Abstract
Alveolar type II epithelial (ATII) cell injury precedes development of pulmonary fibrosis. Mice lacking urokinase-type plasminogen activator (uPA) are highly susceptible, whereas those deficient in plasminogen activator inhibitor (PAI-1) are resistant to lung injury and pulmonary fibrosis. Epithelial–mesenchymal transition (EMT) has been considered, at least in part, as a source of myofibroblast formation during fibrogenesis. However, the contribution of altered expression of major components of the uPA system on ATII cell EMT during lung injury is not well understood. To investigate whether changes in uPA and PAI-1 by ATII cells contribute to EMT, ATII cells from patients with idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease, and mice with bleomycin-, transforming growth factor β–, or passive cigarette smoke–induced lung injury were analyzed for uPA, PAI-1, and EMT markers. We found reduced expression of E-cadherin and zona occludens-1, whereas collagen-I and α-smooth muscle actin were increased in ATII cells isolated from injured lungs. These changes were associated with a parallel increase in PAI-1 and reduced uPA expression. Further, inhibition of Src kinase activity using caveolin-1 scaffolding domain peptide suppressed bleomycin-, transforming growth factor β–, or passive cigarette smoke–induced EMT and restored uPA expression while suppressing PAI-1. These studies show that induction of PAI-1 and inhibition of uPA during fibrosing lung injury lead to EMT in ATII cells.
Idiopathic pulmonary fibrosis (IPF) and other interstitial lung diseases are characterized by destruction of lung architecture due to excessive deposition of extracellular matrix proteins by activated fibroblasts or myofibroblasts, leading to progressive dyspnea and loss of lung function.1–3 The origins of myofibroblasts participating in the pathological remodeling of IPF lungs are not clear. Histopathological evaluation demonstrates that myofibroblasts accumulate in fibroblastic foci. Emerging evidence suggests that polarized type II alveolar epithelial (ATII) cells undergo epithelial–mesenchymal transitions (EMT) after lung injury. The ATII cells assume phenotypic changes such as increased migration, invasion, resistance to apoptosis, and production of elevated levels of extracellular matrix proteins4,5 and therefore serve as a source of myofibroblasts. Understanding the possible mechanisms contributing to EMT in ATII cells may help identify new targets to treat or at least limit fibrogenesis after lung injury.
A number of molecular processes are involved in the initiation of EMT in ATII cells.5 Components of the fibrinolytic system such as urokinase-type plasminogen activator (uPA), uPA plasma membrane receptor (uPAR), and its major inhibitor, plasminogen activator inhibitor (PAI-1) are all elaborated by ATII cells. These proteins independently influence a broad range of biological processes germane to lung injury and its repair.6 However, their role in fibrogenesis via EMT is unclear. Recent publications using bleomycin (BLM)7 and a passive cigarette smoke (PCS)8 or adenovirus expressing constitutively active transforming growth factor β (Ad-TGF-β)1,9 exposure model of lung injury indicate that a coordinate increase in PAI-1 and a decrement in uPA by ATII cells promote lung injury and subsequent pulmonary fibrosis (PF). We also found that caveolin-1 scaffolding domain peptide (CSP) acts as a competitor to caveolin-1, restores expression of uPA and uPAR, and inhibits PAI-1 in ATII cells after lung injury. These changes prevent development of PF after lung injury.7 Recent literature suggests that up to 30% to 50% of myofibroblasts may be derived via EMT during fibrogenesis.10–12 However, an in vivo genetic lineage tracing study reported by Rock et al13 contradicts these findings. Our objective in the current study is to elucidate the role of altered expression of uPA, uPAR, and PAI-1 after lung injury in EMT, and further evaluate whether reinstatement of baseline expression of uPA, uPAR, and PAI-1 by CSP intervention after lung injury reduces EMT in ATII cells.
Materials and Methods
Isolation and Analysis of ATII Cells from IPF and COPD Lung Tissues
IPF or chronic obstructive pulmonary disease (COPD), and control (without overt IPF or COPD) donor lung samples were collected from patients undergoing thoracic surgery at St. Mary's Hospital (Rochester, MN). Protocols were approved by institutional review boards of Mayo Clinic (Rochester, MN) and The UT Health Science Center at Tyler (Tyler, TX). ATII cells were isolated from the IPF, COPD, and control lung tissues, and aliquots were stained for inclusion bodies to assess the purity of cell preparation as we described recently.8 The lysates from these cells were immunoblotted for expression of EMT markers. Lung sections from patients with IPF and COPD, diffused alveolar damage, or healthy donors were provided by the Lung Tissue Research Consortium of the National Heart, Lung, and Blood Institute or by the Department of Pathology at the UT Health Science Center at Tyler. These sections were subjected to immunohistochemical (IHC) analysis to assess changes in antigen levels for uPA, PAI-1, and EMT markers.
BLM- and PCS-Induced Lung Injury
Wild-type (WT) or uPA-, uPAR-, and PAI-1–deficient mice of C57BL/6 background were bred in our facilities or were purchased from The Jackson Laboratory (Bar Harbor, ME). All animal experiments were performed according to approved protocols under the guidelines of the Animal Care and Use Committee of The UT Health Science Center at Tyler. For in vitro experiments, ATII cells were isolated from the lungs of uninjured mice as we reported elsewhere.7,8 The purities of ATII cell preparations were approximately 90% to 95%, based on lithium carbonate staining for inclusion bodies. These cells were treated with 40 μg/mL BLM with or without 10 nmol/L CSP or control peptide (CP), or 2 ng/mL purified TGF-β1 alone in Matrigel-coated culture dishes containing epithelial cell growth–supplemented alveolar epithelial cell medium (AEpiCM) from ScienCell Research Laboratories (Carlsbad, CA) for 3 days.7,8,14–17 For in vivo studies, mice were exposed to 2 U BLM per kg body weight for 3 days or PCS for 20 weeks with or without 18.75 mg of CSP or CP per kg body weight as described previously.7,8 ATII cells were isolated and used for Western blot analysis or real-time PCR analysis to assess changes in the expression of E-cadherin, ZO-1, collagen-I, and α-smooth muscle actin (α-SMA) protein and mRNA, respectively. Real-time PCR using primers for mouse E-cadherin, forward: 5′-AATGGCGGCAATGCAATCCCAAGA-3′ and reverse: 5′-TGCCACAGACCGATTGTGGAGATA-3′; mouse collagen-I, forward: 5′-CCAAGGGTAACAGCGGTGAA-3′ and reverse: 5′-CCTCGTTTTCCTTCTTCTCCG-3′; mouse ZO-1, forward: 5′-ATTCTGAAGAAATGATGAGA-3′ and reverse: 5′-TCCTGATTGACCACTTTTAA-3′; α-SMA, forward: 5′-GGCTCTGGGCTCTGTAAGG-3′ and reverse: 5′-CTCTTGCTCTGGGCTTCATC-3′; and β-actin, forward: 5′-CACCGCAGCTCGTAGCTCTTCTCCAGGG-3′ and reverse: 5′-CCAGCCATGTACGTTGCTATCCAG-3′. Lung sections were separately assessed for collagen-I, E-cadherin, α-SMA, and ZO-1 antigen levels by IHC analysis. Lung homogenates and bronchoalveolar lavage fluids of mice exposed to BLM with or without CSP or CP for 3 days were analyzed for active TGF-β by Western blot analysis. The findings were independently confirmed by enzyme-linked immunosorbent assay (ELISA) for active TGF-β.
Effect of TGF-β Overexpression on ATII Cell EMT and PF
ATII cells isolated from WT mice were exposed to Ad-TGF-β in the presence or absence of 10 nmol/L CSP or CP as described above. ATII cells untreated or treated with empty adenovirus (Ad-EV) for 3 days were used as controls. For in vivo studies, WT mice were exposed to saline or Ad-EV or Ad-TGF-β (109 plaque forming units) through intranasal instillation as described earlier.9 After 24 hours, mice exposed to Ad-TGF-β were treated with or without 18.75 mg CSP or CP per kg body weight. ATII cells were isolated 3 days after instillation of Ad-TGF-β and analyzed for changes in EMT. Lung homogenates of WT mice were analyzed for total hydroxyproline contents 21 days after transduction with Ad-TGF-β to assess changes in PF as we described earlier.7
Effect of p53-Binding uPA, uPAR, and PAI-1 mRNA 3′-UTR Sequences on BLM-Induced ATII Cell EMT in Mice
The competitive inhibition of p53 from binding to endogenous uPA, uPAR, and PAI-1 mRNAs in ATII cells by overexpression of p53-binding chimeric uPA, uPAR, and PAI-1 3′-untranslated region (UTR) sequences, concurrently restores uPA and uPAR, and inhibits PAI-1 expression without affecting BLM- or PCS-induced p53 in mice.8,18 Therefore, we exposed mice to an i.v. (through orbital plexus) injection of lentivirus vector containing SP-B promoter expressing p53-binding or nonbinding control chimeric sequences of uPA, uPAR, and PAI-1 3′-UTR mRNA, and the transduction efficiency was confirmed by expression of luciferase in ATII cells as described previously.18 Twenty-four hours after lentiviral transduction, these mice were exposed to BLM. ATII cells were isolated 72 hours after initiation of BLM injury and analyzed for EMT markers by Western blot analysis.
Effect of Inhibition of uPA or PAI-1 Expression in PAI-1– and uPA-Deficient ATII Cells, Respectively, on EMT
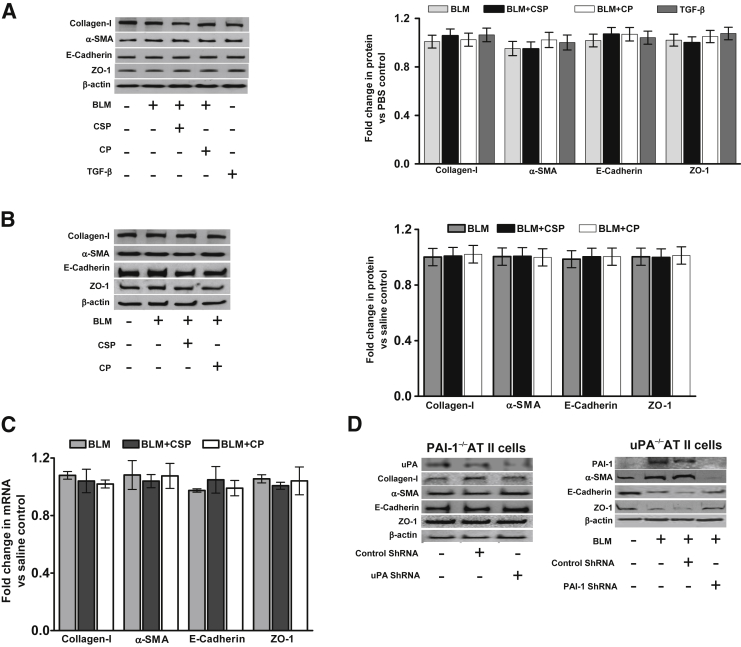
ATII cells isolated from uninjured mice lacking PAI-1 expression were treated with lentiviral vector expressing uPA shRNA to inhibit baseline uPA expression. PAI-1–deficient ATII cells exposed to control shRNA or naive ATII cells were used as controls. Similarly, ATII cells isolated from uPA-deficient mice were exposed to BLM in the presence or absence of PAI-1 shRNA or control shRNA. The lysates from these mice were analyzed for uPA, E-cadherin, ZO-1, and α-SMA expression.
