Summary
The origin of the nucleus at the prokaryote to eukaryote transition represents one of the most important events in the evolution of cellular organization. The nuclear envelope encircles the chromosomes in interphase and is a selectively permeable barrier between the nucleoplasm and cytoplasm and an organizational scaffold for the nucleus. It remains intact in the "closed" mitosis of some yeast but loses its integrity in the "open" mitosis of mammals. Instances of both types of mitosis within two evolutionary clades indicate multiple evolutionary transitions between open and closed mitosis, although the underlying genetic changes that influenced these transitions remain unknown. A survey of the diversity of mitotic nuclei that fall between these extremes is the starting point from which to determine the physiologically relevant characteristics distinguishing open from closed mitosis and to understand how they evolved and why they are retained in present-day organisms. The field is now poised to begin addressing these issues by defining and document patterns of mitotic nuclear variation within and among species and map them onto a phylogenic tree. Deciphering the evolutionary history of open and closed mitosis will complement cell biological and genetic approaches aimed at deciphering the fundamental organizational principles of the nucleus.
Introduction
The presence of a nucleus, which is a specialized part of the endomembrane system, 1), distinguishes eukaryotes, such as plants, animals, fungi, slime molds and a variety of single-celled organisms, from the prokaryotic eubacteria and archaea. Efforts to decipher the nuclear characteristics of the Last Eukaryotic Common Ancestor (LECA), from which all present day nucleated organisms evolved [1], remain challenging, in part because the order of events leading to the origin of the nucleus in the First Eukaryotic Common Ancestor (FECA) remain uncertain and controversial [1–4], and intracellular structure is rarely preserved in the fossil record (for exceptions see [5, 6]). However, characteristics or proteins shared amongst all present day eukaryotes are unlikely to have arisen independently in multiple lineages, and can be traced back to their roots in LECA revealing it to have been a complex organism with nuclear pore complexes (NPCs) and a mechanism for nucleocytoplasmic transport [1]. We also know that the subsequent eukaryotic radiation and the diversification of nuclear structure and function in present-day eukaryotes [7–9] is the product of an estimated 1.5 billion years of evolution from the LECA.
It is worth keeping in mind that this diversity need not have been driven by natural selection, since non-adaptive processes can also shape the course of evolution [10]. For example, gene frequencies change in all populations by means of genetic drift, more so in small than large populations, rendering the former, which is less responsive to natural selection, more vulnerable to biased mutation pressures. Indeed, it has been proposed that many aspects of molecular biology, genome architecture, and cell biology result from constructive neutral evolution, in which complexity increases over the course of evolution without functional consequence [10–12].
The current state of knowledge about the nucleus in present day organisms is limited to the detailed characterization of nuclei in a relatively small number of model organisms [reviewed in 13] and in narrowly focused but comprehensive morphological surveys, for example of fungi [7] or protozoans [8]. The purpose of this review is not to describe the full diversity of mitotic nuclei, but to discuss what is known and what is not known about their evolution. We will first briefly describe the general properties of the nucleus in all cells, and the diverse properties of mitotic nuclei ranging from fully open to fully closed, in order to provide the factual framework within which to consider their evolutionary history. Next, we consider some possible early evolutionary influences on the transitions from one form of mitosis to the other. This will provide a starting point from which to discuss the many challenging questions that remain about the evolution of open and closed mitosis. Reconstructing this evolutionary history will eventually allow the field to address such questions as: What physical constraints and properties of nuclei might have influenced these evolutionary transitions? What adaptive purposes did they serve? Why are these differences retained in present day organisms?
