Abstract
A novel sesquiterpenoid, rumphellaoic acid A (1), was isolated from the gorgonian coral Rumphella antipathies, and was found to possess a carbon skeleton that was obtained for the first time from a natural sources. The structure of 1 was elucidated by spectroscopic methods and this compound and was found to exert a moderate inhibitory effect on the release of elastase by human neutrophils.
Keywords: Rumphella antipathies, gorgonian, rumphellaoic acid, sesquiterpenoid, elastase
1. Introduction
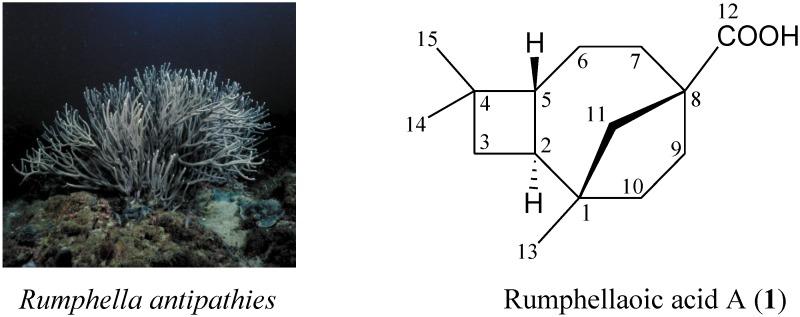
Sesquiterpenoid analogs, particularly caryophyllane- and clovane-type analogs, are major constituents of the extracts of gorgonian coral Rumphella antipathies [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16]. Our continuing studies on the chemical constituents of R. antipathies (family Gorgoniidae) (Chart 1), collected off the waters of Taiwan, have led to the isolation of a novel sesquiterpenoid, rumphellaoic acid A (1) (Chart 1 and Supplementary Figures S1–S7).
Chart 1.
The gorgonian Rumphella antipathies and the structure of rumphellaoic acid A (1).
2. Results and Discussion
Rumphellaoic acid A (1), −32 (c 0.07, CHCl3), was isolated as a colorless oil that gave a protonated molecule [M + H]+ at m/z 237.1832 in the high resolution electronspray ionization mass spectrum (HRESIMS), indicating the molecular formula C15H24O2 (calcd for C15H24O2 + H+, 237.1849) and implying four degrees of unsaturation. Comparison of the 1H NMR (Table 1) and distortionless enhancement by polarization transfer (DEPT) spectral data with the molecular formula indicated that there must be an exchangeable proton, and this deduction was supported by a broad absorption at 2500–3200 cm–1 and a strong absorption at 1696 cm–1 for a carboxyl group in the IR spectrum. From the heteronuclear multiple-bond correlation (HMBC) spectrum of 1 (Table 1), a carbonyl resonance at δC 184.2 (C-12) confirmed the presence of a carboxyl group in 1. Therefore, from the NMR data, a degree of unsaturation was accounted for and 1 must be a tricyclic compound. In addition, three methyl singlets (H3-13, H3-14 and H3-15), two aliphatic methine protons (H-2 and H-5) and six pairs of aliphatic methylene protons (H2-3, H2-6, H2-7, H2-9, H2-10 and H2-11) were observed in the 1H NMR and heteronuclear multiple-quantum coherence (HMQC) spectrum of 1.
Table 1.
1H and 13C NMR data, 1H–1H correlation spectroscopy (COSY) and HMBC correlations for sesquiterpenoid 1.