Generation of Retroviral Plasmid
Retrovirus vector (pLNCX) containing Src mutant Y416F cDNA was cotransfected with packaging plasmid pUMVC and auxiliary plasmid pCMV-VSV-G (Addgene, Cambridge, MA) using Lipofectamine 2000 (Life Technologies, Grand Island, NY) in 293T cells to obtain phage particles. The viral titers were measured using 293 T cells and later transduced ATII cells isolated from mouse lungs.
Localization of Biotin-Labeled CSP in Mouse Lungs
To determine the distribution of CSP in the mouse lungs, mice were exposed to saline or BLM for 72 hours through intranasal instillation. These mice were i.p. injected with biotin-labeled CSP. After 24 hours, mice were euthanized, and the lung sections were analyzed for biotin-labeled CSP using anti-biotin antibody.
Statistical Analysis
The statistical differences between various experimental conditions were analyzed by t-test and one way analysis of variance. P < 0.05 was considered significant.
Results
ATII Cell EMT Is Associated with Increased Expression of PAI-1 and Concurrent Inhibition of uPA in IPF and COPD Lungs
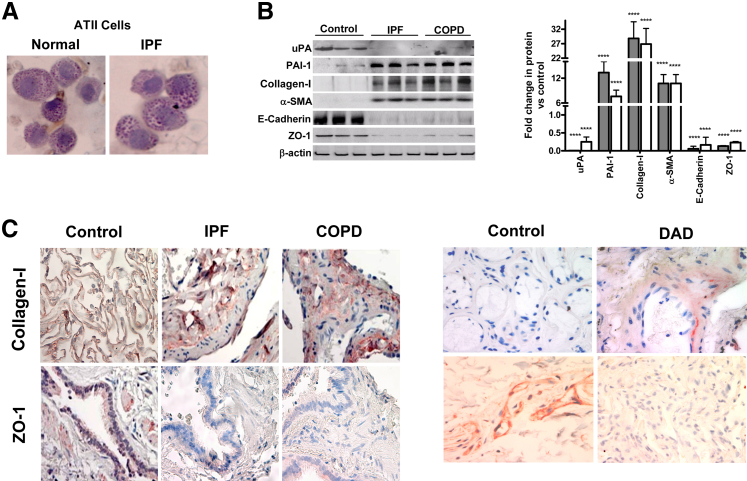
ATII cell damage precedes development of PF, and restoration of uPA and inhibition of PAI-1 expression prevents development of PF after fibrosing lung injury in animal models. Multiple recent studies indicate that EMT in ATII cells promotes fibrogenesis after lung injury. Therefore, we isolated ATII cells from the human lung tissues and stained for inclusion bodies to assess the purity of ATII cell preparation using lithium carbonate, which indicated purity approximately 90% to 95% (Figure 1A). Next, we tested the ATII cell lysates for changes in uPA and PAI-1 expression. ATII cells isolated from human IPF lungs showed a marked increase in PAI-1 and reduction in uPA expression compared to those extracted from histologically normal lungs (Figure 1B). ATII cells from IPF lungs showed suppression of epithelial cell markers (E-cadherin and ZO-1) expression, whereas mesenchymal cell markers (collagen-I and α-SMA) were increased compared to their baseline expressions found in control lungs. We also found similar changes in the expression of uPA, PAI-1, and markers of EMT in ATII cells from the lungs of patients with COPD. Consistent with the results of Western blot analysis of isolated ATII cells, IHC analyses of IPF and COPD lung sections showed increased collagen-I and inhibition of ZO-1 staining compared to their expression in control subjects (Figure 1C). Similar changes were also observed in lung sections of patients with diffused alveolar damage. This indicates a causal link between altered ATII cell collagen-I and ZO-1 expression, and EMT as a result of chronic lung injury.
Figure 1.
Altered expressions of plasminogen activator inhibitor (PAI-1) and urokinase-type plasminogen activator (uPA) are associated with alveolar type II (ATII) cell epithelial–mesenchymal transition in idiopathic pulmonary fibrosis (IPF) and chronic obstructive pulmonary disease (COPD) lungs. A: ATII cells isolated from lung explants were stained for inclusion bodies using lithium carbonate to assess the purity of the cell preparation. B: ATII cells isolated from patients (n = 3) with IPF (gray bars) or COPD (white bars) and from healthy donors. The lysates were analyzed for changes in the expression of uPA, PAI-1, collagen-I, α-SMA, E-cadherin, ZO-1, and β-actin. The fold changes in densities of individual bands are presented as a bar graph after normalization against the corresponding densities of β-actin antigens present in each sample. C: Paraffin-embedded sections from lung tissues of patients with IPF or COPD or diffused alveolar damage (DAD) and healthy donors were subjected to IHC analysis for changes in collagen-I and ZO-1 antigen levels in situ. Representative photomicrograph (from n = 5) is shown. ∗∗∗∗P < 0.0001.
Effect of CSP on BLM-Induced EMT in ATII Cells
Because ATII cells from human IPF lungs showed increased EMT and PAI-1 with reduction in uPA expression, we isolated ATII cells from mouse lungs and exposed them to BLM for 72 hours and analyzed changes in the expression of E-cadherin, ZO-1, collagen-I, and α-SMA to assess EMT in vitro. Exposure of ATII cells to BLM suppressed expression of E-cadherin and ZO-1, while increasing collagen-I and α-SMA (Figure 2A). ATII cells treated with the profibrogenic cytokine, TGF-β, also revealed a significant increase in EMT compared to phosphate-buffered saline (PBS)-treated control ATII cells. Exposure of ATII cells to BLM with CSP restored E-cadherin and ZO-1 expression while significantly reducing the expression of collagen-I and α-SMA. However, the scrambled CP failed to affect BLM-induced EMT in murine ATII cells. Because treatment of mouse ATII cells with purified TGF-β protein increased EMT, we next transduced ATII cells with Ad-TGF-β in the presence or absence of CSP or CP and tested for changes in EMT markers. Ad-TGF-β increased EMT in ATII cells in comparison with those transduced with Ad-EV (Figure 2B). Further, treatment of ATII cells with CSP, but not CP, suppressed TGF-β–induced EMT. To determine whether in vitro culture condition alters phenotypes of ATII cells during the course of our experiments, we maintained control ATII cells isolated from uninjured mice in growth factor–supplemented AEpiCM medium for 0 to 3 days and analyzed for changes in surfactant proteins by Western blot analysis. ATII cells cultured in AEpiCM retained baseline SP-A, SP-B, and SP-C expression at least for 3 days, indicating preservation of cellular phenotypes (Figure 2C).
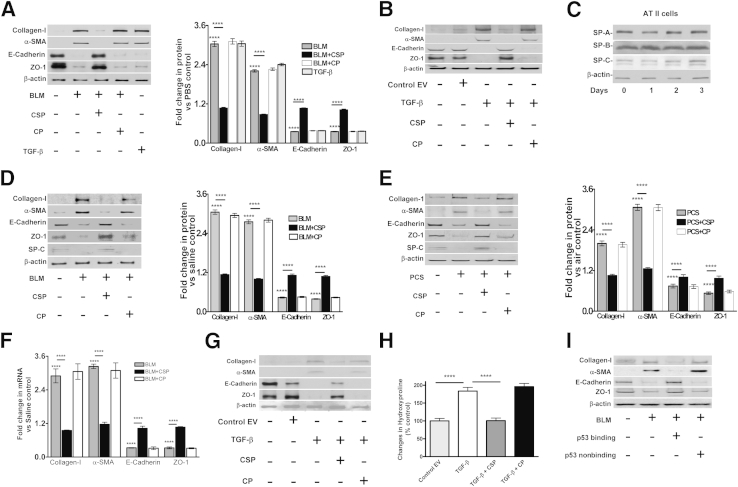
Figure 2.
Inhibition of bleomycin (BLM)-induced epithelial–mesenchymal transitions (EMT) in ATII cells by caveolin-1 scaffolding domain peptide (CSP). A: ATII cells isolated from WT mice were treated with PBS or 40 μg/mL BLM alone or BLM with 10 nmol/L CSP or control peptide (CP) for 72 hours in culture dishes at 37°C. The lysates were immunoblotted for changes in the expression of collagen-I, α-SMA, E-cadherin, ZO-1, and β-actin antigens. The lysates from ATII cells treated with 2 ng/mL transforming growth factor β (TGF-β) were used as a positive control for comparison. The densities of individual bands were normalized against the corresponding densities of β-actin. The fold changes in proteins are presented as a bar graph. Differences between PBS control and BLM or BLM and BLM+CSP groups are statistically significant. B: ATII cells isolated from WT mice were exposed to PBS, Ad-EV, or Ad-TGF-β with or without CSP or CP in culture dishes. After 72 hours, the lysates were analyzed for changes in EMT markers by Western blot analysis. C: ATII cells isolated from WT mice were cultured in Matrigel-coated plastic dishes for 0 to 3 days at 37°C. The lysates were later analyzed for changes in SP-A, SP-B, and SP-C expression by Western blot analysis. D: WT mice were exposed to saline or 2 U/kg body weight BLM through intranasal instillation. After 24 hours, mice exposed to BLM were i.p. injected with or without 18.75 mg/kg body weight of CSP or CP. Three days after BLM injury, ATII cells were isolated and the lysates were immunoblotted for changes in the expression of collagen-I, α-SMA, E-cadherin, ZO-1, and β-actin. The densities of individual bands were measured and normalized against the corresponding densities of β-actin. The fold changes in proteins are presented as a bar graph. E: WT mice were exposed to ambient air or PCS from 40 cigarettes (approximately 90 mg/m3 total solid particulates) using a mechanical smoking chamber over a 2-hour period for 5 days per week. After 4 weeks of PCS exposure, mice exposed to PCS were i.p. injected with or without 18.75 mg/kg body weight of CSP or CP once every week for 4 more weeks. After 20 weeks of PCS exposure, ATII cells were isolated, and the lysates were tested for changes in the expression of collagen-I, α-SMA, E-cadherin, ZO-1 and β-actin by Western blot analysis. F: Total RNA isolated from ATII cells of mice treated with saline, BLM, BLM+CSP, or CP were analyzed for changes in the expression of E-cadherin, collagen-I, ZO-1, and α-SMA mRNAs by quantitative real-time PCR. Changes in their expression levels were normalized to the corresponding levels of β-actin transcripts. The data were presented relative to that of saline-treated control groups. G: WT mice were exposed to saline, Ad-EV, or Ad-TGF-β by intranasal instillation. Twenty-four hours later, mice transduced with Ad-TGF-β were treated with or without CSP or CP. ATII cells were isolated from these mice 72 hours after exposure to TGF-β and analyzed for changes in EMT markers by Western blot analysis. H: WT mice treated with Ad-EV or Ad-TGF-β alone or Ad-TGF-β with CSP or CP as described in G were euthanized 21 days after initial transduction with Ad-TGF-β. The lung homogenates were analyzed for total hydroxylproline contents to assess changes in lung fibrosis. I: Mice were i.v. (via orbital plexus) injected with or without lentivirus expressing p53-binding or non–p53-binding control chimeric 3′-UTR sequences of uPA/uPAR/PAI-1 mRNA 3′-UTR as described elsewhere.14 Twenty-four hours later, the mice were exposed to BLM, and ATII cells were isolated 72 hours after inception of BLM injury. ATII cells isolated from mice exposed to saline were used as control for comparison. The lysates were tested for changes in the expression of collagen-I, α-SMA, E-cadherin, ZO-1, and β-actin. ∗∗∗∗P < 0.0001 versus control.
