Toxic shock syndrome (TSS) is an acute-onset, multisystem illness characterized by fever (>102°F), hypotension (systolic pressure, <90 mm of Hg), sunburn-like rash, peeling of the skin, and a variable multiorgan component that often initially appears flu-like, perhaps including vomiting and diarrhea (10). The illness is caused by Staphylococcus aureus bacteria that make superantigens (SAgs), usually TSS toxin-1 (TSST-1), enterotoxin B (SEB), or SEC (7); these toxins exert systemic effects on the host (2). Probable TSS has been described as the same illness but lacking one of the defining criteria of TSS (6). Parsonnet suggests that the term toxin-mediated disease be used for cases of apparent TSS in which more than one clinical feature is absent though it can be established that S. aureus is present and that the patient lacks protective antibodies (5).
TSS is subdivided into two major categories, menstrual and nonmenstrual. In tests of thousands of S. aureus isolates, our laboratory has demonstrated that the SAg TSST-1 is associated with nearly all cases of menstrual TSS (mTSS). Nonmenstrual TSS may be caused by any of the 15 described SAgs but is most often associated with S. aureus strains that make TSST-1, SEB, or SEC (7). There are many subsets of nonmenstrual TSS, including those associated with routine skin infections (such as boils and minor wounds), recalcitrant erythematous desquamating syndrome of AIDS patients, and postsurgical and postrespiratory viral infections (1, 4).
According to the Centers for Disease Control and Prevention definition, mTSS occurs in TSST-1-serosusceptible women within 2 days of onset of menses, during menses, or within 2 days of termination of menses. A small number of mTSS cases are associated with S. aureus infections at other body sites (usually mucous membranes).
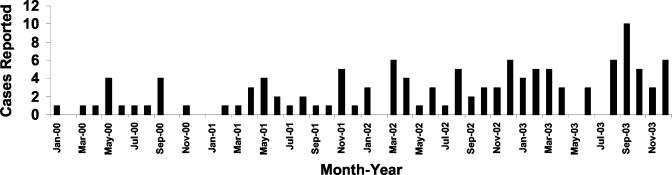
In the early 1980s, the yearly incidence of mTSS in the United States was approximately 10/100,000 women of menstrual age (9). With the removal from the market of tampons of highest absorbency by 1984, the incidence dropped, and by the mid- to late 1980s the yearly incidence was estimated to be approximately 1/100,000 women of menstrual age (3). Since mid-2002, our laboratory has been contacted by several physicians from across the United States who indicate they are seeing increasing numbers of both mTSS and nonmenstrual TSS cases. Due to these reports, the number of cases of TSS in the Minneapolis-St. Paul (Twin Cities) metropolitan area (with a population of approximately 3,000,000) from 2000 through 2003 was evaluated by a previously used surveillance system. This system is based (i) on the knowledge that the clinical features of TSS are highly distinctive and (ii) on a high correlation between physician-submitted S. aureus strains that produce SAgs (e.g., TSST-1, SEB, or SEC) and a diagnosis of TSS (8). The Schlievert laboratory provides TSS toxin testing for physicians in the Twin Cities metropolitan area. Of isolates submitted for toxin testing across this entire time period (total, 126 isolates), all but two isolates (98.4%) made one or more of TSST-1, SEB, and SEC. This percentage is similar to that we reported in a previous study (8). The two toxin-negative cases were not included in subsequent studies in this report. As shown in Fig. 1, there was approximately 1 case of TSS (menstrual or nonmenstrual) per month in the Twin Cities area during 2000 (total number of cases, 15). In contrast, there were an average of 2 cases per month during 2001 (total number of cases, 22) and 3 cases per month during 2002 (total number of cases, 37). Finally, there were 4 cases per month during 2003 (total number of cases, 50). On the basis of these numbers, the calculated incidence of TSS (menstrual and nonmenstrual) in the Twin Cities was approximately 0.8/100,000 women of menstrual age in 2000, 1.6/100,000 women of menstrual age in 2001, 2.4/100,000 women of menstrual age in 2002, and 3.4/100,000 women of menstrual age in 2003.