| Position | δH (J in Hz) | δC, Multiple | 1H-1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 41.9, C | |||
| 2 | 1.58 m | 46.0, CH | H2-3, H-5 | C-1, -6, -10, -13 |
| 3α | 1.56 m | 37.2, CH2 | H-2, H-3β | C-1, -2 |
| β | 1.35 m | H-2, H-3α | C-2, -4, -14, -15 | |
| 4 | 33.5, C | |||
| 5 | 1.55 m | 48.9, CH | H-2, H2-6 | C-2, -14 |
| 6a | 1.32 m | 25.8, CH2 | H-5, H-6b, H2-7 | C-2, -4, -7 |
| b | 1.64 m | H-5, H-6a, H2-7 | C -2, -4, -5, -7, -8 | |
| 7a | 1.61 m | 34.2, CH2 | H2-6, H-7b | C-5, -6, -8, -9, -12 |
| b | 1.78 dd (12.8, 5.6) | H2-6, H-7a | C-5, -8 | |
| 8 | 52.6, C | |||
| 9a | 1.68 m | 29.4, CH2 | H-9b, H2-10 | C-7, -8, -10, -11 |
| b | 2.14 dd (9.6, 7.6) | H-9a, H2-10 | C-1, -8, -10, -11, -12 | |
| 10a | 1.44 m | 45.4, CH2 | H2-9, H-10b | n.o. a |
| b | 1.68 m | H2-9, H-10a | C-8, -9, -11 | |
| 11α | 1.59 d (12.8) | 48.9, CH2 | H-11β | C-1, -2, -7, -8, -9, -10, -12 |
| β | 1.94 dd (12.8, 2.4) | H-11α | C-9, -10 | |
| 12 | 184.2, C | |||
| 13 | 0.93 s | 22.0, CH3 | C-1, -2, -10, -11 | |
| 14 | 0.98 s | 20.3, CH3 | C-3, -4, -5, -15 | |
| 15 | 0.98 s | 30.5, CH3 | C-3, -4, -5, -14 |
a n.o. = not observed.
The gross structure of 1 and all 1H and 13C NMR data associated with the molecule were determined and verified by 2D NMR studies. 1H NMR coupling information in the 1H-1H COSY spectrum of 1 enabled identification of C2-C5-C6-C7 and C9-C10 (Table 1). These data, together with the HMBC correlations between H-2/C-1, -6, -10; H-5/C-2; H2-6/C-2, -5, -7, -8; H2-7/C-5, -6, -8, -9; H2-9/C-1, -7, -8, -10; and H2-10/C-8, -9, established the connectivity within the eight-membered ring (Table 1). The cyclobutane ring, which is fused to the eight-membered ring at C-2 and C-5, was established by the 1H-1H COSY correlation between H-2 and H2-3, and by the HMBC correlations between H2-6/C-4 and H2-3/C-1, -2. The two tertiary methyls at C-4 were elucidated by the HMBC correlations between H3-14/C-3, -4, -5, -15 and H3-15/C-3, -4, -5, -14. Moreover, the tertiary methyl at C-1 was confirmed by the HMBC correlations between H3-13/C-1, -2, -10, -11. The presence of a carboxyl group at C-8 was deduced from the HMBC correlations between the C-7, C-9 and C-11 methylene protons and the carbonyl carbon of the carboxyl group at δC 184.2 (C-12). The C-11 methylene bridge between C-1 and C-8 was linked by the HMBC correlations between H2-9, H-10a, H3-13/C-11; H-11α/C-1, -2, -7, -8, -9, -10, -12; and H-11β/C-9, -10. Based on the above observations, the planar structure of 1 was elucidated unambiguously.