Next, we exposed the mice to BLM through intranasal instillation, and some mice were exposed to CSP or CP 24 hours after initiation of BLM injury. Saline-treated mice were used as controls. The lung sections from these mice were subjected to IHC analysis for changes in EMT markers in situ. Following BLM-induced injury, lung sections of mice showed a marked inhibition of ZO-1 antigen expression, whereas collagen-I antigen levels were markedly increased in the lung parenchyma (Supplemental Figure S1). Further, treatment of mice with CSP markedly reduced BLM-induced EMT, which was confirmed by the changes in ZO-1 or collagen-I expression, whereas the response of CP to BLM-induced EMT was negative. Because PCS exposure causes lung injury, albeit to a lesser extent than BLM-induced lung injury in mice,7,8 and ATII cells isolated from patients with COPD also showed increased EMT (Figure 1, B and C), we exposed mice to PCS and analyzed the lung sections for changes in markers for EMT. PCS exposure increased collagen-I with concurrent suppression of ZO-1 expression (Supplemental Figure S2). However, treatment with CSP markedly reversed PCS-induced changes in the EMT markers.
To further show these changes for alveolar epithelium, we isolated ATII cells from mice exposed to BLM (Figure 2D) or PCS (Figure 2E) and analyzed for changes in expression of EMT markers. ATII cells collected from mice exposed to intranasal BLM or PCS showed increased expression of collagen-I and α-SMA, whereas epithelial cell markers such as E-cadherin and ZO-1 were significantly suppressed compared to baseline levels in mice without lung injury (Figure 2, D and E). Further, treatment of mice with CSP after BLM or PCS exposure injury significantly prevented EMT. We next evaluated changes in E-cadherin, ZO-1, collagen-I, and α-SMA expression at the mRNA level. Consistent with protein expression and mRNA analysis by quantitative real-time PCR, the results indicated that BLM injury increased EMT (Figure 2F). In addition, treatment of mice with CSP attenuated BLM-mediated induction of collagen-I and α-SMA mRNA, and restored the expression of E-cadherin and ZO-1 mRNA in ATII cells. There were no significant changes in the expression of EMT markers in BLM-injured mice treated with CP. Because TGF-β induces EMT in ATII cells in vitro (Figure 2B), and is a major mediator of fibrosis in diverse organs, including lungs,19,20 we next tested whether CSP reverses ATII cell EMT in mice exposed to Ad-TGF-β. We found increased EMT in ATII cells from mice transduced with Ad-TGF-β compared to those exposed to Ad-EV (Figure 2G). Consistent with BLM-induced injury, treatment of mice exposed to Ad-TGF-β with CSP, but not CP, suppressed EMT in ATII cells. Further analysis of lung tissues for total hydroxyproline contents confirmed increased PF in mice exposed to Ad-TGF-β 21 days later (Figure 2H). This was significantly suppressed in mice exposed to Ad-TGF-β and later treated with CSP.
ATII cells isolated from IPF and COPD showed increased EMT associated with a parallel increase in PAI-1 and inhibition of uPA expression (Figure 1). p53 concurrently inhibits uPA and uPAR while inducing PAI-1 expression in ATII cells during BLM or PCS exposure injury by binding unique 3′-UTR sequences.7,8,18 CSP inhibits BLM- or PCS-induced ATII cell injury and prevents BLM-induced development of PF. The process involves inhibition of p53 expression and reversal of p53-mediated downstream changes in uPA, uPAR, and PAI-1. To independently confirm whether p53-mediated changes in uPA, uPAR, and PAI-1 expression in ATII cells contribute to EMT after BLM lung injury, we transduced mouse lung ATII cells with lentivirus expressing p53-binding sequences under the control of SP-B promoter in vivo. These mice were later exposed to BLM, and ATII cells were isolated and tested for changes in EMT. Overexpression of p53-binding 3′-UTR sequences significantly suppressed BLM-induced EMT in ATII cells (Figure 2I). However, mice exposed to control sequence showed increased EMT in ATII cells after exposure to BLM.
Role of uPA in CSP-Mediated Inhibition of BLM-Induced ATII Cell EMT
ATII cells from COPD and IPF lungs showed reduction in uPA expression (Figure 1). Studies suggests that mice lacking uPA expression are highly susceptible to BLM-induced PF. We further found that treatment of uPA-deficient mice with CSP after BLM injury failed to prevent the development of PF.7 To determine whether CSP-mediated inhibition of ATII cell EMT in WT mice required restoration of uPA expression, we isolated ATII cells from uninjured uPA-deficient mice. The purity of ATII cell preparation was confirmed by lithium carbonate staining for inclusion bodies (Supplemental Figure S3A). These cells were treated with BLM in the presence or absence of CSP or CP in vitro. Treatment of uPA-deficient mouse ATII cells with either BLM or TGF-β in vitro, significantly induced EMT (Figure 3A). Interestingly, unlike WT ATII cells, ATII cells lacking uPA expression with BLM injury failed to respond to CSP treatment. Our results suggest that restoration of uPA expression after BLM injury is critical in the control of EMT.
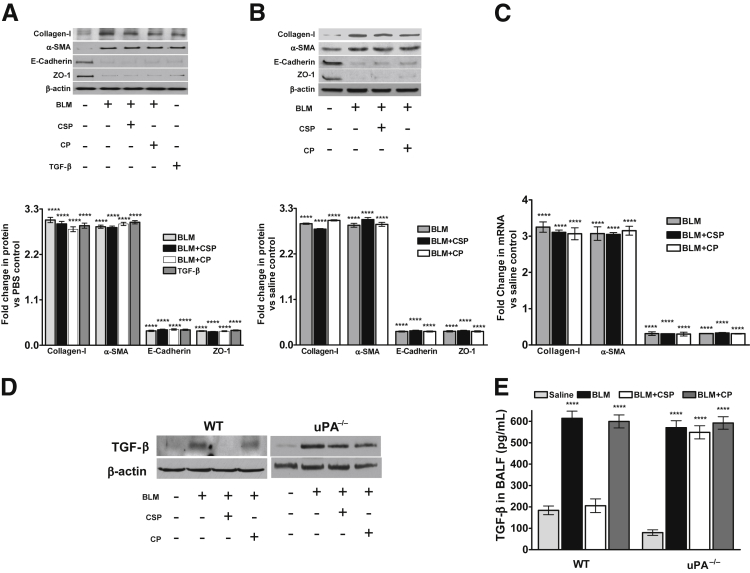
Figure 3.
Role of urokinase-type plasminogen activator (uPA) in ATII cell epithelial–mesenchymal transitions after bleomycin (BLM) injury. A: ATII cells isolated from mice deficient in uPA expression were treated with PBS, BLM, BLM+CSP, or BLM+CP in vitro. The lysates were tested for the expression of collagen-I, α-SMA, E-cadherin, ZO-1, and β-actin antigens by Western blot analysis. The lysates from uPA-deficient ATII cells treated with TGF-β were used as a control for comparison. The individual bands were quantitated and normalized to the corresponding values of β-actin loading controls and the fold changes in proteins are presented as a bar graph. Differences between treatments versus control are statistically significant. B: Mice deficient in uPA expression were exposed to saline or BLM as described in Figure 2D. Twenty-four hours after initial exposure to BLM, mice were i.p. injected with or without caveolin-1 scaffolding domain peptide (CSP) or control peptide (CP). Seventy-two hours after initiation of BLM injury, ATII cells were isolated and the lysates were evaluated for changes in the expression of collagen-I, α-SMA, E-cadherin, ZO-1, and β-actin by Western blot analysis. The densities of individual bands were normalized against the corresponding densities of β-actin protein, and the fold changes in proteins are presented as a bar graph. C: Total RNA isolated from ATII cells of uPA-deficient mice exposed to saline, BLM, BLM+CSP, or CP were analyzed for changes in the expression of E-cadherin, collagen-I, ZO-1, and α-SMA mRNA levels by real-time PCR. The data were normalized with corresponding levels of β-actin mRNA. The fold changes of mRNA are presented as a bar graph. D: The lung homogenates of WT and uPA-deficient mice exposed to saline, BLM, BLM+CSP, or BLM+CP were tested for active TGF-β by Western blot analysis. E: The bronchoalveolar lavage fluids (BALF) of WT and uPA-deficient mice exposed to saline, BLM, BLM+CSP, or BLM+CP were analyzed for active TGF-β byELISA. ∗∗∗∗P < 0.0001 versus control (A, D, E, and F).
We then exposed uPA-deficient mice to BLM with or without CSP or CP, and analyzed the lung tissues for EMT in situ by IHC. uPA-deficient mice exposed to BLM showed increased expression of collagen-I and α-SMA, whereas ZO-1 antigen levels were significantly reduced after BLM injury (Supplemental Figure S4). Interestingly, unlike WT mice, uPA-deficient mice with BLM-induced lung injury failed to respond to CSP treatment. Analysis of isolated ATII cells from these mice, for changes in the expression of EMT markers by Western blot analysis, indicated an increased EMT compared to their baseline levels in uPA-deficient mice without lung injury (Figure 3B). Treatment of uPA-deficient mice with either CSP or CP after BLM injury failed to reverse ATII cell EMT that was due to BLM injury. mRNA analyses for changes in the expression of EMT markers in uPA-deficient mice with BLM injury further confirmed that CSP failed to restore their expression (Figure 3C). These data indicate the importance of uPA expression by ATII cells to prevent EMT during BLM-induced lung injury. The literature21,22 suggests that uPA through plasmin generation can activate extracellular matrix–trapped TGF-β, whereas ATII cells lacking uPA expression resist CSP-mediated suppression of EMT after BLM injury. Therefore, we analyzed lung homogenates and bronchoalveolar lavage fluids from WT and uPA-deficient mice for active TGF-β by Western blot analysis (Figure 3D) and ELISA (Figure 3E). Our results confirmed that BLM lung injury increased active TGF-β levels in WT and uPA-deficient mice, which was significantly suppressed in WT mice with BLM injury exposed to CSP.