Nuclei of cells that undergo open or closed mitosis have common and unique properties
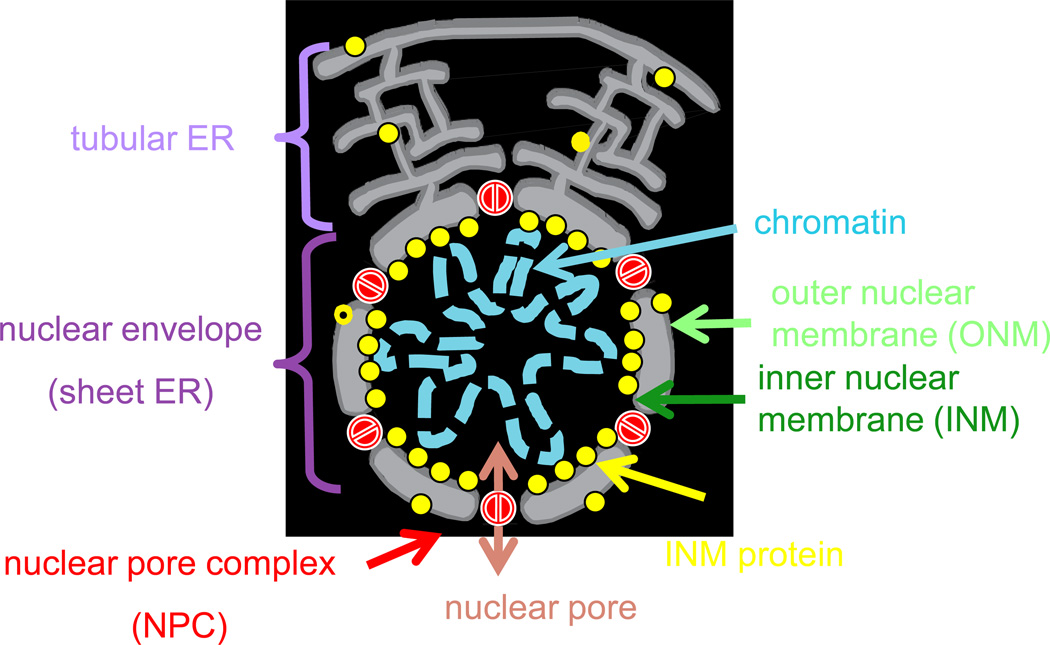
In the nuclei of interphase (non-mitotic) cells, the decondensed chromosomes are completely surrounded by and anchored to the nuclear envelope (NE) [13] (Figure 1). The non-random organization of chromatin in the nucleus during interphase [14] depends, in part, on a set of proteins enriched at the inner nuclear membrane (INM) [15, 16] (Figure 1). These INM proteins interact directly or indirectly with specific chromatin domains, anchor them to the nuclear periphery [15, 17] where the genes are generally transcriptionally inactive [18, 19], and may also provide structural support for the NE. The NE is perforated by selectively permeable pores (Figure 1) [13] and precise regulation of nuclear protein import and export through them allows cells to establish concentration gradients of soluble proteins across the NE [13].
Figure 1. The nucleus in interphase.
The double phospholipid bilayer-bound nucleus is a specialized region of the endoplasmic reticulum (ER) that harbors the chromosomes of eukaryotic cells in interphase. The membranes of the inner (INM) and outer (ONM) nuclear envelope are continuous with one another and with the ER, but the INM is enriched with a specialized collection of INM proteins [15, 16] that are synthesized in the ER (as shown), transit to the ONM and then to the INM where they are retained by association with chromatin and/or other proteins at the nuclear periphery. The nuclear envelope (NE) is perforated by nuclear pore complexes (NPC), which surround the nuclear pores, aqueous channels that form a selectively-permeable barrier between the nucleoplasm and the cytoplasm. With few exceptions [36, 47], the exchange of material across the intact NE is restricted to the NPCs that allow the free diffusion of some small molecules and proteins and the selective Ran-GTPase dependent exchange of larger cargoes [13]. During interphase of the cell cycle (the time when cells are not in mitosis), the chromosomes are decondensed, the NE is intact, and some proteins of the INM (e.g. the nuclear lamina proteins of mammalian cells or telomere or heterochromatin-binding proteins of yeast), anchor specific chromosome domains, such as non-transcribed heterochromatin and telomeres, to the nuclear periphery.
As the cell prepares for mitosis, the chromosomes are released from the NE and condense into their mitotic configurations whereas the cytoplasmic microtubules of the cytoskeleton are re-organized into the mitotic spindle. During mitosis, the spindle elongates and segregates the chromosomes into what will be the two daughter cells (Figure 2).