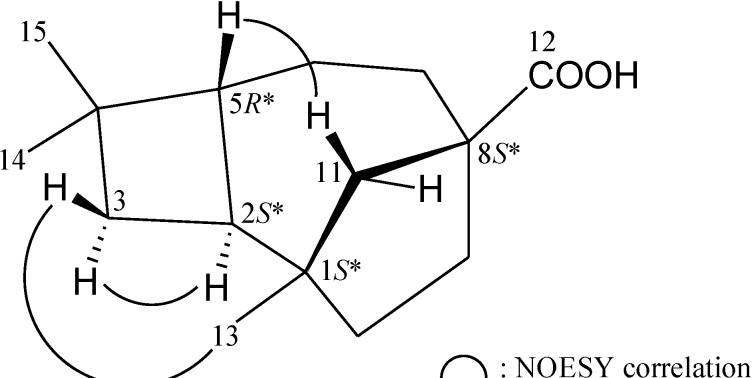
The relative configuration of 1 was established from the interactions observed in nuclear Overhauser effect spectroscopy (NOESY) spectra (Figure 1). In the NOESY spectra of 1, the correlation of H-5 with one proton of the C-11 methylene (δH 1.94), but not with H-2, indicated that these protons were situated on the same face, and these were assigned as β protons, since H-2 is α-substituted at C-2. It was found that one of the methylene protons at C-3 (δH 1.56) exhibited a correlation with H-2, and therefore it was assigned as H-3α, and the other C-3 proton (δH 1.35) as H-3β. The C-13 methyl showed a correlation with H-3β, but not with H-3α, demonstrating that the C-1 chiral carbon possesses an S*-configuration. Furthermore, the carboxyl group at C-8 was proven to possess an S*-configuration by modeling analysis. Based on the above findings, the structure of 1 was elucidated and the chiral carbons for 1 were assigned as 1S*, 2S*, 5R* and 8S*.
Figure 1.
Selective key NOESY correlations of 1.
It is worth noting that a sesquiterpenoid analog possessing the carbon skeleton descried for 1 was obtained from a natural source for the first time in this study. The in vitro anti-inflammatory effect of 1 was tested and this compound was found to display a modest inhibitory effect on the release of elastase (inhibition rate = 29.2%) by human neutrophils at a concentration of 10 μg/mL.
3. Experimental Section
3.1. General Experimental Procedures
Optical rotation values were measured with a Jasco P-1010 digital polarimeter (Japan Spectroscopic Corporation, Tokyo, Japan). IR spectra were obtained on a Varian Digilab FTS 1000 FT-IR spectrophotometer (Varian Inc., Palo Alto, CA, USA); peaks are reported in cm−1. NMR spectra were recorded on a Varian Mercury Plus 400 NMR spectrometer (Varian Inc., Palo Alto, CA, USA) using the residual CHCl3 signal (δH 7.26 ppm) as the internal standard for 1H NMR and CDCl3 (δC 77.1 ppm) for 13C NMR. Coupling constants (J) are given in Hz. ESIMS and HRESIMS were recorded using a Bruker 7 Tesla solariX FTMS system (Bruker, Bremen, Germany). Column chromatography was performed on silica gel (230–400 mesh, Merck, Darmstadt, Germany). TLC was carried out on precoated Kieselgel 60 F254 (0.25 mm, Merck, Darmstadt, Germany); spots were visualized by spraying with 10% H2SO4 solution followed by heating. Normal-phase HPLC (NP-HPLC) was performed using a system comprised of a Hitachi L-7110 pump (Hitachi Ltd., Tokyo, Japan), a Hitachi L-7455 photodiode array detector (Hitachi Ltd., Tokyo, Japan) and a Rheodyne 7725 injection port (Rheodyne LLC, Rohnert Park, CA, USA). A semi-preparative normal-phase column (Hibar 250 × 10 mm, LiChrospher Si 60, 5 μm, Merck, Darmstadt, Germany) was used for HPLC.
3.2. Animal Material
Specimens of the gorgonian coral Rumphella antipathies (Nutting) were collected by hand using scuba equipment off the coast of Pingtung, Southern Taiwan. This organism was identified by comparison with previous descriptions [17]. A voucher specimen (Specimen No. NMMBA-TWGC-010) was deposited in the National Museum of Marine Biology and Aquarium, Taiwan.
3.3. Extraction and Isolation
Sliced bodies of the gorgonian R. antipathies (wet weight 402 g, dry weight 144 g) were extracted with a mixture of methanol (MeOH) and dichloromethane (CH2Cl2) (1:1) at room temperature. The extract was partitioned with ethyl acetate (EtOAc) and H2O. The EtOAc layer was separated by silica gel and eluted using n-hexane/EtOAc (stepwise, 25:1–pure EtOAc) to yield 29 fractions. Every fraction was checked using the 1H NMR spectra. Fraction 15 was re-purified by normal-phase HPLC (NP-HPLC) using a mixture of n-hexane and EtOAc as the mobile phase to afford 1 (1.6 mg, 5:1).
