Abstract
Caspase-8 is an initiator and apical activator caspase that plays a central role in apoptosis. Caspase-8–deficient mice are embryonic lethal, which makes study of caspase-8 in primary immune cells difficult. Recent advances have rescued caspase-8–deficient mice by crossing them to mice deficient in receptor-interacting serine-threonine kinase 3 (RIPK3). These genetic tools have made it possible to study the role of caspase-8 in vivo and in primary immune cells. Several recent studies have identified novel roles for caspase-8 in modulating IL-1β and inflammation, showing that caspase-8 directly regulates IL-1β independent of inflammasomes or indirectly through the regulation of inflammasomes, depending on the stimulus or stimuli that initiate the signaling cascade. Here, we address recent findings on caspase-8 and its role in modulating IL-1β and inflammation.
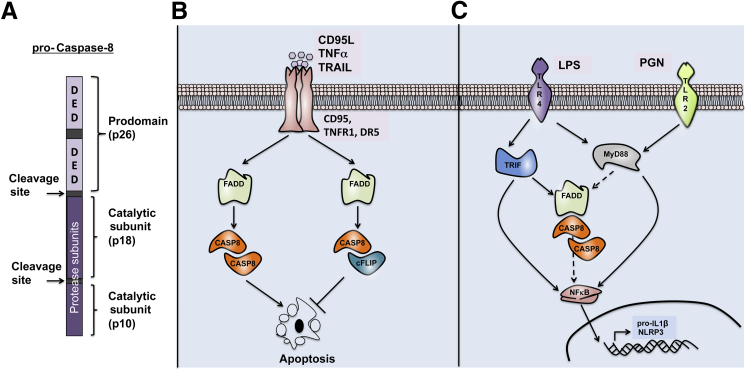
Caspase-8 is an initiator and apical activator caspase that plays a central role in apoptosis. It consists of two N-terminal death effector domains (DEDs), which are followed by a large (p18) and a small (p10) protease subunit at the C-terminal end (Figure 1). First described in 1996, caspase-8 is essential for death receptor–induced activation of the extrinsic cell-death pathway.1,2 On activation of death receptors (CD95, TNFR1, or DR5), caspase-8 is recruited to the receptors via the adaptor protein FAS-associated death domain (FADD). Caspase-8 and FADD both contain DEDs, which mediate DED–DED homotypic interactions and coordinate complex formation of death receptors. Caspase-8 homodimer formation in this complex results in activation and autocleavage, which further stabilizes the active dimer. Active caspase-8 then processes and cleaves downstream executioner caspases, or the BCL2 family member BID, to initiate apoptosis. Because apoptosis is central for development and survival of the host, caspase-8 activation is tightly regulated. cFLIP, a homolog of caspase-8, blocks caspase-8 apoptotic function by forming heterodimeric complexes3 (Figure 1). It has also been proposed that caspase-8 is cleaved, and in some instances activated by other caspases, such as caspase-34,5 and caspase-6,5,6 as well as by the proteases granzyme B7 and cathepsin D.8
Figure 1.
Role for caspase-8 (CASP8) in inducing apoptosis and regulating signaling pathways. A: Procaspase-8 consists of two N-terminal death-effector domain (DED) prodomains, which are followed by the catalytic subunits p18 and p10, respectively. On dimerization, caspase-8 is cleaved at the sites between the DED and p18, and between p18 and p10. B: Death receptor (CD95, TNFR1, DR5) engagement with the respective ligand [CD95L, tumor necrosis factor alpha (TNF-α), TNF-related apoptosis inducing ligand (TRAIL)] results in recruitment of FAS-associated death domain (FADD) and caspase-8 homodimers. Activation of caspase-8 results in induction of apoptosis. cFLIP can bind to caspase-8 to form cFLIP–caspase-8 heterodimers. The formation of cFLIP–caspase-8 heterodimers inhibits apoptosis. C: Activation of Toll-like receptor 4 (TLR4) or TLR2 results in recruitment of TIR domain-containing adaptor-inducing interferon-β (TRIF) and myeloid differentiation primary response protein MyD88 (MyD88) to the receptors. TRIF and MyD88 signaling results in downstream nuclear factor-κB (NF-κB) signaling events that induce mRNA expression of pro–IL-1β and NLRP3. Evidence suggests that FADD and caspase-8 are required for optimal expression of pro–IL-1β and NLRP3 mRNA, possibly through their role in NF-κB activation. LPS, lipopolysaccharide; PGN, peptidoglycan.