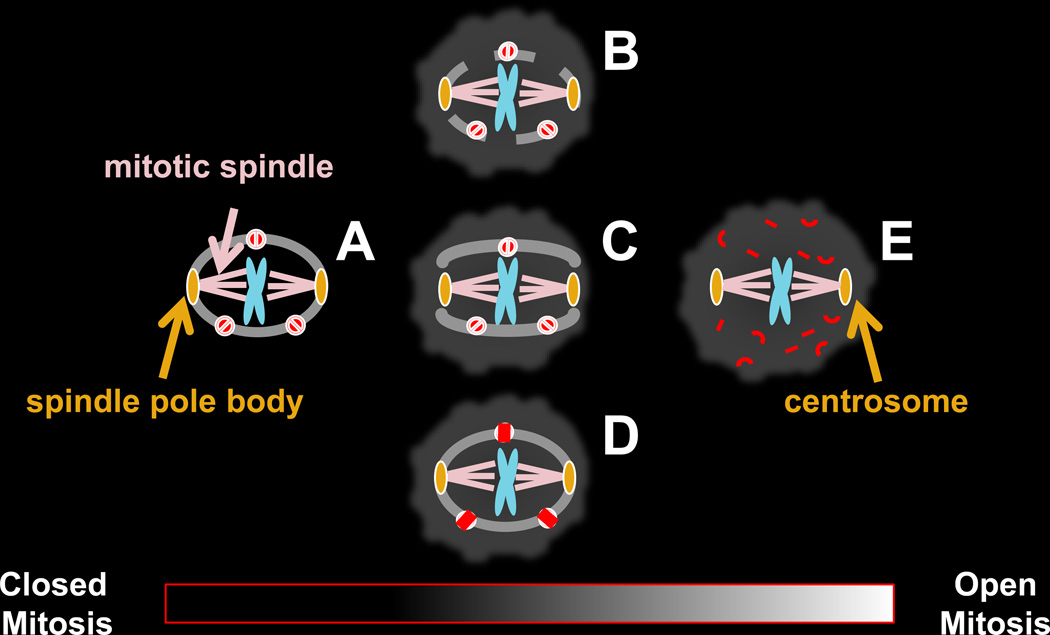
Figure 2. The nucleus in mitosis.
The nucleus changes dramatically from its interphase state (Figure 1) as cells enter mitosis. The chromatin previously tethered to the nuclear periphery is released, the chromosomes condense into their mitotic metaphase configuration, and the microtubule cytoskeleton is typically (but not always [44, 48]) reorganized by the microtubule organizing center, called the centrosome or spindle pole body (SPB), into the mitotic spindle that attaches to and then segregates the duplicated chromosomes to opposite sides of the cell.
The two best-characterized examples of mitosis-specific nuclear changes are the closed mitosis of the fission yeast Schizosaccharomyces pombe and the budding yeast Saccharomyces cerevisiae in which the NE remains intact (2-A) and the open mitosis of mammalian cells (2-E) in which it breaks down. There are also examples of mitosis that for a variety of reasons cannot be characterized as strictly open or strictly closed (2-B, 2-C, 2-D).
A. In the closed mitosis of some yeast cells, the duplicated SPBs are embedded in the nuclear membrane at mitosis and nucleate mitotic spindle formation within the confines of the nucleus. By definition, the presence of the SPB in the NE is essential for this type of closed mitosis. In some organisms the SPB is embedded in the NE during interphase and mitosis (e.g. S. cerevisiae) whereas in others it lies in the cytoplasm in close proximity to the NE in interphase, and enters the NE at mitosis (e.g. S. pombe)[30]. As the spindle elongates and applies pressure to opposite sides of the nucleus the spherical nucleus divides into two smaller spheres. These properties of closed mitosis are best characterized in S. cerevisiae and S. pombe but are not necessarily typical of other yeast or fungi or other organisms that undergo closed mitosis, some of which do not form a spindle inside of the nucleus [7].
B, C, D. Some types of mitosis are neither open nor closed. There are instances of cells that assemble an intranuclear mitotic spindle from NE embedded SPBs, but later in mitosis holes form in the NE, as in the fission yeast Schizosaccharomyces japonicas [24, 25]. In some cell types, such as multinucleated Drosophila melanogaster embryos [23] (B), nuclei undergo only partial NEBD. In other cell types, such as Chitridiales [7, 26], polar openings form in the NE, through which the cytoplasmic spindle extends (C). Cells with an ultrastructurally intact NE can also have a disrupted permeability barrier, resulting in the mixing of nucleoplasm and cytoplasm due to changes in NPC composition and/or permeability (D) as in the mitotic cycle of the filamentous fungus, Aspergillus nidulans [29], and during meiosis in the fission yeast S. pombe [27, 28].