Rumphellaoic acid A (1): Colorless oil; −32 (c 0.07, CHCl3); IR (neat) νmax 2500–3200 (broad), 1696 cm−1; 1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data, see Table 1; ESIMS: m/z 237 [M + H]+; HRESIMS: m/z 237.1832 (calcd for C15H24O2 + H+, 237.1849).
3.4. Human Neutrophil Elastase Release
Human neutrophils were obtained by means of dextran sedimentation and Ficoll centrifugation. Briefly, elastase release experiments were performed using MeO-Suc-Ala-Ala-Pro-Val-p-nitroanilide as the elastase substrate [18,19]. Elastatinal was used as a reference compound in the anti-inflammatory test of the inhibitory effects on the release of elastase (IC50 = 60.0 μM) by human neutrophils in response to fMet-Leu-Phe/Cytochalastin B (fMLP/CB). In the in vitro anti-inflammatory bioassay, the inhibitory effects on the release of elastase by activated neutrophils were used as indicators. At a concentration of 10 μg/mL, for the significant activity of pure compounds, an inhibition rate ≥50% is required (inhibition rate ≤ 10%, not active; 20% ≥ inhibition rate ≥ 10%, weakly anti-inflammatory; 50% ≥ inhibition rate ≥ 20%, modestly anti-inflammatory).
4. Conclusions
In continuing studies of new substances from marine invertebrates collected off the waters of Taiwan, a new sesquiterpenoid, rumphellaoic acid A (1), was isolated from R. antipathies. The structure of sesquiterpenoid 1 was elucidated on the basis of spectroscopic methods, and this compound was found to display an inhibitory effect on the release of elastase by human neutrophils. The sesquiterpenoid analogues prepared by chemical methods and biotransformation by Collado’s group [20,21,22] possessed the same carbon skeleton as that of 1. However, to the best of our knowledge, this is the first time that compound 1 has been obtained from a natural source.
Acknowledgments
This research was supported by grants from the National Museum of Marine Biology and Aquarium; National Dong Hwa University; Asia-Pacific Ocean Research Center, National Sun Yat-sen University; the Ministry of Science and Technology (Grant No. NSC101-2320-B-291-001-MY3 and MOST 103-2325-B-291-001); and China Medical University under the Aim for Top University Plan of the Ministry of Education, Taiwan, awarded to Y.-C.W. and P.-J.S.
Supplementary Files
Author Contributions
Yang-Chang Wu and Ping-Jyun Sung designed the whole experiment and contributed to manuscript preparation. Hsu-Ming Chung and Wei-Hsien Wang researched data and wrote the manuscript. Tsong-Long Hwang, Lee-Shing Fang, Zhi-Hong Wen and Jih-Jung Chen analyzed the data and performed data acquisition.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Chuang L.-F., Fan T.-Y., Li J.-J., Sung P.-J. Kobusone: Occurrence of a norsesquiterpenoid in the gorgonian coral Rumphella antipathies (Gorgoniidae) Biochem. Syst. Ecol. 2007;35:470–471. doi: 10.1016/j.bse.2007.01.007. [DOI] [Google Scholar]
