) chloride channel by calcium is not mediated by calmodulin. J. Gen. Physiol. 143:253–267 10.1085/jgp.201311047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Kuan A.S., and Chen T.Y.. 2014b. Calcium-calmodulin does not alter the anion permeability of the mouse TMEM16A calcium-activated chloride channel. J. Gen. Physiol. 144:115–124 10.1085/jgp.201411179 [DOI] [PMC free article] [PubMed] [Google Scholar]