E. In the open mitosis of mammalian cells, brought about by NEBD, the nuclear lamins (that line the inner NE in interphase) depolymerize, the NPCs disassemble and the NE is reorganized into the mitotic ER [21, 37] to which some of the membrane associated components of the nuclear lamina and NPC also relocalize. Although there are exceptions (e.g. Planarians lack centrosomes [48]) in animal cells the mitotic spindle is typically organized by centrosomes and can gain access to the chromosomes only after NEBD. Unlike the functional equivalent of the centrosomes in yeast (the spindle pole body) the centrosome is not embedded in the NE, even in interphase, although it lies in the cytoplasm in close proximity and tethered to the NE [44, 49]. Following mitosis, membrane associated components of the lamina re-associate with the condensed mitotic chromosomes to nucleate reassembly of the NE [50].
Based on the morphological continuity of the NE in mitosis, as assessed by electron microscopy, cells have historically been categorized as undergoing either closed mitosis, in which the NE remains intact surrounding the chromosomes, as in some yeast (Figure 2-A), or open mitosis, in which the NE breaks down, as in mammalian cells (Figure 2-E). Mitoses that fall between these extreme examples (Figures 2-B, 2-C, 2-D), in which the NE is present, but not continuous [7–9, 20], have been variously described as semi-open, semi-closed or partially open. As discussed below, even the seemingly straightforward terms "open" and "closed" need to be used with care, as we are now learning that there are many differences in the way the NE behaves in mitosis beyond its appearance in the electron microscope.
During closed mitosis in yeasts, (Figure 2-A), the spindle pole bodies (SPBs) are embedded in the NE and nucleate spindle formation inside of the nucleus. As the spindle elongates, it changes the shape of the nucleus that eventually divides into two daughter nuclei. During the entire cell cycle, the NE remains intact. In contrast, upon complete NE breakdown (NEBD) in the open mitosis of mammalian cells (Figure 2-E), the INM-associated nuclear lamina scaffold and the NPCs are disassembled, leading to the dissipation of interphase protein gradients across the NE and the reorganization of the NE into the mitotic endoplasmic reticulum (ER) [21, 22].
The multiple mitotic functions of the NE are interdependent, and there are a variety of types of mitosis that do not fit into either the open or closed categories (Figures 2-B, 2-C, 2-D). For example, the permeability barrier across the NE, that separates the nucleoplasm from the cytoplasm (Figures 2-A, 2-E) can be breached in mitosis by partial NEBD [23] in some cell types or by the formation of transient holes in the NE (Figure 2-B, 2-C) [7, 8, 24–26] in others. The permeability barrier of the NE can also be lost in cells undergoing closed mitosis or meiosis with ultrastructurally intact NEs but altered nucleocytoplasmic trafficking or NPC composition (Figure 2-D) [25, 27–29]. There could also be as yet undetected small or transient breaches in the NE, perhaps accompanying the insertion or extrusion of the SPB from the NE in yeast and other fungi [30]. In these situations, but not in cells with complete NEBD, the NE retains the ability to physically separate the chromosomes from cytoplasmic structures.
What properties of the nucleus may have influenced the evolution of open vs. closed mitosis?
Even though we cannot yet reconstruct the evolutionary history of open, closed or other forms of mitosis, and we do not even know whether LECA underwent open or closed mitosis [31], we do know that there have been multiple evolutionary transitions between open and closed mitosis. For example, the Opisthokonta clade includes metazoans, such as humans that undergo open mitosis, but also fungi, such as budding and fission yeast, that undergo closed mitosis. Similarly, the Archaeplastida clade includes land plants, such as Arabidopsis with open mitosis and some algae, such as the red algae C. merolae [32], with closed mitosis. Because eukaryotes are monophyletic, meaning that they have a common ancestor, their shared properties were most likely present in this ancient ancestor and their differences reflect evolutionary transitions. Because all species in a clade also share a common ancestor, the presence of some organisms that undergo open mitosis and others that undergo closed mitosis with these clades indicates that there must have been at least one transition between open and closed mitosis within that group. Closed mitosis and open mitosis can even coexist in a single organism at different life cycle stages, as in the slime mold Physarum polycephalum [33].
Documenting the structural and functional differences that distinguish open from closed mitosis or the variations within each category (Figure 2) is an important starting point for formulating questions about nuclear evolution. Although it is tempting to speculate about the relative benefits of a particular nuclear property to the cell, it is important to remember that present-day cells can also reflect the influence of neutral or even non-adaptive evolutionary changes.