- 2.Chuang L.-F., Fan T.-Y., Li J.-J., Kuo J., Fang L.-S., Wang W.-H., Sung P.-J. Isokobusone, a caryophyllane-type norsesquiterpenoid from the gorgonian coral Rumphella antipathies (Gorgoniidae) Platax. 2007;4:61–67. [Google Scholar]
- 3.Sung P.-J., Chuang L.-F., Kuo J., Chen J.-J., Fan T.-Y., Li J.-J., Fang L.-S., Wang W.-H. Rumphellolides A–F, six new caryophyllane-related derivatives from the Formosan gorgonian coral Rumphella antipathies. Chem. Pharm. Bull. 2007;55:1296–1301. doi: 10.1248/cpb.55.1296. [DOI] [PubMed] [Google Scholar]
- 4.Sung P.-J., Chuang L.-F., Fan T.-Y., Chou H.-N., Kuo J., Fang L.-S., Wang W.-H. Rumphellolide G, a new caryophyllane-type tetrahydropyran norsesquiterpenoid from the gorgonian coral Rumphella antipathies (Gorgoniidae) Chem. Lett. 2007;36:1322–1323. doi: 10.1246/cl.2007.1322. [DOI] [Google Scholar]
- 5.Hwang T.-L., Su Y.-D., Hu W.-P., Chuang L.-F., Sung P.-J. Rumphellolide H, a new natural caryophyllane from the gorgonian Rumphella antipathies. Heterocycles. 2009;78:1563–1567. doi: 10.3987/COM-08-11636. [DOI] [Google Scholar]
- 6.Sung P.-J., Su Y.-D., Hwang T.-L., Chuang L.-F., Chung H.-M., Chen J.-J., Li J.-J., Fang L.-S., Wang W.-H. Rumphellolide I, a novel caryophyllane-related tetrahydropyran norsesquiterpenoid from gorgonian coral Rumphella antipathies. Chem. Lett. 2009;38:282–283. doi: 10.1246/cl.2009.282. [DOI] [Google Scholar]
- 7.Sung P.-J., Chuang L.-F., Kuo J., Fan T.-Y., Hu W.-P. Rumphellatin A, the first chloride-containing caryophyllane-type norsesquiterpenoid from Rumphella antipathies. Tetrahedron Lett. 2007;48:3987–3989. doi: 10.1016/j.tetlet.2007.04.040. [DOI] [Google Scholar]
- 8.Sung P.-J., Chuang L.-F., Hu W.-P. Rumphellatins B and C, two new caryophyllane-type hemiketal norsesquiterpenoids from the Formosan gorgonian coral Rumphella antipathies. Bull. Chem. Soc. Jpn. 2007;80:2395–2399. doi: 10.1246/bcsj.80.2395. [DOI] [Google Scholar]
- 9.Sung P.-J., Su Y.-D., Hwang T.-L., Chuang L.-F., Chen J.-J., Li J.-J., Fang L.-S., Wang W.-H. Rumphellatin D, a novel chlorinated caryophyllane from gorgonian coral Rumphella antipathies. Chem. Lett. 2008;37:1244–1245. doi: 10.1246/cl.2008.1244. [DOI] [Google Scholar]
- 10.Chung H.-M., Chen Y.-H., Lin M.-R., Su J.-H., Wang W.-H., Sung P.-J. Rumphellaone A, a novel caryophyllane-related derivative from the gorgonian coral Rumphella antipathies. Tetrahedron Lett. 2010;51:6025–6027. doi: 10.1016/j.tetlet.2010.09.032. [DOI] [Google Scholar]
- 11.Chung H.-M., Wang W.-H., Hwang T.-L., Li J.-J., Fang L.-S., Wu Y.-C., Sung P.-J. Rumphellaones B and C, new 4,5-seco-carophyllane sesquiterpenoids from Rumphella antipathies. Molecules. 2014;19:12320–12327. doi: 10.3390/molecules190812320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung H.-M., Wang W.-H., Hwang T.-L., Chen J.-J., Fang L.-S., Wen Z.-H., Wang Y.-B., Wu Y.-C., Sung P.-J. Rumphellols A and B, new carophyllene sesquiterpenoids from a Formosan gorgonian coral, Rumphella antipathies. Int. J. Mol. Sci. 2014;15:15679–15688. doi: 10.3390/ijms150915679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung H.-M., Chen Y.-H., Hwang T.-L., Chuang L.-F., Wang W.-H., Sung P.-J. Rumphellclovane A, a novel clovane-related sesquiterpenoid from the gorgonian coral Rumphella antipathies. Tetrahedron Lett. 2010;51:2734–2736. doi: 10.1016/j.tetlet.2010.03.046. [DOI] [Google Scholar]