Role of uPAR in ATII Cell EMT
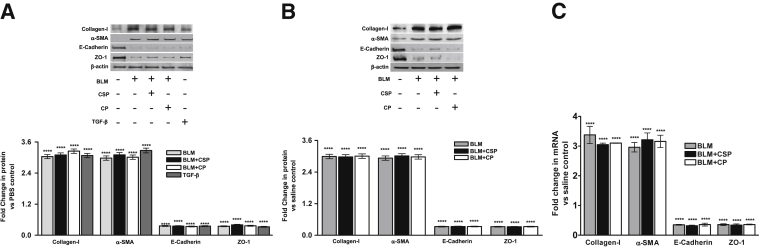
Because most of the cellular functions of uPA are dependent on its binding to uPAR, we also isolated ATII cells from uPAR-deficient mice, confirmed the purity by lithium carbonate staining for inclusion bodies (Supplemental Figure S3B), and used these cells to study the role of uPAR in EMT. Consistent with the responses of WT and uPA-deficient ATII cells, ATII cells lacking uPAR showed increased EMT following exposure to BLM. Treatment with either CSP or CP failed to significantly attenuate EMT induced in ATII cells because of BLM (Figure 4A). To further confirm the above findings in vivo, we exposed uPAR-deficient mice to BLM and treated them with CSP or CP. ATII cells were isolated and analyzed for changes in EMT markers. Reduced E-cadherin and ZO-1 with increased collagen-I and α-SMA expression indicated EMT in ATII cells isolated from mice after BLM injury (Figure 4B). Further, uPAR-deficient mice exposed to BLM resisted CSP treatment and showed increased ATII cell EMT. The analyses of ATII cells for changes in E-cadherin, ZO-1, collagen-I, and α-SMA mRNAs further proved that ATII cell-specific uPAR expression is also required for CSP to inhibit BLM-induced EMT (Figure 4C).
Figure 4.
Effect of uPA plasma membrane receptor (uPAR) expression on bleomycin (BLM)-induced ATII cell epithelial–mesenchymal transitions. A: ATII cells isolated from mice lacking uPAR expression were exposed to PBS, BLM, BLM+CSP, or BLM+CP in vitro. The lysates were immunoblotted for the expression of collagen-I, α-SMA, E-cadherin, ZO-1, and β-actin antigens. The lysates from uPAR-deficient ATII cells treated with transforming growth factor β (TGF-β) were used as a control. The densities of individual bands were compiled and normalized against corresponding values of β-actin loading controls. The fold changes in proteins are presented as a bar graph, and the differences between treatments versus control were statistically significant. B: uPAR-deficient mice were treated with saline or BLM and 24 hours after the initial exposure to BLM, mice with BLM injury were i.p. injected with or without caveolin-1 scaffolding domain peptide (CSP) or control peptide (CP). Three days after the initiation of BLM injury, ATII cells were isolated from these mice, and the cell lysates were immunoblotted for altered expression of collagen-I, α-SMA, E-cadherin, ZO-1, and β-actin. The fold changes of proteins are presented as a bar graph. C: ATII cell RNA from uPAR-deficient mice exposed to saline, BLM, BLM+CSP, or CP were tested for changes in the expression of E-cadherin, collagen-I, ZO-1, and α-SMA mRNAs by real-time PCR. The fold changes of mRNA are presented as a bar graph. ∗∗∗∗P < 0.0001 versus control.
Role of PAI-1 Expression in ATII Cell EMT
PAI-1 expression is increased during BLM- or PCS-induced lung injury, and mice that lack PAI-1 expression resist ATII cell injury and subsequent development of PF.7,8 We investigated whether PAI-1 expression contributes to EMT in ATII cells. ATII cells lacking PAI-1 when exposed to BLM in vitro, resisted inhibition of E-cadherin and ZO-1 (Figure 5A). Interestingly, baseline expression levels of collagen-I and α-SMA in PAI-1–deficient ATII cells without BLM injury were highly elevated and comparable with WT ATII cells with BLM injury (Figure 2A). However, treatment of PAI-1–deficient ATII cells with BLM alone, or BLM+CSP, or BLM+CP failed to significantly alter the baseline levels of E-cadherin, ZO-1, collagen-I, and α-SMA expression.
Figure 5.
The contribution of plasminogen activator inhibitor (PAI-1) in bleomycin (BLM)-induced ATII cell epithelial–mesenchymal transitions (EMT). A: ATII cells isolated from PAI-1–deficient mice were treated with PBS, BLM, BLM+CSP, or BLM+CP in vitro. The lysates were immunoblotted for changes in collagen-I, α-SMA, E-cadherin, ZO-1, and β-actin expression. The lysates from ATII cells lacking PAI-1 expression that were treated with transforming growth factor β (TGF-β) were used as a control. The individual densities of bands were quantitated and normalized to the corresponding values of β-actin. The fold changes in protein expression are presented as a bar graph. Differences between treatments versus control are not statistically significant. B: Mice lacking PAI-1 expression were treated with saline or BLM, and 24 hours later, mice exposed to BLM were i.p. injected with or without caveolin-1 scaffolding domain peptide (CSP) or control peptide (CP). Three days after initiation of BLM injury, ATII cells were isolated from saline, BLM-, BLM+CSP-, or BLM+CP-treated mice. The lysates were examined for the expression of collagen-I, α-SMA, E-cadherin, ZO-1, and β-actin by Western blot analysis. The densities of bands were normalized against the corresponding levels of β-actin. The bar graph represents the fold change of proteins. C: Total RNA isolated from ATII cells of PAI-1–deficient mice exposed to saline, BLM, BLM+CSP, or CP were analyzed for changes in the expression of E-cadherin, ZO-1, collagen-I, and α-SMA mRNA levels by real-time PCR. The data were normalized with corresponding levels of β-actin mRNA. The fold changes of RNA are presented as a bar graph. D: ATII cells isolated from PAI-1–deficient or uPA-deficient mice were treated with lentivirus expressing uPA or PAI-1 shRNA. ATII cells obtained from both uPA-deficient and PAI-1–deficient mice exposed to nonspecific shRNA or naive ATII cells were used as controls for comparison. Because BLM injury causes EMT in uPA-deficient mice, whereas those lacking PAI-1 expression resist BLM-induced EMT, ATII cells from uPA-deficient mice treated with or without PAI-1 shRNA or control shRNA were later exposed to BLM for 72 hours. The lysates from PAI-1–deficient and uPA-deficient ATII cells were analyzed for changes in EMT markers by Western blot analysis.
Analysis of ATII cells isolated from the lungs of PAI-1–deficient mice exposed to BLM showed little inhibition of basal E-cadherin and ZO-1 expression in vivo (Figure 5B). Similarly, in mice lacking PAI-1 expression, BLM failed to augment baseline levels of collagen-I and α-SMA, which were unusually high in ATII cells. Treatment of PAI-1–deficient mice, exposed to BLM with either CSP or CP, failed to affect baseline expression of E-cadherin, ZO-1, collagen-I, and α-SMA further. Analysis of ATII cell mRNA for EMT markers (Figure 5C) likewise demonstrated resistance of PAI-1–deficient mice to BLM.
Mice deficient in uPA expression exhibit increased EMT and develop PF after BLM injury, whereas those lacking PAI-1 resist both EMT and PF. Therefore, we next inhibited uPA expression in ATII cells obtained from PAI-1–deficient mice to determine whether increased uPA expression, as a result of a lack of PAI-1, provided resistance against BLM-induced EMT. Inhibition of uPA expression by at least 75% to 80% using shRNA failed to alter E-cadherin, ZO-1, collagen-I, and α-SMA expression in PAI-1–deficient ATII cells (Figure 5D). On the contrary, inhibition of PAI-1 expression using shRNA in uPA-deficient ATII cells resisted BLM-induced EMT, supporting the pivotal role played by increased PAI-1 expression in the induction of ATII cell EMT.
Role of Activation of Src Kinase in BLM-Induced ATII Cell EMT
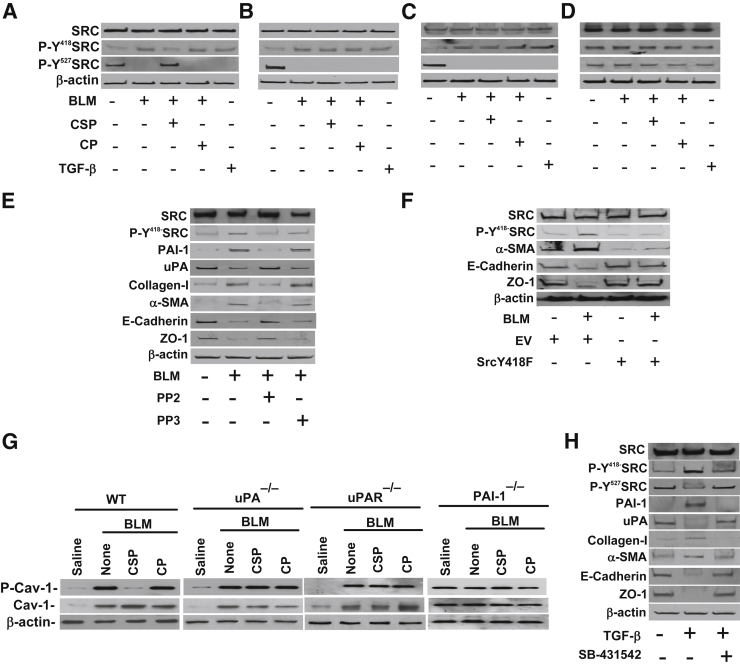
BLM and TGF-β lung injuries increase Src kinase activation and induction of PAI-1 expression is further dependent on activation of Src kinases.23 To investigate the involvement of Src kinase activation in ATII cell EMT, we tested ATII cell lysates for changes in the phosphorylation of tyrosine residues 418 (Y418) and 527 (Y527). We found that ATII cells isolated from WT (Figure 6A), uPA-deficient (Figure 6B), and uPAR-deficient (Figure 6C) mice exposed to BLM show increased Y418 phosphorylation, indicating activation of Src kinase. This was reversed after treatment of WT mice with CSP through inhibitory phosphorylation (Y527) of Src kinase. However, treatment of uPA- or uPAR-deficient mice with CSP failed to inhibit BLM-induced activation of Src kinase (Figure 6, B and C) or EMT (Figures 3 and 4). Interestingly, BLM failed to increase phosphorylation of Y418 and inhibit phosphorylation of Y527 Src kinases from baseline levels observed in control mice lacking PAI-1 expression (Figure 6D). Our data therefore suggest that induction of Src activation in ATII cells by BLM contributes to EMT. This is reversed by CSP-mediated inhibitory (Y527) phosphorylation of Src kinases. The process involves inhibition of BLM-induced PAI-1 expression and restoration of baseline levels of uPA and uPAR expression.
Figure 6.