Might transposable elements influence the transition from closed to open mitosis?
It is not clear which evolutionary processes have driven the variations in the properties of the NE during mitosis that are seen in present day organisms. As plants and animals, which are in different clades, both undergo open mitosis, it is tempting to speculate that some common evolutionary force is responsible for this shared form of division. One striking feature of both clades is they have bloated genomes that can largely be accounted for by the high proportion of transposable elements they contain [34, 35]. The prevalence of transposable elements and open mitosis in both plants and animals might not be a coincidence: it is possible that transposable elements are responsible for a transition from closed to open mitosis in these two lineages.
In principle, a closed NE may act as a barrier for transposable elements that transfer between the cytoplasm and the nucleus. A transposable element that gains the ability to induce perforations in the envelope would acquire enhanced access to the genome, potentially endowing it with a large selective advantage. NEBD associated with open mitosis could be the result of such an induction of perforations that became fixed when the associated transposable elements became fixed. This mechanism seems plausible, as present day parvoviruses act in analogous fashion by creating transient gaps in the inner and outer NE through which they pass [reviewed in 36]. The transposable element hypothesis could most clearly be demonstrated by reconstructing the evolutionary history of the factors responsible for NEBD and identifying those that arose from transposable elements. This may be challenging, because the factors that originally caused the transition to open mitosis could be different from those that regulate this process in extant organisms [22, 37]. Furthermore, the upstream signals, which determine the timing of NEBD, could have a different evolutionary history from the factors that are directly involved in rupturing the nuclear membrane. This transposable-element hypothesis might explain transitions from closed to open mitosis, but it does not provide a mechanism to understand transitions between different types of open mitosis, which seem equally adequate at providing access to the genome, for example, transitions between open mitoses that do or do not involve complete NEBD or between mitotic cells that have lost or changed their permeability barrier function due to alterations in NPC structure (Figure 2-D).
Might incompatible allometries drive transitions from closed to open mitosis?
While the transposable element hypothesis proposes a common cause for genome expansion and open mitosis, another possibility is that increasing genome size could directly drive a transition to open mitosis. One way this might occur is through incompatible allometries (biological scaling relationships) driven by increasing genome size. The density of DNA in the nucleus of eukaryotes is approximately constant [38–40], and both nuclear volume and spindle length scale with genome size, although they do so in different ways. Nuclear volume scales linearly with genome size [38, 40], so the nuclear radius grows as the cubed root of genome size. In contrast, spindle length seems to scale approximately linearly with cell volume [41, 42], which changes roughly linearly with genome size [43]. This means that as genome size increases, spindle length will increase faster than the nuclear radius. If the ancestral state is a cell with a small genome and a closed mitosis, then, as genome size increases over the course of evolution, it will inevitably reach a state where the spindle would not be able to fit into the nucleus. The disassembly of the NE during mitosis allows both allometries to be satisfied. A clear prediction of the incompatible allometries hypothesis is that, when mapped onto phylogeny, changes from closed to open mitosis should be correlated with the genome passing a critical size. Unfortunately, such a test cannot currently be performed as there is insufficient information on closely related organisms of known phylogeny that differ in the behavior of the NE during mitosis.
Conclusions
Because the mitotic nucleus has been well characterized in only a relatively small number of extant organisms, whether LECA underwent open or closed mitosis is not known. However, the presence of organisms that undergo open mitosis and others that undergo closed mitosis within two evolutionary supergroups is evidence that there have been multiple transitions between open and closed mitosis since LECA.
Because intracellular structure is rarely preserved in the fossil record, reconstructing the evolutionary history of the nucleus by constructing a phylogenetic tree upon which to map nuclear characteristics and eventually correlating them with underlying genetic changes will require surveying both widely and deeply in the tree of life: widely because the relatively small number of eukaryotic model organisms are very divergent from one another and represent just a tiny fraction of the present-day diversity of life on earth; and deeply because analyses of organisms with close relationships within carefully selected branches of the tree will be most informative in revealing the likely steps of cellular divergence.
A meaningful phylogenetic tree will make it possible to test hypotheses related to the evolution of open and closed mitosis, to correlate genomic changes with the transition points between these two forms of mitosis in closely related organisms, and to eventually discover the molecular mechanisms that distinguish them. It will also be informative to compare these evolutionary patterns with those of other nuclear proteins and structures known to impact the mitotic nucleus, such as the centriole/centrosome/spindle pole body [44, 45], and the nuclear scaffold, inner NE localized proteins and NPC components [1, 2, 46].