The importance of caspase-8 is highlighted by the fact that knockout mice die at approximately embryonic day 10.5.9 In seminal studies, the Mocarski10 and Green11 research groups showed that deletion of receptor-interacting serine-threonine kinase 3 (RIPK3, involved in necroptotic cell death) rescues caspase-8 deficient mice. These studies established a nonapoptotic role for caspase-8, namely, to rescue the lethality induced by RIPK3-mediated pathways. The generation of double-knockout Ripk3−/−Casp8−/− mice has provided an invaluable tool for investigating the role of caspase-8 in vivo and in primary immune cells.
Here, we discuss inflammasome-mediated IL-1β production and the novel roles of caspase-8 in modulating inflammasomes, IL-1β, and inflammation.
Inflammasomes and IL-1β
Inflammasomes
The term inflammasome was coined to describe a multimeric protein complex containing a Nod-like receptor (NLR), an adaptor protein (ASC), and a protease (caspase-1).12 Inflammasomes now also include AIM2, a member of the HIN-200 family. Inflammasomes result in the activation of caspase-1, which cleaves pro–IL-1β and pro–IL-18 into their mature bioactive forms. NLRP1b,12 NLRP3,13–15 NLRC4,16 and AIM217,18 are the most well studied inflammasomes that form this multimeric protein complex. Other inflammasomes containing NLRP12,19 NLRP6,20 and pyrin21 have also been identified, although further research is needed to establish these NLRs as true inflammasomes. All of these upstream receptors sense various stimuli that ultimately result in the formation of the inflammasome complex. For example, NLRP1b senses lethal toxin from Bacillus anthracis22; NLRP3 senses various stimuli ranging from ATP, nigericin, uric acid crystals, Escherichia coli, and Citrobacter rodentium13,15,23,24; NLRC4 senses flagellin components of Salmonella typhimurium and Legionella pneumophila16,25; and AIM2 senses free DNA in the cytoplasm.17,18
Alternative Sources of IL-1β
Inflammasome-induced caspase-1 activation is a major source of IL-1β. However, recent evidence suggests alternative sources of IL-1β release, independent of inflammasome-induced caspase-1 activation. In particular, serine proteases such as cathepsin C, cathepsin D, cathepsin G, neutrophil elastases, and collagenases have been shown to be important in processing IL-1β independent of caspase-1.26–30 In autoimmune osteomyelitis, proline-serine-threonine phosphatase-interacting protein 2 (PSTPIP2) deficiency induces IL-1β that is produced independent of the inflammasomes.31,32 Moreover, caspase-8 has also been identified as an alternative protease that can process IL-1β either in the inflammasomes or independently.33–35 Recent reports suggest that caspase-8 is critical for the NLRP3 inflammasome activation, which also requires caspase-1 for IL-1β processing.23,36 In the following sections, we discuss novel roles of caspase-8 as a direct protease for IL-1β, as well as its role as a direct regulator of the NLRP3 inflammasome.
Caspase-8–Mediated Regulation of IL-1β
Nonapoptotic Functions of Caspase-8 in Regulating IL-1β Expression
In addition to regulating cell death, active caspase-8 regulates inflammation by modulating IL-1β mRNA expression. It is proposed that caspase-8 regulates activation of nuclear factor-κB (NF-κB) to modulate inflammation. This nonapoptotic function, however, does not require cleavage.37 Studies using transgenic mice that express noncleavable caspase-8 (Casp8D387A) showed that these mice are born normal.37 Although Casp8D387A cells exhibited impaired apoptosis in response to CD95 ligand (CD95L), CD95-induced activation of NF-κB and ERK signaling pathways was intact.37 Initial studies in HEK293T cells demonstrated that overexpression of caspase-8 induces activation of NF-κB.38–40 The alternative function of caspase-8 is not dependent on its protease subunits, but rather on the prodomain containing the DED domains.38 Furthermore, siRNA-mediated knockdown of caspase-8 demonstrated that caspase-8 regulates inflammation induced by viral components that activate intracellular receptors such as RIG-I and MDA-5.41,42 Other studies hint at possible roles for caspase-8 in NLRC4-mediated NF-κB activation.43,44 Nonetheless, a direct role for caspase-8 in modulating NF-κB activation in a physiological condition has yet to be identified.