- 14.Chung H.-M., Hwang T.-L., Chen Y.-H., Su J.-H., Lu M.-C., Chen J.-J., Li J.-J., Fang L.-S., Wang W.-H., Sung P.-J. Rumphellclovane B, a novel clovane analogue from the gorgonian coral Rumphella antipathies. Bull. Chem. Soc. Jpn. 2011;84:119–121. doi: 10.1246/bcsj.20100253. [DOI] [Google Scholar]
- 15.Chung H.-M., Su J.-H., Hwang T.-L., Li J.-J., Chen J.-J., Chen Y.-H., Chang Y.-C., Su Y.-D., Chen Y.-H., Fang L.-S., et al. Rumphellclovanes C–E, new clovane-type sesquiterpenoids from the gorgonian coral Rumphella antipathies. Tetrahedron. 2013;69:2740–2744. doi: 10.1016/j.tet.2013.01.087. [DOI] [Google Scholar]
- 16.Chung H.-M., Wang W.-H., Hwang T.-L., Wu Y.-C., Sung P.-J. Natural clovanes from the gorgonian coral Rumphella antipathies. Nat. Prod. Commun. 2013;8:1037–1040. [PubMed] [Google Scholar]
- 17.Bayer F.M. Key to the genera of Octocorallia exclusive of Pennatulacea (Coelenterata: Anthozoa), with diagnoses of new taxa. Proc. Biol. Soc. Wash. 1995;94:902–947. [Google Scholar]
- 18.Yang S.-C., Chung P.-J., Ho C.-M., Kuo C.-Y., Hung M.-F., Huang Y.-T., Chang W.-Y., Chang Y.-W., Chan K.-H., Hwang T.-L. Propofol inhibits superoxide production, elastase release, and chemotaxis in formyl peptide-activated human neutrophils by blocking formyl peptide receptor 1. J. Immunol. 2013;190:6511–6519. doi: 10.4049/jimmunol.1202215. [DOI] [PubMed] [Google Scholar]
- 19.Yu H.-P., Hsieh P.-W., Chang Y.-J., Chung P.-J., Kuo L.-M., Hwang T.-L. 2-(2-Fluorobenzamido)benzoate ethyl ester (EFB-1) inhibits superoxide production by human neutrophils and attenuates hemorrhagic shock-induced organ dysfunction in rats. Free Radic. Biol. Med. 2011;50:1737–1748. doi: 10.1016/j.freeradbiomed.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 20.Ascari J., Boaventura M.A.D., Takahashi J.A., Durán-Patrón R., Hernández-Galán R., Macías-Sánchez A.J., Collado I.G. Phytotoxic activity and metabolism of Botrytis cinerea and structure-activity relationships of isocaryolane derivatives. J. Nat. Prod. 2013;76:1016–1024. doi: 10.1021/np3009013. [DOI] [PubMed] [Google Scholar]
- 21.Racero J.C., Macías-Sánchez A.J., Hernández-Galán R., Hitchcock P.B., Hanson J.R., Collado I.G. Novel rearrangement of an isocaryolane sesquiterpenoid under Mitsunobu conditions. J. Org. Chem. 2000;65:7786–7791. doi: 10.1021/jo000765p. [DOI] [PubMed] [Google Scholar]
- 22.Collado I.G., Hanson J.R., Hernández-Galán R., Hitchcock P.B., Macías-Sánchez A.J., Racero J.C. Novel methoxyl and hydroxyl directed pinacol rearrangements of an isocaryolane sesquiterpenoid under Mitsunobu conditions. Tetrahedron Lett. 1999;40:6497–6498. doi: 10.1016/S0040-4039(99)01335-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.