We have suggested two hypotheses for the evolution of open and closed mitosis involving adaptive processes. With the acquisition of additional data it should be possible to determine the validity of these scenarios, and it will be interesting to explore the implications of alternative hypothesis as well. More generally, it remains to be determined if variation in the behavior of the NE has been driven by lineage specific variation in the pressures of natural selection.
Acknowledgements
Research in our laboratories is supported by the NIH (RO1GM036873 to ML and 1R01GM104976-01 to DN), the National Science Foundation (MCB-1051962 to SS; MCB-1050161 to ML; DMR-0820484, PHY-0847188, and PHY-130525 to DN), HFSP (RGP0034/2010 to DN) and BSF (BSF 2009271 to DN). Any opinions, findings and conclusions or recommendations expressed in this article are those of the authors and do not necessarily reflect the views of the National Science Foundation. We apologize to those of our colleagues whose important contributions could not be acknowledged due to space constraints.
References
- 1.Koumandou VL, Wickstead B, Ginger ML, van der Giezen M, Dacks JB, Field MC. Molecular paleontology and complexity in the last eukaryotic common ancestor. Crit. Rev. Biochem. Mol. Biol. 2013;48:373–396. doi: 10.3109/10409238.2013.821444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devos DP, Graf R, Field MC. Evolution of the nucleus. Curr. Opin. Cell Biol. 2014;28:8–15. doi: 10.1016/j.ceb.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koonin EV. The origin and early evolution of eukaryotes in the light of phylogenomics. Genome Biol. 2010;11:209. doi: 10.1186/gb-2010-11-5-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavalier-Smith T. Origin of the cell nucleus, mitosis and sex: roles of intracellular coevolution. Biol. Direct. 2010;5:7. doi: 10.1186/1745-6150-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allwood AC, Walter MR, Kamber BS, Marshall CP, Burch IW. Stromatolite reef from the Early Archaean era of Australia. Nature. 2006;441:714–718. doi: 10.1038/nature04764. [DOI] [PubMed] [Google Scholar]
- 6.Bomfleur B, McLoughlin S, Vajda V. Fossilized nuclei and chromosomes reveal 180 million years of genomic stasis in royal ferns. Science. 2014;343:1376–1377. doi: 10.1126/science.1249884. [DOI] [PubMed] [Google Scholar]
- 7.Heath I. Variant mitosis in lower eukaryotes: indicators of the evolution of mitosis? Int. Rev. Cytol. 1980;64:1–80. doi: 10.1016/s0074-7696(08)60235-1. [DOI] [PubMed] [Google Scholar]
- 8.Raikov IB. Morphology of Eukaryotic Protozoan Nuclei. Vol. 9 WeinNew York: Springer-Verlag; 1982. [Google Scholar]
- 9.Sazer S. Nuclear membrane: nuclear envelope PORosity in fission yeast meiosis. Curr. Biol. 2010;20:R923–R925. doi: 10.1016/j.cub.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Lynch M. The frailty of adaptive hypotheses for the origins of organismal complexity. Proc. Natl. Acad. Sci. U. S. A. 2007;104(Suppl 1):8597–8604. doi: 10.1073/pnas.0702207104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoltzfus A. On the possibility of constructive neutral evolution. J. Mol. Evol. 1999;49:169–181. doi: 10.1007/pl00006540. [DOI] [PubMed] [Google Scholar]
- 12.Lukes J, Archibald JM, Keeling PJ, Doolittle WF, Gray MW. How a neutral evolutionary ratchet can build cellular complexity. IUBMB Life. 2011;63:528–537. doi: 10.1002/iub.489. [DOI] [PubMed] [Google Scholar]
- 13.Hetzer MW, Walther TC, Mattaj IW. Pushing the envelope: structure, function, and dynamics of the nuclear periphery. Annu. Rev. Cell Dev. Biol. 2005;21:347–380. doi: 10.1146/annurev.cellbio.21.090704.151152. [DOI] [PubMed] [Google Scholar]
- 14.Hubner MR, Eckersley-Maslin MA, Spector DL. Chromatin organization and transcriptional regulation. Curr. Opin. Genet. Dev. 2013;23:89–95. doi: 10.1016/j.gde.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simon DN, Wilson KL. The nucleoskeleton as a genome-associated dynamic 'network of networks'. Nat. Rev. Mol. Cell Biol. 2011;12:695–708. doi: 10.1038/nrm3207. [DOI] [PubMed] [Google Scholar]