Effect of tyrosine phosphorylation of Src kinases on bleomycin (BLM)-induced ATII cell epithelial–mesenchymal transitions (EMT) in WT, urokinase-type plasminogen activator (uPA)-, uPA plasma membrane receptor (uPAR)-, and plasminogen activator inhibitor (PAI-1)–deficient mice. A–D: ATII cells isolated from WT (A), uPA-deficient (B), uPAR-deficient (C) and PAI-1–deficient (D) mice were treated with saline or BLM with or without caveolin-1 scaffolding domain peptide (CSP) or control peptide (CP) in culture dishes. The lysates were tested for changes in the phosphorylation of tyrosine (Y418 and Y527) residues of Src kinases by Western blot analysis using phospho-specific antibodies. The same membrane was stripped and tested for total Src kinase and β-actin proteins. ATII cell lysates treated with TGF-β were also used as controls. E: ATII cells were treated with PBS, BLM, BLM in the presence of 10 μmol/L Src kinase inhibitor PP2 or control PP3 in culture dishes for 72 hours. The lysates were tested for changes in tyrosine Y418 phosphorylation of Src kinases, PAI-1, uPA, collagen-I, α-SMA, E-cadherin, ZO-1, and β-actin by Western blot analysis. F: ATII cells isolated from WT mice were transduced with retrovirus expressing dominant-negative Y418F mutant Src kinase. ATII cells from WT mice exposed to empty vector (EV) were used as controls for comparison. After 24 hours, these cells were treated with BLM, and the lysates were analyzed for changes in EMT markers 72 hours after BLM injury. WT ATII cells treated with PBS or BLM alone were used as controls. The lysates were analyzed for changes in EMT markers. G: ATII cells isolated from WT and uPA-deficient mice were exposed to saline or BLM or BLM with CSP or CP for 72 hours, and the lysates were immunoblotted for total and phosphorylated caveolin-1 (P-Cav-1), and β-actin using specific antibodies. H: ATII cells were treated with PBS, TGF-β, or TGF-β in the presence of 10 μmol/L inhibitor of TGF-β receptor kinase (SB-431542) in culture dishes for 72 hours. The lysates were immunoblotted for changes in tyrosine phosphorylation of Src kinases, PAI-1, uPA, collagen-I, α-SMA, E-cadherin, ZO-1, and β-actin by Western blot analysis.
Microscopic examination of lung sections of mice i.p. injected with biotin-labeled CSP revealed that the peptide CSP was predominantly distributed in the alveolar and airway epithelium of the BLM-injured lungs compared with uninjured epithelium of the saline-treated lungs. This indicates that the lung injury facilitates CSP adsorption (Supplemental Figure S5).
To further confirm that BLM-induced activation of Src kinase contributes to ATII cell EMT, we treated ATII cells with BLM in the presence of Src kinase inhibitor PP2,24 and the control cells received PP3. Inhibition of Src activation significantly suppressed BLM-induced EMT in ATII cells (Figure 6E). These changes were associated with parallel inhibition of BLM-induced PAI-1 expression and restoration of uPA expression. Because BLM induces Src activation through Y418, we transduced ATII cells with dominant-negative Y418F mutant Src kinase and exposed these cells to BLM. The lysates were tested for changes in EMT markers. Our data indicate that transduction of ATII cells with Y418F mutant Src kinase failed to induce EMT after exposure to BLM (Figure 6F). Because activated Src kinase phosphorylates caveolin-1 during renal epithelial cell EMT,25,26 and CSP inhibits activation of Src kinase, we analyzed ATII cells for changes in total and Y14 phosphorylated caveolin-1. BLM injury increased both total and Y14 phosphorylated forms of caveolin-1 in ATII cells of WT and uPA-deficient mice (Figure 6G). However in WT mice, CSP suppressed Y14 phosphorylation of caveolin-1 probably through inhibition of Src kinase activation without affecting total caveolin-1, which is otherwise increased after BLM lung injury. Interestingly, CSP failed to suppress Src activation or Y14 phosphorylation of caveolin-1 in uPA- or uPAR-deficient mice, indicating the inevitability of CSP-mediated uPA and uPAR expression in inactivation of Src kinase through Y527 phosphorylation7 and downstream Y14 phosphorylation of caveolin-1. Consistent with Src activation, BLM or BLM+CSP failed to alter the baseline expression of the total or Y14 phosphorylated caveolin-1 level in PAI-1–deficient mice.
The expression of TGF-β, a profibrotic cytokine that induces EMT in ATII cells, was significantly increased after BLM injury and is a major contributor to the development of PF in multiple organs, including lungs. We therefore exposed the ATII cells to TGF-β alone or TGF-β in the presence of TGF-β receptor kinase inhibitor (SB-431542)27 and found that TGF-β increased EMT, indicated by the changes in the markers as well as Src activation (Figure 6H). These changes were associated with parallel induction of PAI-1 and inhibition of uPA. However, inhibition of TGF-β receptor kinase significantly suppressed TGF-β–induced EMT, expression of PAI-1, and activation of Src kinase with restoration of baseline uPA expression.
Discussion
uPA is mainly involved in extravascular proteolysis and tissue remodeling.28–30 uPAR, a glycosylphosphatidylinositol-linked receptor, is implicated in multiple uPA-mediated cellular functions. Bronchoalveolar lavage fluids normally contain high levels of uPA activity, but it is markedly reduced in patients with IPF, COPD, sarcoidosis, and adult respiratory distress syndrome,2,31,32 as well as in mice with BLM- and PCS-induced lung injury because of increased local expression of PAI-1.7,33 Proinflammatory stimuli such as TNF-α and TGF-β augment uPA, uPAR, and PAI-1 expression, and PAI-1 inhibits uPA activity and promotes cycling of uPA–uPAR–PAI-1.30 Lung epithelial cells are often targets of acute and chronic lung injuries and a key driver of normal repair because of their constant contact with the outside environment and their ability to suppress fibroblast growth.34
IPF is one of the most common forms of interstitial lung diseases, characterized by apoptosis of ATII cells, alveolar fibrin deposition, depressed fibrinolysis, inflammation, myofibroblast accumulation, and progressive loss of lung function as a result of accumulation of extracellular matrix proteins.2,35,36 Myofibroblasts play a vital role in fibrogenesis after lung injury. The literature suggests that myofibroblasts arise from activation of resident lung fibroblasts, from differentiation of bone-marrow–derived stromal cells and also from ATII cells via EMT.1,37,38 Other reports suggest increased expression of mesenchymal marker proteins and mRNAs by hyperplastic ATII cells in human IPF lungs4 and that approximately 30% to 50% of fibroblasts are also derived via EMT in BLM-induced lung injury.11 This supports the importance of transformation of ATII cells into myofibroblasts via EMT in the pathogenesis of PF12 in addition to apoptosis of ATII cells.7,8 Most recent in vivo genetic lineage tracing studies using single-dose BLM in mice13 suggest that epithelial cells do not generate myofibroblasts, thus undermining the importance of EMT in the context of PF. However, others studies using a mouse model of either a single dose or repeated doses of BLM-induced lung injury indicate that EMT promotes fibrogenesis.11,39,40 The discrepancies between studies could be technical, which is beyond the scope of the present study.
Irrespective of whether or not reversible phenotypic changes, such as EMT in ATII cells during lung injury, contribute to fibrosis, ATII cells in IPF and COPD lungs show EMT with elevated PAI-1 and concurrent suppression of uPA. Recently, Wang et al41 showed association of epithelial overexpression of uPAR with EMT in COPD lungs. Although CS is a risk factor for lung fibrosis,42,43 it is a major contributor for lung carcinogenesis, and mice exposed to CS do not develop lung fibrosis despite the increased EMT. In addition, increased senescence and apoptosis, rather than excess proliferation, of lung epithelial cells are hallmarks of COPD. Further, uPA and uPAR provides proproliferative and prosurvival signals in diverse cell types, including lung epithelial cells,44,45 and mice lacking uPA or uPAR resist tumor growth,46 whereas uPA and uPAR deficiency, rather than overexpression, contributes to multiple organ fibrosis, including PF. This is further supported by the fact that lung injuries, including BLM- or CS-induced lung injury, are often associated with reduced uPA and uPAR expression because of excess expression of PAI-1 and PAI-1–mediated turnover of the uPA–uPAR complex.18,47 Besides, DNA damage caused by BLM or CS or other injuries augments p53 in airway epithelial cells. p53 in turn concurrently inhibits uPA and uPAR while inducing PAI-1 expression both at the transcriptional48,49 and posttranscriptional levels.50–52 Therefore, EMT associated with increased uPAR in COPD lungs may be more related to carcinogenesis than fibrosis of lungs or other organs.41
We further found that BLM-, TGF-β–, and PCS-induced lung injury drives ATII cells to EMT and, later, BLM- and TGF-β–induced PF in WT and uPA-deficient mice, whereas PAI-1–deficient mice resist EMT and PF. We found that tissues, as well as isolated ATII cells from the lungs of WT mice with BLM- or PCS-exposure injury, show induction of PAI-1, and reciprocal suppression of uPA and uPAR expression and apoptosis.7,8,18 Treatment of WT mice with CSP inhibited BLM-induced PAI-1 while augmenting uPA and uPAR, and protected the mice against ATII cell apoptosis and development of PF.7 However, in uPA- and uPAR-deficient mice, CSP failed to mitigate PAI-1 expression or apoptosis in ATII cells 3 days after BLM injury, and they exhibited severe PF when tested 21 days later.7 This is consistent with increased PAI-1, and inhibition of uPA and uPAR appears to promote ATII cell apoptosis, EMT, and fibrosis after lung injury in mice.
Our findings suggest that CSP, an intervention that targets this pathway, concurrently protects the lung epithelium from apoptosis7,8 as well as EMT, and prevents PF after BLM-induced lung injury via uPA-mediated inhibition of PAI-1. The paradigms that drive ATII cells to these two opposite fates during lung injury are still unclear. However, we speculate that EMT is part of a naturally evolving adaptive safeguard response to protect ATII cells against apoptosis during chronic lung injury. This postulate is supported by the fact that simultaneous apoptosis and EMT in ATII cells as a result of endoplasmic reticulum stress contribute to the development of PF.18,53 In addition, BLM and TGF-β concurrently promote apoptotic death54 and EMT55–57 in lung epithelial cells, and apoptosis alone could contribute to the development of PF.7,58 PAI-1, a downstream product of TGF-β, simultaneously causes apoptosis,7,8,40 senescence,59,60 and EMT61 in diverse cell types, including ATII cells.
It has been previously reported62 that macrophage surface activation of TGF-β is dependent on uPA–uPAR-mediated plasmin generation. However, we found that BLM lung injury increased active TGF-β in WT and uPA-deficient mice, which is consistent with findings of silica-induced fibrosing injury reported earlier.21,63 In addition, despite having increased plasminogen activator activity, mice lacking PAI-1 expression resist EMT and organ fibrosis.18,64,65 On the contrary, those deficient in uPA and uPAR expression exhibit accelerated fibrosis7,18,65,66 and lung-specific expression of uPA protecting them against development of PF after BLM injury.67 PAI-1 expression is disproportionately increased in injured lungs33 and also induced by TGF-β,23 which in turn irreversibly suppresses both uPA activity and its steady-state level by PAI-1–mediated turnover of the uPA–uPAR complex. The protective effects of increased uPA and uPAR may involve suppression of PAI-1 through increased uPA- and uPAR-mediated turnover of PAI-1 protein,47 or inhibition of p53 expression and reversal of p53-mediated induction of PAI-1 expression by increased uPA and uPAR,7,44,51,52 or both.