More recent studies using Ripk3−/−Casp8−/− primary macrophages have confirmed a role for caspase-8 as transcriptional regulator of the Il1b gene in response to lipopolysaccharide (LPS), Pam3CSK4 (a synthetic triacylated lipopeptide that mimics bacterial lipopeptide), or infection with Gram-negative bacteria such as S. typhimurium, C. rodentium, and E. coli.36,45 Specifically, pro–IL-1β up-regulation on stimulation with S. typhimurium, C. rodentium, or E. coli is dramatically reduced in Ripk3−/−Casp8−/− macrophages, compared with Ripk3−/− macrophages. LPS and enteropathogens engage the TLR4–TRIF [Toll-like receptor 4–Toll-interleukin-1 receptor (TIR) domain-containing adaptor protein inducing interferon-β (alias TICAM-1)] signaling pathway,23 and it has been proposed that caspase-8 can engage with TRIF.46,47 Our research group recently showed that Il1b up-regulation induced by TLR4–MyD88 (induced by Pam3CSK4) or NOD2 (induced by MDP) is similarly hampered in Ripk3−/−Casp8−/− macrophages.36 Further studies are needed to elucidate how caspase-8 regulates these signaling pathways and whether caspase-8 also interacts with MyD88 and NOD2. Nonetheless, the various independent studies noted here suggest a positive role for caspase-8 in regulating IL-1β expression by potentially regulating the NF-κB signaling axis (Figure 1).
Caspase-8 Directly Cleaves IL-1β during Fungal Infection
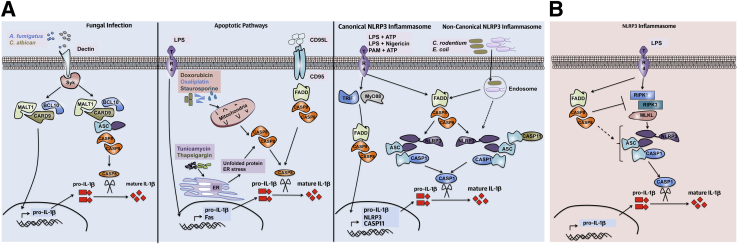
Caspase-8 can directly cleave pro–IL-1β.33–35,48 Studies with HEK293T cells overexpressing both caspase-8 and pro–IL-1β suggest that caspase-8 directly cleaves pro–IL-1β in response to stimulation by TLR3 or TLR4.49 Downstream TRIF-dependent signaling is important for activation of caspase-8, which cleaves pro–IL-1β at the same sites as recombinant caspase-1 and produces similar mature IL-1β fragments in vitro.49 Caspase-8 is the major protease that cleaves pro–IL-1β during infection with fungal pathogens such as Candida albicans and Aspergillus fumigatus.48 However, these functions of caspase-8 are not limited to fungal pathogens; indeed, caspase-8 is also important for IL-1β processing during infection with Mycobacterium bovis and M. leprae. Specifically, fungal components activate dectin-1 receptor signaling to induce a noncanonical CARD9–BCL10–MALT1–ASC-caspase-8 complex.48 In this complex, Syk signaling induces transcription of pro–IL-1β promoted by the CARD9–BCL10–MALT1 complex. Additional recruitment of ASC and caspase-8 to the CARD9–BCL10–MALT1 complex activates caspase-8. Activated caspase-8 then cleaves pro–IL-1β independent of both caspase-1 and the inflammasome complex (Figure 2). These studies did not use primary Casp8−/− macrophages, a limitation that impedes our understanding of caspase-8 and its regulation of IL-1β in vivo. These functions should be addressed in further studies taking advantage of Ripk3−/−Casp8−/− macrophages.
Figure 2.