- 16.Schirmer EC, Florens L, Guan T, Yates JR, 3rd, Gerace L. Nuclear membrane proteins with potential disease links found by subtractive proteomics. Science. 2003;301:1380–1382. doi: 10.1126/science.1088176. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez Y, Saito A, Sazer S. Fission yeast Lem2 and Man1 perform fundamental functions of the animal cell nuclear lamina. Nucleus. 2012;3:60–76. doi: 10.4161/nucl.18824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steglich B, Sazer S, Ekwall K. Transcriptional regulation at the yeast nuclear envelope. Nucleus. 2013;4:379–389. doi: 10.4161/nucl.26394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deniaud E, Bickmore WA. Transcription and the nuclear periphery: edge of darkness? Curr. Opin. Genet. Dev. 2009;19:187–191. doi: 10.1016/j.gde.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Arnone JT, Walters AD, Cohen-Fix O. The dynamic nature of the nuclear envelope: lessons from closed mitosis. Nucleus. 2013;4:261–266. doi: 10.4161/nucl.25341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu L, Ladinsky MS, Kirchhausen T. Cisternal organization of the endoplasmic reticulum during mitosis. Mol. Biol. Cell. 2009;20:3471–3480. doi: 10.1091/mbc.E09-04-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Güttinger S, Laurell E, Kutay U. Orchestrating nuclear envelope disassembly and reassembly during mitosis. Nat. Rev. Mol. Cell Biol. 2009;10:178–191. doi: 10.1038/nrm2641. [DOI] [PubMed] [Google Scholar]
- 23.Orr-Weaver TL. Developmental modification of the Drosophila cell cycle. Trends Genet. 1994;10:321–327. doi: 10.1016/0168-9525(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 24.Yam C, He Y, Zhang D, Chiam KH, Oliferenko S. Divergent strategies for controlling the nuclear membrane satisfy geometric constraints during nuclear division. Curr. Biol. 2011;21:1314–1319. doi: 10.1016/j.cub.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 25.Aoki K, Hayashi H, Furuya K, Sato M, Takagi T, Osumi M, Kimura A, Niki H. Breakage of the nuclear envelope by an extending mitotic nucleus occurs during anaphase in Schizosaccharomyces japonicus. Genes Cells. 2011;16:911–926. doi: 10.1111/j.1365-2443.2011.01540.x. [DOI] [PubMed] [Google Scholar]
- 26.Powell MJ. Mitosis in the aquatic fungus Rhizophydium spherotheca. Am J Botany. 1980;67:839–853. [Google Scholar]
- 27.Asakawa H, Kojidani T, Mori C, Osakada H, Sato M, Ding DQ, Hiraoka Y, Haraguchi T. Virtual breakdown of the nuclear envelope in fission yeast meiosis. Curr. Biol. 2010;20:1919–1925. doi: 10.1016/j.cub.2010.09.070. [DOI] [PubMed] [Google Scholar]
- 28.Arai K, Sato M, Tanaka K, Yamamoto M. Nuclear compartmentalization is abolished during fission yeast meiosis. Curr. Biol. 2010;20:1913–1918. doi: 10.1016/j.cub.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 29.De Souza CP, Osmani AH, Hashmi SB, Osmani SA. Partial nuclear pore complex disassembly during closed mitosis in Aspergillus nidulans. Curr. Biol. 2004;14:1973–1984. doi: 10.1016/j.cub.2004.10.050. [DOI] [PubMed] [Google Scholar]
- 30.McIntosh JR, O'Toole ET. Life cycles of yeast spindle pole bodies: getting microtubules into a closed nucleus. Biol. Cell. 1999;91:305–312. [PubMed] [Google Scholar]
- 31.Devos DP, Reynaud EG. Evolution. Intermediate steps. Science. 2010;330:1187–1188. doi: 10.1126/science.1196720. [DOI] [PubMed] [Google Scholar]
- 32.Yagisawa F, Fujiwara T, Kuroiwa H, Nishida K, Imoto Y, Kuroiwa T. Mitotic inheritance of endoplasmic reticulum in the primitive red alga Cyanidioschyzon merolae. Protoplasma. 2012;249:1129–1135. doi: 10.1007/s00709-011-0359-1. [DOI] [PubMed] [Google Scholar]