We found that dysregulation of uPA and PAI-1 expression in ATII cells promotes both apoptosis and EMT after BLM-, TGF-β–, or PCS-induced lung injury, and reversal of these changes by CSP intervention prevents BLM- and TGF-β–induced EMT and PF.7 Therefore, inhibition of EMT by CSP intervention may be associated with resolution of lung injury. In addition, CSP requires uPA expression to inhibit BLM-induced PAI-1 and apoptosis7 or EMT. Thus, dysregulated alveolar fibrinolysis, as a result of increased expression of PAI-1 or inhibition of uPA and uPAR as a consequence of lung injury, contributes to ATII cell apoptosis, EMT, and development of PF. uPA can induce itself or its cell surface receptor, uPAR,44,68 and inhibit PAI-1 expression,69 which requires uPA interaction with uPAR. Further, uPA- and uPAR-deficient mice resist CSP treatment after BLM injury, whereas CSP is effective in WT mice. We therefore believe that protection against BLM-induced EMT by CSP requires both uPA and uPAR expression. On the basis of our recent work,7 we also suspect that the inability of CSP to mitigate ATII cell EMT, in both uPA- or uPAR-deficient mice after BLM injury, is due to a lack of uPA-mediated inhibition of PAI-1 expression. Interestingly, the basal expression of collagen-I and α-SMA is highly elevated in PAI-1–deficient ATII cells, which is comparable to or even higher than that of WT ATII cells exposed to BLM. However, PAI-1–deficient mice resist spontaneous or inducible ATII cell apoptosis and development of PF. Further, protection against development of PF in mice by transplantation of healthy ATII cells into lungs of mice with BLM- or silica-induced injury57,63 underscores the importance of initial ATII cell apoptosis for subsequent PF. On the basis of these findings and a recent lineage study13 using a single-hit BLM model, we believe ATII cell apoptosis, rather than EMT, mediated by increased PAI-1 probably plays a dominant role in the subsequent development of PF. EMT may be a reversible safeguard mechanism evolved to provide transitory protection against apoptosis and ATII cell damage through undergoing phenotypic changes without significant impact on PF per se.
Although several pathways could concurrently operate, our studies indicate a direct role for uPA and PAI-1 in the process. We provide evidence that supports the role of activated Src kinase in facilitating BLM- or TGF-β–induced EMT in ATII cells. PP2 and Y418F mutant Src kinase attenuated BLM- and TGF-β–induced phosphorylation of β-cateninY654 and EMT, and inhibited PAI-1,24,70–72 while restoring ATII cell uPA expression. BLM-induced activation of Src kinase and phosphorylation of caveolin-1 was inhibited by CSP through phosphorylation of Y527.7 These results suggest a role for increased Src kinase activation in EMT via induction of PAI-1 and inhibition of uPA and uPAR expression (Figure 7). This supports the contention that apoptosis and EMT, as a result of increased PAI-1 by ATII cells, contributes to fibrogenesis after BLM injury.
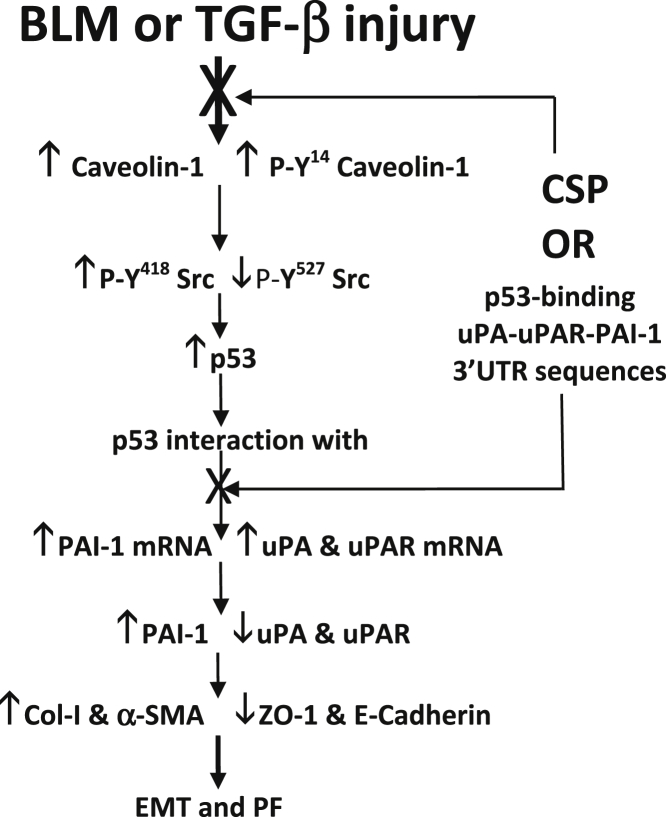
Figure 7.
Regulation of ATII cell epithelial–mesenchymal transitions (EMT) and pulmonary fibrosis (PF) through p53–uPA–fibrinolytic system cross talk. Increased expression and phosphorylation of caveolin-1 by activated Src kinases augments p53 expression in ATII cells during fibrosing lung injury. p53 in turn binds to urokinase-type plasminogen activator (uPA), uPA plasma membrane receptor (uPAR), and plasminogen activator inhibitor (PAI-1) mRNAs leading to suppression of uPA and uPAR, and increased PAI-1 expression.49–51 This results in enhanced ATII cell EMT and development of PF. Inhibition of p53 interaction with endogenous uPA, uPAR, and PAI-1 mRNA in ATII cells either by inhibiting p53 expression using CSP or competitive inhibition through overexpression of p53-binding 3′-UTR sequences of uPA, uPA, and PAI-1 mRNA restores uPA, uPAR, and PAI-1 expression and mitigates ATII cell EMT and development of PF after fibrosing lung injury.
Acknowledgments
We thank Dr. Rui-Ming Liu (University of Alabama, Birmingham, AL) for sharing the AdTGF-β1 virus.
A.S.M., Y.P.B., and S.K.S. performed experiments, analyzed data, and participated in the presentation of the manuscript; S.S. designed the study, interpreted the results, and drafted the manuscript; J.F. provided critical reagents and cDNA constructs; V.S. and Y.S.P. classified patients and provided critical patient samples and participated in the interpretation of results, writing, and critical revision of the manuscript; and all authors reviewed and approved the final content of the manuscript.
Footnotes
Supported in part by Flight Attendant Medical Research Institute Clinical Innovator Award FAMRI-ID-082380 (S.S.), American Heart Association grant GRNT19020001 (S.S.), and NIH/National Heart, Lung, and Blood Institute grant HL093547 (S.S.).
Disclosures: None declared.
Supplemental Data
Inhibition of bleomycin (BLM)-induced epithelial–mesenchymal transitions in ATII cells by caveolin-1 scaffolding domain peptide (CSP). Wild-type mice were exposed to saline or BLM (2 U/kg body weight) through intranasal instillation. After 24 hours, mice exposed BLM were i.p. injected with or without 18.75 mg/kg body weight of CSP or control peptide (CP). Three days after BLM treatment, the lungs were inflated and the lung sections subjected to immunohistochemical analysis using anti–collagen-I and anti–ZO-1 antibodies.
Inhibition of passive cigarette smoke (PCS)-induced epithelial–mesenchymal transitions in ATII cells by caveolin-1 scaffolding domain peptide (CSP). Wild-type mice were exposed to ambient air or PCS from 40 cigarettes (approximately 90 mg/m3 total solid particulates) using a mechanical smoking chamber over a 2-hour period for 5 days per week. After 4 weeks of PCS exposure, mice exposed to PCS were i.p. injected with or without 18.75 mg/kg body weight of CSP or control peptide (CP) once every week for 4 more weeks. After 20 weeks of PCS exposure, the lungs were inflated and the lung sections subjected to immunohistochemical analysis using anti–collagen-I and anti–ZO-1 antibodies.
Isolation of ATII cells lacking urokinase-type plasminogen activator (uPA) or uPA plasma membrane receptor (uPAR) expression. ATII cells isolated from uPA-deficient (A) or uPAR-deficient (B) mice were stained with lithium carbonate for inclusion bodies to assess the purity of cell preparation.
Role of urokinase-type plasminogen activator (uPA) in ATII cell epithelial–mesenchymal transitions after bleomycin (BLM) injury. Mice deficient in uPA expression were exposed to saline or BLM (2 U/kg body weight) through intranasal instillation. Twenty-four hours after initial exposure to BLM, mice were i.p. injected with or without caveolin-1 scaffolding domain peptide (CSP) or control peptide (CP). Seventy-two hours after initiation of BLM injury, the lung sections were subjected to immunohistochemical analysis using anti–ZO-1, collagen-I, and α-SMA antibodies.
Distribution of biotin-labeled caveolin-1 scaffolding domain peptide (CSP) in the lungs of mice. Mice exposed to saline or bleomycin (BLM) for 3 days were i.p. injected with vehicle or biotin-labeled CSP; 24 hours after biotin-CSP, lung sections were analyzed for biotin-CSP using anti-biotin antibody. Lung sections from the vehicle-treated mice were used as controls. Representative fields from one of the six sections are shown (n = 2 per group). Arrows indicate alveolar epithelial reactivity. Original magnification: ×200.