Novel roles for caspase-8 in positive (A) and negative (B) regulation of IL-1β and the NLRP3 inflammasome. A: Fungal infection (left panel) induces signaling through the dectin receptor and Syk activation. CARD9–BCL10–MALT1 complex formation induces NF-κB signaling and up-regulates pro–IL-1β. Recruitment of apoptosis-associated speck-like protein containing CARD (ASC) and caspase-8 to the CARD9–BCL10–MALT1 complex induces caspase-8 activation, which then cleaves pro–IL-1β to its mature form. Induction of apoptosis (middle panel) in the presence of LPS priming is accompanied by caspase-8–dependent IL-1β processing. CD95L signals through CD95 to recruit FADD and caspase-8 to the receptor and to induce apoptosis. In the presence of LPS, CD95L induces activation of caspase-8 and caspase-8–dependent IL-1β release. Chemotherapeutic drugs induce mitochondrial dependent intrinsic cell death. In the presence of LPS, doxorubicin and oxaliplatin induce caspase-8 activation. Treatment of cells with tunicamycin and thapsigargin [known inducers of endoplasmic reticulum (ER) stress] in the presence of LPS triggers caspase-8 activation. Active caspase-8 cleaves pro–IL-1β to release mature IL-1β. In the NLRP3 inflammasome (right panel), caspase-8 is required for both canonical and noncanonical NLRP3 inflammasome activation. Stimulation of TLR results in NF-κB activation and up-regulation of pro–IL-1β and NLRP3 mRNA that is partially dependent on caspase-8. Stimulation of the canonical NLRP3 (LPS+ATP, LPS+nigericin, Pam3CSK4+ATP) or noncanonical NLRP3 (C. rodentium, E. coli) inflammasome requires caspase-8 for assembly and activation of the NLRP3 inflammasome complex. Caspase-8 activation is required for activation of caspase-1 and caspase-11. B: Caspase-8 is a negative regulator of LPS-induced activation of the NLRP3 inflammasome. In the absence of caspase-8, dendritic cells are hyper-responsive to LPS stimulation and activate the NLRP3 inflammasome in a RIPK1-, RIPK3-, and MLKL-dependent manner. PAM, Pam3CSK4.
Death Signal–Induced IL-1β Processing Requires Caspase-8
CD95-induced signaling induces caspase-8–mediated cell death.1,2 Caspase-8 is directly involved in cleavage and activation of IL-1β during CD95L-induced IL-1β maturation of TLR-primed macrophages and dendritic cells.34 These studies show that CD95 is up-regulated in both dendritic cells and macrophages on TLR priming. Primed myeloid cells can then activate caspase-8 on ligation of the CD95 to its ligand CD95L (Figure 2). Although significant IL-1β production is observed in CD95L-stimulated wild-type (WT) or Ripk3−/− cells, IL-1β production in Ripk3−/−Casp8−/− cells is dramatically reduced. Furthermore, CD95L-provoked IL-1β secretion is independent of ASC, caspase-1, and caspase-11. Consistently, proapoptotic chemotherapeutic drugs also induce IL-1β production by LPS-primed dendritic cells.35 This IL-1β production is independent of the inflammasome components NLRP3, NLRC4, and ASC and is also independent of caspase-1. Similar to the CD95L findings, doxorubicin-induced IL-1β processing35 is reduced in Ripk3−/−Casp8−/− dendritic cells, compared with Ripk3−/− or WT dendritic cells, suggesting a direct role for caspase-8 as a protease for pro–IL-1β processing. Taken together, these studies indicate that caspase-8 acts as a major protease for processing pro–IL-1β and that caspase-8 can initiate inflammation in TLR-primed macrophages or dendritic cells in response to death signals. These results are paradoxical, considering that CD95 and death receptor ligation induce apoptosis, whereas the release of IL-1β is associated with pyroptosis (ie, inflammatory cell death). Studies are needed to elucidate how TLR priming before triggering of death receptors (CD95, TNFR1, or DR5) or death signaling pathways (doxorubicin, staurosporine, oxaliplatin) induces inflammation by caspase-8. Understanding the mechanistic and molecular underpinnings of these pathways should be invaluable in determining the multiple regulatory roles of caspase-8.