FIG. 1.
Cases of staphylococcal toxic shock from January 2000 through December 2003 in the Minenapolis-St. Paul metropolitan area.
The cases considered in this study included both mTSS and nonmenstrual TSS, and prior attempts to establish clearly the precise number of cases in each category have been unsuccessful (8). Our observation of increased numbers of cases of TSS across this 4-year time period is consistent with Centers for Disease Control and Prevention findings of an 18% increase in the incidence of TSS from 2002 to 2003.
The data suggest that the medical practitioners and women who use tampons should be aware that the TSS incidence is increasing. The reason for this increase is unclear, but it is possible that S. aureus strains capable of causing TSS are reemerging, as was reported to have happened in the early 1970s. It is unlikely that increases in menstrual cases are due to changes in tampons since there have not been significant changes in these products since 1984; it is possible, however, that there are changes in tampon usage patterns.
Acknowledgments
This work was supported by USPHS research grant HL36611 from the National Heart, Lung, and Blood Institute.
REFERENCES
- 1.Cone, L. A., D. R. Woodard, R. G. Byrd, K. Schulz, S. M. Kopp, and P. M. Schlievert. 1992. A recalcitrant, erythematous, desquamating disorder associated with toxin-producing staphylococci in patients with AIDS. J. Infect. Dis. 165:638-643. [DOI] [PubMed] [Google Scholar]
- 2.Dinges, M. M., P. M. Orwin, and P. M. Schlievert. 2000. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 13:16-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaventa, S., A. L. Reingold, A. W. Hightower, C. V. Broome, B. Schwartz, C. Hoppe, J. Harwell, L. K. Lefkowitz, S. Makintubee, D. R. Cundiff, et al. 1989. Active surveillance for toxic shock syndrome in the United States, 1986. Rev. Infect. Dis. 11:S28-S34. [DOI] [PubMed] [Google Scholar]
- 4.MacDonald, K. L., M. T. Osterholm, C. W. Hedberg, C. G. Schrock, G. F. Peterson, J. M. Jentzen, S. A. Leonard, and P. M. Schlievert. 1987. Toxic shock syndrome. A newly recognized complication of influenza and influenzalike illness. JAMA 257:1053-1058. [DOI] [PubMed] [Google Scholar]
- 5.Parsonnet, J. 1998. Case definition of staphylococcal TSS: a proposed revision incorporating laboratory findings. Int. Congr. Symp. Ser. 29:15-16. [Google Scholar]
- 6.Reingold, A. L., N. T. Hargrett, B. B. Dan, K. N. Shands, B. Y. Strickland, and C. V. Broome. 1982. Nonmenstrual toxic shock syndrome: a review of 130 cases. Ann. Intern. Med. 96:871-874. [DOI] [PubMed] [Google Scholar]
- 7.Schlievert, P. M. 1986. Staphylococcal enterotoxin B and toxic-shock syndrome toxin-1 are significantly associated with non-menstrual TSS. Lancet i:1149-1150. [DOI] [PubMed] [Google Scholar]
- 8.Schlievert, P. M., and M. H. Kim. 1991. Reporting of toxic shock syndrome Staphylococcus aureus in 1982 to 1990. J. Infect. Dis. 164:1245-1246. [DOI] [PubMed] [Google Scholar]
- 9.Shands, K. N., G. P. Schmid, B. B. Dan, D. Blum, R. J. Guidotti, N. T. Hargrett, R. L. Anderson, D. L. Hill, C. V. Broome, J. D. Band, and D. W. Fraser. 1980. Toxic-shock syndrome in menstruating women: association with tampon use and Staphylococcus aureus and clinical features in 52 cases. N. Engl. J. Med. 303:1436-1442. [DOI] [PubMed] [Google Scholar]
- 10.Todd, J. K., F. A. Kapral, M. Fishaut, and T. R. Welch. 1978. Toxic shock syndrome associated with phage group 1 staphylococci. Lancet ii:1116-1118. [DOI] [PubMed] [Google Scholar]