References
- 1.Kim K.K., Kugler M.C., Wolters P.J., Robillard L., Galvez M.G., Brumwell A.N., Sheppard D., Chapman H.A. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapman H.A. Disorders of lung matrix remodeling. J Clin Invest. 2004;113:148–157. doi: 10.1172/JCI20729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalluri R., Neilson E.G. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Konigshoff M., Kramer M., Balsara N., Wilhelm J., Amarie O.V., Jahn A., Rose F., Fink L., Seeger W., Schaefer L., Günther A., Eickelberg O. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest. 2009;119:772–787. doi: 10.1172/JCI33950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhong Q., Zhou B., Ann D.K., Minoo P., Liu Y., Banfalvi A., Krishnaveni M.S., Dubourd M., Demaio L., Willis B.C., Kim K.J., duBois R.M., Crandall E.D., Beers M.F., Borok Z. Role of endoplasmic reticulum stress in epithelial-mesenchymal transition of alveolar epithelial cells: effects of misfolded surfactant protein. Am J Respir Cell Mol Biol. 2011;45:498–509. doi: 10.1165/rcmb.2010-0347OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shetty S., Bdeir K., Cines D.B., Idell S. Induction of plasminogen activator inhibitor-1 by urokinase in lung epithelial cells. J Biol Chem. 2003;278:18124–18131. doi: 10.1074/jbc.M207445200. [DOI] [PubMed] [Google Scholar]
- 7.Bhandary Y.P., Shetty S.K., Marudamuthu A.S., Gyetko M.R., Idell S., Gharaee-Kermani M., Shetty R.S., Starcher B.C., Shetty S. Regulation of alveolar epithelial cell apoptosis and pulmonary fibrosis by coordinate expression of components of the fibrinolytic system. Am J Physiol Lung Cell Mol Physiol. 2012;302:L463–L473. doi: 10.1152/ajplung.00099.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shetty S.K., Bhandary Y.P., Marudamuthu A.S., Abernathy D., Velusamy T., Starcher B., Shetty S. Regulation of airway and alveolar epithelial cell apoptosis by p53-induced plasminogen activator inhibitor-1 during cigarette smoke exposure injury. Am J Respir Cell Mol Biol. 2012;47:474–483. doi: 10.1165/rcmb.2011-0390OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang W.T., Vayalil P.K., Miyata T., Hagood J., Liu R.M. Therapeutic value of small molecule inhibitor to plasminogen activator inhibitor-1 for lung fibrosis. Am J Respir Cell Mol Biol. 2012;46:87–95. doi: 10.1165/rcmb.2011-0139OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Degryse A.L., Tanjore H., Xu X.C., Polosukhin V.V., Jones B.R., McMahon F.B., Gleaves L.A., Blackwell T.S., Lawson W.E. Repetitive intratracheal bleomycin models several features of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2010;299:L442–L452. doi: 10.1152/ajplung.00026.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanjore H., Xu X.C., Polosukhin V.V., Degryse A.L., Li B., Han W., Sherrill T.P., Plieth D., Neilson E.G., Blackwell T.S., Lawson W.E. Contribution of epithelial-derived fibroblasts to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med. 2009;180:657–665. doi: 10.1164/rccm.200903-0322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radisky D.C., Kenny P.A., Bissell M.J. Fibrosis and cancer: do myofibroblasts come also from epithelial cells via EMT? J Cell Biochem. 2007;101:830–839. doi: 10.1002/jcb.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rock J.R., Barkauskas C.E., Cronce M.J., Xue Y., Harris J.R., Liang J., Noble P.W., Hogan B.L. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci U S A. 2011;108:E1475–E1483. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corti M.1, Brody A.R., Harrison J.H. Isolation and primary culture of murine alveolar type II cells. Am J Respir Cell Mol Biol. 1996;14:309–315. doi: 10.1165/ajrcmb.14.4.8600933. [DOI] [PubMed] [Google Scholar]
- 15.Dobbs L.G. Isolation and culture of alveolar type II cells. Am J Physiol. 1990;258:L134–L147. doi: 10.1152/ajplung.1990.258.4.L134. [DOI] [PubMed] [Google Scholar]
- 16.Rice W.R., Conkright J.J., Na C.L., Ikegami M., Shannon J.M., Weaver T.E. Maintenance of the mouse type II cell phenotype in vitro. Am J Physiol Lung Cell Mol Physiol. 2002;283:L256–L264. doi: 10.1152/ajplung.00302.2001. [DOI] [PubMed] [Google Scholar]
- 17.Meng X., Ezzati P., Wilkins J.A. Requirement of podocalyxin in TGF-beta induced epithelial mesenchymal transition. PLoS One. 2011;6:e18715. doi: 10.1371/journal.pone.0018715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhandary Y.P., Shetty S.K., Marudamuthu A.S., Ji H.L., Neuenschwander P.F., Boggaram V., Morris G.F., Fu J., Idell S., Shetty S. Regulation of lung injury and fibrosis by p53-mediated changes in urokinase and plasminogen activator inhibitor-1. Am J Pathol. 2013;183:131–143. doi: 10.1016/j.ajpath.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim K.K., Wei Y., Szekeres C., Kugler M.C., Wolters P.J., Hill M.L., Frank J.A., Brumwell A.N., Wheeler S.E., Kreidberg J.A., Chapman H.A. Epithelial cell alpha3beta1 integrin links beta-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J Clin Invest. 2009;119:213–224. doi: 10.1172/JCI36940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyoshi K., Yanagi S., Kawahara K., Nishio M., Tsubouchi H., Imazu Y., Koshida R., Matsumoto N., Taguchi A., Yamashita S., Suzuki A., Nakazato M. Epithelial Pten controls acute lung injury and fibrosis by regulating alveolar epithelial cell integrity. Am J Respir Crit Care Med. 2013;187:262–275. doi: 10.1164/rccm.201205-0851OC. [DOI] [PubMed] [Google Scholar]
- 21.Matrat M., Lardot C., Huaux F., Broeckaert F., Lison D. Role of urokinase in the activation of macrophage-associated TGF-beta in silica-induced lung fibrosis. J Toxicol Environ Health A. 1998;55:359–371. doi: 10.1080/009841098158403. [DOI] [PubMed] [Google Scholar]
- 22.Munger J.S., Harpel J.G., Gleizes P.E., Mazzieri R., Nunes I., Rifkin D.B. Latent transforming growth factor-beta: structural features and mechanisms of activation. Kidney Int. 1997;51:1376–1382. doi: 10.1038/ki.1997.188. [DOI] [PubMed] [Google Scholar]
- 23.Samarakoon R., Higgins C.E., Higgins S.P., Kutz S.M., Higgins P.J. Plasminogen activator inhibitor type-1 gene expression and induced migration in TGF-beta1-stimulated smooth muscle cells is pp60 (c-src)/MEK-dependent. J Cell Physiol. 2005;204:236–246. doi: 10.1002/jcp.20279. [DOI] [PubMed] [Google Scholar]
- 24.Kong L., Deng Z., Shen H., Zhang Y. Src family kinase inhibitor PP2 efficiently inhibits cervical cancer cell proliferation through down-regulating phospho-Src-Y416 and phospho-EGFR-Y1173. Mol Cell Biochem. 2011;348:11–19. doi: 10.1007/s11010-010-0632-1. [DOI] [PubMed] [Google Scholar]
- 25.Bailey K.M., Liu J. Caveolin-1 up-regulation during epithelial to mesenchymal transition is mediated by focal adhesion kinase. J Biol Chem. 2008;283:13714–13724. doi: 10.1074/jbc.M709329200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J., Chen J.K., Harris R.C. Angiotensin II induces epithelial-to-mesenchymal transition in renal epithelial cells through reactive oxygen species/Src/caveolin-mediated activation of an epidermal growth factor receptor-extracellular signal-regulated kinase signaling pathway. Mol Cell Biol. 2012;32:981–991. doi: 10.1128/MCB.06410-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inman G.J., Nicolas F.J., Callahan J.F., Harling J.D., Gaster L.M., Reith A.D., Laping N.J., Hill C.S. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- 28.Irigoyen J.P., Munoz-Canoves P., Montero L., Koziczak M., Nagamine Y. The plasminogen activator system: biology and regulation. Cell Mol Life Sci. 1999;56:104–132. doi: 10.1007/PL00000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andreasen P.A., Kjoller L., Christensen L., Duffy M.J. The urokinase-type plasminogen activator system in cancer metastasis: a review. Int J Cancer. 1997;72:1–22. doi: 10.1002/(sici)1097-0215(19970703)72:1<1::aid-ijc1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 30.Andreasen P.A., Egelund R., Petersen H.H. The plasminogen activation system in tumor growth, invasion, and metastasis. Cell Mol Life Sci. 2000;57:25–40. doi: 10.1007/s000180050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gharaee-Kermani M., Hu B., Phan S.H., Gyetko M.R. The role of urokinase in idiopathic pulmonary fibrosis and implication for therapy. Expert Opin Investig Drugs. 2008;17:905–916. doi: 10.1517/13543784.17.6.905. [DOI] [PubMed] [Google Scholar]
- 32.Bertozzi P., Astedt B., Zenzius L., Lynch K., LeMaire F., Zapol W., Chapman H.A., Jr. Depressed bronchoalveolar urokinase activity in patients with adult respiratory distress syndrome. N Engl J Med. 1990;322:890–897. doi: 10.1056/NEJM199003293221304. [DOI] [PubMed] [Google Scholar]
- 33.Olman M.A., Mackman N., Gladson C.L., Moser K.M., Loskutoff D.J. Changes in procoagulant and fibrinolytic gene expression during bleomycin-induced lung injury in the mouse. J Clin Invest. 1995;96:1621–1630. doi: 10.1172/JCI118201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caniggia I., Tseu I., Rolland G., Edelson J., Tanswell A.K., Post M. Inhibition of fibroblast growth by epithelial cells in fetal rat lung. Am J Respir Cell Mol Biol. 1995;13:91–98. doi: 10.1165/ajrcmb.13.1.7598942. [DOI] [PubMed] [Google Scholar]
- 35.Thannickal V.J., Toews G.B., White E.S., Lynch J.P., III, Martinez F.J. Mechanisms of pulmonary fibrosis. Annu Rev Med. 2004;55:395–417. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- 36.Selman M., Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc Am Thorac Soc. 2006;3:364–372. doi: 10.1513/pats.200601-003TK. [DOI] [PubMed] [Google Scholar]
- 37.Aghajanova L., Horcajadas J.A., Esteban F.J., Giudice L.C. The bone marrow-derived human mesenchymal stem cell: potential progenitor of the endometrial stromal fibroblast. Biol Reprod. 2010;82:1076–1087. doi: 10.1095/biolreprod.109.082867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wynn T.A. Integrating mechanisms of pulmonary fibrosis. J Exp Med. 2011;208:1339–1350. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanjore H., Cheng D.S., Degryse A.L., Zoz D.F., Abdolrasulnia R., Lawson W.E., Blackwell T.S. Alveolar epithelial cells undergo epithelial-to-mesenchymal transition in response to endoplasmic reticulum stress. J Biol Chem. 2011;286:30972–30980. doi: 10.1074/jbc.M110.181164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willis B.C., duBois R.M., Borok Z. Epithelial origin of myofibroblasts during fibrosis in the lung. Proc Am Thorac Soc. 2006;3:377–382. doi: 10.1513/pats.200601-004TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Q., Wang Y., Zhang Y., Zhang Y., Xiao W. The role of uPAR in epithelial-mesenchymal transition in small airway epithelium of patients with chronic obstructive pulmonary disease. Respir Res. 2013;14:67. doi: 10.1186/1465-9921-14-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baumgartner K.B., Samet J.M., Stidley C.A., Colby T.V., Waldron J.A. Cigarette smoking: a risk factor for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1997;155:242–248. doi: 10.1164/ajrccm.155.1.9001319. [DOI] [PubMed] [Google Scholar]