Caspase-8 as a Protease during ER Stress–Induced Inflammation
Accumulation of unfolded proteins within the endoplasmic reticulum (ER) stimulates the unfolded protein response pathway known as ER stress. These programs are tightly regulated for proper folding and generation of functional proteins. Caspase-8 is central to the inflammation generated during ER stress induced by the drugs tunicamycin or thapsigargin in LPS-primed macrophages33 (Figure 2). More specifically, caspase-8 is involved in direct processing of pro–IL-1β into its mature form. The processing and maturation of IL-1β are largely independent of both ASC and caspase-1. ER stress contributes to various disease conditions ranging from obesity, diabetes, Alzheimer disease, Parkinson disease, and neurological damage more generally to several types of infection. Thus, exploring the roles of caspase-8 is important for the generation of novel therapeutics.50 Similar to stimulation with CD95L or chemotherapeutic drugs, ER stress also induces apoptosis.51
These studies suggest a novel role for caspase-8 in promoting inflammation instead of apoptosis. The stimuli (CD95L, chemotherapeutic drugs, ER stress) induce apoptosis, whereas LPS triggers caspase-8–induced inflammatory process that involves cleavage of pro–IL-1β. Furthermore, caspase-8 appears to be involved in several different complexes, depending on the stimuli involved. Although the dectin pathway comprises a large multiprotein complex involving CARD9–BCL10–MALT1–ASC and caspase-8,48 ligation of CD95 or induction of apoptosis via chemotherapeutic drugs or ER stress induces a unique complex that does not require ASC.33–35 In this regard, caspase-8 is promiscuous in its ability to form inflammatory complexes to potentiate inflammation. Improved understanding of the conditions and milieu that promote these complexes is needed, to shed light on the complex caspase-8 biology.
Caspase-8 and Regulation of the NLRP3 Inflammasome
NLRP3 Inflammasome
The NLRP3 inflammasome is one of the best-studied inflammasomes, partly because of the promiscuity of NLRP3 in its ability to be activated in response to a wide array of stimuli.52 These stimuli range from environmental factors (silica and asbestos crystals), endogenous danger signals (uric acid and cholesterol crystals, ATP, reactive oxygen species, and protein aggregates), and infections (bacteria, viruses, and fungi). In the canonical NLRP3 inflammasome, activation of the inflammasome in response to stimuli such as LPS+ATP or LPS+nigericin does not require caspase-11; by contrast, activation of the noncanonical NLRP3 inflammasome during C. rodentium or E. coli infection does require caspase-11 (Figure 2).53 The ligand directly recognized by NLRP3 remains elusive. Different stimuli result in common cellular perturbations, such as potassium efflux and calcium mobilization, that ultimately activate the NLRP3 inflammasome.54,55 Several mutations in the NLRP3 gene have been found to cause autoinflammatory disorders, including Muckle–Wells syndrome and familial cold autoinflammatory syndrome, both of which are associated with elevated IL-1β.13,56–58
Caspase-8 as a Positive Regulator of the NLRP3 Inflammasome
Caspase-8 regulates the NLRP3 inflammasome.36,45,59,60 Genetic deletion of caspase-8 or its adaptor FADD on a Ripk3−/− background generates Ripk3−/−Casp8−/− and Ripk3−/−Fadd−/− mice. Studies using macrophages and dendritic cells from Ripk3−/−Casp8−/− and Ripk3−/−Fadd−/− mice demonstrate drastically reduced activation of both the canonical (LPS+ATP, LPS+nigericin) and noncanonical (C. rodentium, E. coli) NLRP3 inflammasomes in Ripk3−/−Casp8−/− and Ripk3−/−Fadd−/− cells, but not in Ripk3−/− or WT cells.36 Mechanistic studies revealed the role of caspase-8 and FADD in both priming and activation of the NLRP3 inflammasome complex.36 Specifically, both caspase-8 and FADD are required for optimal induction of pro–IL-1β mRNA and protein after LPS stimulation or C. rodentium and E. coli infection. Coimmunoprecipitation and confocal studies confirmed that caspase-8 is present in the NLRP3 inflammasome complex, where it is involved in cleavage and processing of procaspase-1. Furthermore, caspase-8 is able to directly and specifically cleave caspase-1 in in vitro assays, which suggests a direct role for caspase-8 in caspase-1 processing. Another study confirmed the role for caspase-8 in promoting S. typhimurium–induced IL-1β production.45 S. typhimurium–induced pro–IL-1β expression is blunted in Ripk3−/−Casp8−/− but not Ripk3−/− or WT macrophages. Confocal studies confirmed that caspase-8 colocalizes with ASC puncta after S. typhimurium infection; caspase-8 is thus activated and is involved in the processing of pro–IL-1β to its mature form. Furthermore, Yersinia pestis–induced caspase-1 activation and IL-1β production is also dependent on caspase-8.61,62 In accord, Listeria monocytogenes infection induces the production of reactive oxygen species, which instigates association of caspase-8 with ASC to promote IL-1β and IL-18 processing.63 These findings variously confirm a role for caspase-8 in promoting NLRP3 inflammasome activation via regulation of both priming and post-transcriptional activation of the inflammasome components.