- 33.Burland TG, Solnica-Krezel L, Bailey J, Cunningham DB, Dove WF. Patterns of inheritance, development and the mitotic cycle in the protist Physarum polycephalum. Adv. Microb. Physiol. 1993;35:1–69. doi: 10.1016/s0065-2911(08)60096-x. [DOI] [PubMed] [Google Scholar]
- 34.Lynch M. The Origins of Genome Architecture. Sunderland, MA: Sinauer Associates, Inc.; 2007. [Google Scholar]
- 35.Agren JA, Wright SI. Co-evolution between transposable elements and their hosts: a major factor in genome size evolution? Chromosome Res. 2011;19:777–786. doi: 10.1007/s10577-011-9229-0. [DOI] [PubMed] [Google Scholar]
- 36.Cohen S, Au S, Pante N. How viruses access the nucleus. Biochim. Biophys. Acta. 2011;1813:1634–1645. doi: 10.1016/j.bbamcr.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 37.Kutay U, Hetzer MW. Reorganization of the nuclear envelope during open mitosis. Curr. Opin. Cell Biol. 2008;20:669–677. doi: 10.1016/j.ceb.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cavalier-Smith T. Economy, speed and size matter: evolutionary forces driving nuclear genome miniaturization and expansion. Ann Bot. 2005;95:147–175. doi: 10.1093/aob/mci010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szarski H. Cell size and nuclear DNA content in vertebrates. Int. Rev. Cytol. 1976;44:93–111. doi: 10.1016/s0074-7696(08)61648-4. [DOI] [PubMed] [Google Scholar]
- 40.Sparrow AH, Price HJ, Underbrink AG. A survey of DNA content per cell and per chromosome of prokaryotic and eukaryotic organisms: some evolutionary considerations. Brookhaven Symp. Biol. 1972;23:451–494. [PubMed] [Google Scholar]
- 41.Good MC, Vahey MD, Skandarajah A, Fletcher DA, Heald R. Cytoplasmic volume modulates spindle size during embryogenesis. Science. 2013;342:856–860. doi: 10.1126/science.1243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hazel J, Krutkramelis K, Mooney P, Tomschik M, Gerow K, Oakey J, Gatlin JC. Changes in cytoplasmic volume are sufficient to drive spindle scaling. Science. 2013;342:853–856. doi: 10.1126/science.1243110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gregory TR. The bigger the C-value, the larger the cell: genome size and red blood cell size in vertebrates. Blood Cells Mol. Dis. 2001;27:830–843. doi: 10.1006/bcmd.2001.0457. [DOI] [PubMed] [Google Scholar]
- 44.Azimzadeh J. Exploring the evolutionary history of centrosomes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369 doi: 10.1098/rstb.2013.0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carvalho-Santos Z, Azimzadeh J, Pereira-Leal JB, Bettencourt-Dias M. Evolution: Tracing the origins of centrioles, cilia, and flagella. J. Cell Biol. 2011;194:165–175. doi: 10.1083/jcb.201011152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mans B, Anantharaman V, Aravind L, Koonin E. Comparative genomics, evolution and origins of the nuclear envelope and nuclear pore complex. Cell Cycle. 2004;3:1612–1637. doi: 10.4161/cc.3.12.1316. [DOI] [PubMed] [Google Scholar]
- 47.Speese SD, Ashley J, Jokhi V, Nunnari J, Barria R, Li Y, Ataman B, Koon A, Chang YT, Li Q, et al. Nuclear envelope budding enables large ribonucleoprotein particle export during synaptic Wnt signaling. Cell. 2012;149:832–846. doi: 10.1016/j.cell.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Azimzadeh J, Wong ML, Downhour DM, Sanchez Alvarado A, Marshall WF. Centrosome loss in the evolution of planarians. Science. 2012;335:461–463. doi: 10.1126/science.1214457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agircan FG, Schiebel E, Mardin BR. Separate to operate: control of centrosome positioning and separation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369 doi: 10.1098/rstb.2013.0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anderson DJ, Hetzer MW. Nuclear envelope formation by chromatin-mediated reorganization of the endoplasmic reticulum. Nat. Cell Biol. 2007;9:1160–1166. doi: 10.1038/ncb1636. [DOI] [PubMed] [Google Scholar]