- 43.Zhang H., Liu H., Borok Z., Davies K.J., Ursini F., Forman H.J. Cigarette smoke extract stimulates epithelial-mesenchymal transition through Src activation. Free Radic Biol Med. 2012;52:1437–1442. doi: 10.1016/j.freeradbiomed.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shetty S., Idell S. Urokinase induces expression of its own receptor in Beas2B lung epithelial cells. J Biol Chem. 2001;276:24549–24556. doi: 10.1074/jbc.M101605200. [DOI] [PubMed] [Google Scholar]
- 45.Tkachuk N., Kiyan J., Tkachuk S., Kiyan R., Shushakova N., Haller H., Dumler I. Urokinase induces survival or pro-apoptotic signals in human mesangial cells depending on the apoptotic stimulus. Biochem J. 2008;415:265–273. doi: 10.1042/BJ20071652. [DOI] [PubMed] [Google Scholar]
- 46.Gutierrez L.S., Schulman A., Brito-Robinson T., Noria F., Ploplis V.A., Castellino F.J. Tumor development is retarded in mice lacking the gene for urokinase-type plasminogen activator or its inhibitor, plasminogen activator inhibitor-1. Cancer Res. 2000;60:5839–5847. [PubMed] [Google Scholar]
- 47.Shetty S., Kumar A., Johnson A., Pueblitz S., Idell S. Urokinase receptor in human malignant mesothelioma cells: role in tumor cell mitogenesis and proteolysis. Am J Physiol. 1995;268:L972–L982. doi: 10.1152/ajplung.1995.268.6.L972. [DOI] [PubMed] [Google Scholar]
- 48.Kunz C., Pebler S., Otte J.,, von der Ahe D. Differential regulation of plasminogen activator and inhibitor gene transcription by the tumor suppressor p53. Nucleic Acids Res. 1995;23:3710–3717. doi: 10.1093/nar/23.18.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parra M., Jardí M., Koziczak M., Nagamine Y., Muñoz-Cánoves P. p53 phosphorylation at serine 15 is required for transcriptional induction of the plasminogen activator inhibitor-1 (PAI-1) gene by the alkylating agent N-methyl-N'-nitro-N-nitrosoguanidine. J Biol Chem. 2001;276:36303–36310. doi: 10.1074/jbc.M103735200. [DOI] [PubMed] [Google Scholar]
- 50.Shetty S., Velusamy T., Idell S., Shetty P., Mazar A.P., Bhandary Y.P., Shetty R.S. Regulation of urokinase receptor expression by p53: novel role in stabilization of uPAR mRNA. Mol Cell Biol. 2007;27:5607–5618. doi: 10.1128/MCB.00080-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shetty S., Shetty P., Idell S., Velusamy T., Bhandary Y.P., Shetty R.S. Regulation of plasminogen activator inhibitor-1 expression by tumor suppressor protein p53. J Biol Chem. 2008;283:19570–19580. doi: 10.1074/jbc.M710268200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shetty P., Velusamy T., Bhandary Y.P., Shetty R.S., Liu M.C., Shetty S. Urokinase expression by tumor suppressor protein p53: a novel role in mRNA turnover. Am J Respir Cell Mol Biol. 2008;39:364–372. doi: 10.1165/rcmb.2007-0406OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomas A.Q., Lane K., Phillips J., III, Prince M., Markin C., Speer M., Schwartz D.A., Gaddipati R., Marney A., Johnson J., Roberts R., Haines J., Stahlman M., Loyd J.E. Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am J Respir Crit Care Med. 2002;165:1322–1328. doi: 10.1164/rccm.200112-123OC. [DOI] [PubMed] [Google Scholar]
- 54.Hagimoto N., Kuwano K., Inoshima I., Yoshimi M., Nakamura N., Fujita M., Maeyama T., Hara N. TGF-beta 1 as an enhancer of Fas-mediated apoptosis of lung epithelial cells. J Immunol. 2002;168:6470–6478. doi: 10.4049/jimmunol.168.12.6470. [DOI] [PubMed] [Google Scholar]
- 55.Willis B.C., Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L525–L534. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 56.Alipio Z.A., Jones N., Liao W., Yang J., Kulkarni S., Sree K.K., Hauer-Jensen M., Ward D.C., Ma Y., Fink L.M. Epithelial to mesenchymal transition (EMT) induced by bleomycin or TGF-β1/EGF in murine induced pluripotent stem cell-derived alveolar Type II-like cells. Differentiation. 2011;82:89–98. doi: 10.1016/j.diff.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 57.Kasai H., Allen J.T., Mason R.M., Kamimura T., Zhang Z. TGF-beta1 induces human alveolar epithelial to mesenchymal cell transition (EMT) Respir Res. 2005;6:56. doi: 10.1186/1465-9921-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Serrano-Mollar A., Nacher M., Gay-Jordi G., Closa D., Xaubet A., Bulbena O. Intratracheal transplantation of alveolar type II cells reverses bleomycin-induced lung fibrosis. Am J Respir Crit Care Med. 2007;176:1261–1268. doi: 10.1164/rccm.200610-1491OC. [DOI] [PubMed] [Google Scholar]
- 59.Elzi D.J., Lai Y., Song M., Hakala K., Weintraub S.T., Shiio Y. Plasminogen activator inhibitor 1-insulin-like growth factor binding protein 3 cascade regulates stress-induced senescence. Proc Natl Acad Sci U S A. 2012;109:12052–12057. doi: 10.1073/pnas.1120437109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kortlever R.M., Higgins P.J., Bernards R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat Cell Biol. 2006;8:877–884. doi: 10.1038/ncb1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jeong S.S., Chun M.K. Inhibition of plasminogen activator inhibitor-1 expression in smoke exposed alveolar type II cells attenuates epithelial–mesenchymal transition. Tuberc Respir Dis. 2011;70:462–473. [Google Scholar]
- 62.Venkatraman L., Chia S.M., Narmada B.C., White J.K., Bhowmick S.S., Forbes Dewey C., Jr., So P.T., Tucker-Kellogg L., Yu H. Plasmin triggers a switch-like decrease in thrombospondin-dependent activation of TGF-β1. Biophys J. 2012;103:1060–1068. doi: 10.1016/j.bpj.2012.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spitalieri P., Quitadamo M.C., Orlandi A., Guerra L., Giardina E., Casavola V., Novelli G., Saltini C., Sangiuolo F. Rescue of murine silica-induced lung injury and fibrosis by human embryonic stem cells. Eur Respir J. 2012;39:446–457. doi: 10.1183/09031936.00005511. [DOI] [PubMed] [Google Scholar]
- 64.Eitzman D.T., McCoy R.D., Zheng X., Fay W.P., Shen T., Ginsburg D., Simon R.H. Bleomycin-induced pulmonary fibrosis in transgenic mice that either lack or overexpress the murine plasminogen activator inhibitor-1 gene. J Clin Invest. 1996;97:232–237. doi: 10.1172/JCI118396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang G., Kim H., Cai X., López-Guisa J.M., Alpers C.E., Liu Y., Carmeliet P., Eddy A.A. Urokinase receptor deficiency accelerates renal fibrosis in obstructive nephropathy. J Am Soc Nephrol. 2003;14:1254–1271. doi: 10.1097/01.asn.0000064292.37793.fb. [DOI] [PubMed] [Google Scholar]
- 66.Mondino A., Blasi F. uPA and uPAR in fibrinolysis, immunity and pathology. Trends Immunol. 2004;25:450–455. doi: 10.1016/j.it.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 67.Sisson T.H., Hanson K.E., Subbotina N., Patwardhan A., Hattori N., Simon R.H. Inducible lung-specific urokinase expression reduces fibrosis and mortality after lung injury in mice. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1023–L1032. doi: 10.1152/ajplung.00049.2002. [DOI] [PubMed] [Google Scholar]
- 68.Shetty S., Pendurthi U.R., Halady P.K., Azghani A.O., Idell S. Urokinase induces its own expression in Beas2B lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2002;283:L319–L328. doi: 10.1152/ajplung.00395.2001. [DOI] [PubMed] [Google Scholar]
- 69.Shetty S., Gyetko M.R., Mazar A.P. Induction of p53 by urokinase in lung epithelial cells. J Biol Chem. 2005;280:28133–28141. doi: 10.1074/jbc.M413190200. [DOI] [PubMed] [Google Scholar]
- 70.Ulsamer A., Wei Y., Kim K.K., Tan K., Wheeler S., Xi Y., Thies R.S., Chapman H.A. Axin pathway activity regulates in vivo pY654-ß-catenin accumulation and pulmonary fibrosis. J Biol Chem. 2012;287:5164–5172. doi: 10.1074/jbc.M111.322123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Samarakoon R., Chitnis S.S., Higgins S.P., Higgins C.E., Krepinsky J.C., Higgins P.J. Redox-induced Src kinase and caveolin-1 signaling in TGF-β1-initiated SMAD2/3 activation and PAI-1 expression. PLoS One. 2011;6:e22896. doi: 10.1371/journal.pone.0022896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hu M., Che P., Han X., Cai G.Q., Liu G., Antony V., Luckhardt T., Siegal G.P., Zhou Y., Liu R.M., Desai L.P., O'Reilly P.J., Thannickal V.J., Ding Q. Therapeutic targeting of Src kinase in myofibroblast differentiation and pulmonary fibrosis. J Pharmacol Exp Ther. 2014;351:87–95. doi: 10.1124/jpet.114.216044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Inhibition of bleomycin (BLM)-induced epithelial–mesenchymal transitions in ATII cells by caveolin-1 scaffolding domain peptide (CSP). Wild-type mice were exposed to saline or BLM (2 U/kg body weight) through intranasal instillation. After 24 hours, mice exposed BLM were i.p. injected with or without 18.75 mg/kg body weight of CSP or control peptide (CP). Three days after BLM treatment, the lungs were inflated and the lung sections subjected to immunohistochemical analysis using anti–collagen-I and anti–ZO-1 antibodies.
Inhibition of passive cigarette smoke (PCS)-induced epithelial–mesenchymal transitions in ATII cells by caveolin-1 scaffolding domain peptide (CSP). Wild-type mice were exposed to ambient air or PCS from 40 cigarettes (approximately 90 mg/m3 total solid particulates) using a mechanical smoking chamber over a 2-hour period for 5 days per week. After 4 weeks of PCS exposure, mice exposed to PCS were i.p. injected with or without 18.75 mg/kg body weight of CSP or control peptide (CP) once every week for 4 more weeks. After 20 weeks of PCS exposure, the lungs were inflated and the lung sections subjected to immunohistochemical analysis using anti–collagen-I and anti–ZO-1 antibodies.
Isolation of ATII cells lacking urokinase-type plasminogen activator (uPA) or uPA plasma membrane receptor (uPAR) expression. ATII cells isolated from uPA-deficient (A) or uPAR-deficient (B) mice were stained with lithium carbonate for inclusion bodies to assess the purity of cell preparation.
Role of urokinase-type plasminogen activator (uPA) in ATII cell epithelial–mesenchymal transitions after bleomycin (BLM) injury. Mice deficient in uPA expression were exposed to saline or BLM (2 U/kg body weight) through intranasal instillation. Twenty-four hours after initial exposure to BLM, mice were i.p. injected with or without caveolin-1 scaffolding domain peptide (CSP) or control peptide (CP). Seventy-two hours after initiation of BLM injury, the lung sections were subjected to immunohistochemical analysis using anti–ZO-1, collagen-I, and α-SMA antibodies.
Distribution of biotin-labeled caveolin-1 scaffolding domain peptide (CSP) in the lungs of mice. Mice exposed to saline or bleomycin (BLM) for 3 days were i.p. injected with vehicle or biotin-labeled CSP; 24 hours after biotin-CSP, lung sections were analyzed for biotin-CSP using anti-biotin antibody. Lung sections from the vehicle-treated mice were used as controls. Representative fields from one of the six sections are shown (n = 2 per group). Arrows indicate alveolar epithelial reactivity. Original magnification: ×200.