Caspase-8 as a Negative Regulator of the NLRP3 Inflammasome and Inflammation
Kang et al64 reported a negative role for caspase-8 in LPS-induced IL-1β production; Casp8−/− dendritic cells (conditional deletion of floxed caspase-8 driven by CD11c-Cre) exhibited hyperactive production of IL-1β, compared with WT controls. Mechanistic studies have shown that spontaneous activation of the NLRP3 inflammasome by LPS alone is dependent on both RIPK1 and RIPK3.64 Indeed, genetic deletion of RIPK3 in Casp8−/− dendritic cells rescues LPS-induced IL-1β production from Casp8−/− dendritic cells, and necrostatin-1 (a chemical inhibitor of RIPK1 kinase activity) treatment inhibits LPS-induced IL-1β production from Casp8−/− dendritic cells.64 Furthermore, siRNA-mediated deletion of MLKL (a molecule that contributes to RIPK3-dependent necrosis) also reduces LPS-induced IL-1β production from Casp8−/− dendritic cells. Therefore, caspase-8 negatively regulates RIPK1–RIPK3–MLKL-mediated activation of the NLRP3 inflammasome.64 Findings from another study support the negative role of caspase-8 in regulation of dendritic cell activation.65 Several studies using conditional caspase-8 deletion have demonstrated overt inflammation in vivo, which supports a negative regulatory role for caspase-8 in controlling inflammation.66–69 However, it remains to be determined whether these inflammatory disorders are the result of specific activation of NLRP3 inflammasome in the absence of caspase-8.
Explanations for Discrepancies among Studies
LPS-induced spontaneous activation of NLRP3 inflammasomes differs from LPS+ATP–induced activation of NLRP3 inflammasomes, in that the levels of IL-1β produced by LPS stimulation alone are much less.64 Furthermore, LPS stimulation alone fails to induce appreciable amounts of IL-1β in Ripk3−/−Casp8−/− macrophages.36 Several differences in experimental systems could account for the observed role of caspase-8 as either a positive or a negative regulator of NLRP3 inflammasome, such as i) use of Casp8−/− dendritic cells versus the use of Ripk3−/−Casp8−/− macrophages; ii) LPS-induced IL-1β production versus LPS+ATP-induced IL-1β production; and iii) incomplete deletion of floxed caspase-8. Regardless of the direction of regulation, these studies point toward caspase-8 playing a very important role in regulating the NLRP3 inflammasome and IL-1β. The present understanding of the roles of caspase-8 in regulating inflammatory processes is far from complete, and further research is needed to sort out the differential roles of caspase-8 in regulating the NLRP3 inflammasomes.
Caspase-8 in Disease Pathogenesis
Caspase-8 is a critical modulator of cell death. Although several caspase-8 mutations associated with tumors have been attributed to loss of apoptotic functions,70–72 some caspase-8 mutations have been observed to promote enhanced NF-κB signaling in cancer cells.73 By contrast, D302H mutations in caspase-8 have been associated with reduced rates of cancer, although the precise molecular mechanisms are not understood.74,75 Mice lacking caspase-8 on a RIPK3-deficient background (Ripk3−/−Casp8−/−)11 or with caspase-8 conditionally deleted in dendritic cells (Casp8fl/−:Itgax-Cre)64 have defects in cell death and accumulation of lymphocytes, resulting in splenomegaly and lymphadenopathy. Interestingly, patients with homozygous mutations in caspase-8 that result in caspase-8 deficiency are developmentally normal, suggesting redundancy in human caspase-8 function.76 Although such patients exhibit the expected defective lymphocyte apoptosis and homeostasis, the lymphocytes are unable to undergo activation, resulting in severe immunodeficiency and increased rates of infection.76 Studies using Ripk3−/−Casp8−/− mice have shown a critical role for caspase-8 in potentiating inflammation and protection in response to C. rodentium36 and Y. pestis.61,62 Furthermore, Ripk3−/−Casp8−/− mice elicit a blunted response to LPS shock and produce significantly less IL-1β than littermate controls.36
Studies using mice with conditional deletion (driven by Cre) of floxed caspase-8 (Casp8fl/fl) in specific cells have shown an opposite role. Mice with conditional deletion of caspase-8 in dendritic cells (Casp8fl/−:Itgax-Cre mice) are highly sensitive to LPS and succumb to LPS shock.64 Mice expressing enzymatically inactive caspase-8 (Casp8C363S) or mice with caspase-8 deleted in keratinocytes (Casp8fl/−:Keratin5-Cre mice) develop inflammatory skin disease.67 Acute deletion of caspase-8 in the skin of adult mice (tamoxifen-induced deletion of floxed caspase-8; Rosa26.CreER+.Casp8fl/fl mice) also induces keratinocyte death and inflammation.68 Similarly, acute deletion of caspase-8 in the gut induces massive cell death, inflammation, sepsis, and death in adult mice.68
These studies variously reveal the importance of caspase-8 in cancer, infection, and tissue homeostasis. In light of such novel and controversial roles of caspase-8 in regulating inflammasome and inflammation, further research is needed to elucidate the molecular underpinnings of caspase-8 and their roles in cancer and infectious diseases.
Perspectives
Recent studies have indicated alternative roles for caspase-8 in signaling, metabolism, homeostasis, and controlling necrotic cell death, and various roles have been identified for caspase-8 in regulation of inflammasomes and IL-1β. Caspase-8 forms novel complexes, depending on the type of receptors and signaling pathways that are engaged. On ligation of dectin receptors during fungal infections, caspase-8 assembles into a noncanonical multiprotein complex comprising CARD9–BCL10–MALT1 and ASC.48 Once activated within this complex, caspase-8 assumes an inflammatory role to further cleave and process pro–IL-1β and to initiate inflammation. In other settings, LPS-primed myeloid cells activate caspase-8 on ligation with CD95,34 on treatment with chemotherapeutic drugs such as doxorubicin or oxaliplatin,35 or on induction of ER stress by tunicamycin or thapsigargin.33 Instead of inducing apoptosis, active caspase-8 cleaves pro–IL-1β to initiate inflammation under these conditions. It is unclear, however, how caspase-8 is activated under such stimulatory conditions or what kind of complexes are formed. Intrinsic cell-death pathways engaged by chemotherapeutic drugs or ER-stress inducers are independent of caspase-8; however, caspase-8 is engaged when these stimuli are present in combination with LPS stimulation. The nature of caspase-8 activation in these settings or of the complex within which caspase-8 is present are not yet known. Understanding of these pathways can be expected to contribute to improved therapeutics.
Several studies have shed light on the role of caspase-8 in directly regulating the NLRP3 inflammasome, in addition to its ability to directly regulate IL-1β up-regulation and cleavage.36,45 During LPS+ATP stimulation (canonical NLRP3 activation) or C. rodentium infection (noncanonical NLRP3 activation), caspase-8 is present in the NLRP3 inflammasome complex. In the inflammasome complex, caspase-8 promotes caspase-1 cleavage and IL-1β processing. Identification of the ability of caspase-8 to directly modulate the NLRP3 inflammasome reveals a previously unknown role for caspase-8, and these studies demonstrate how the apoptotic and inflammatory pathways are tightly linked and regulated by caspase-8.
IL-1β is a pleiotropic cytokine, and deregulated IL-1β production has been linked with several autoinflammatory disorders.77–80 Several diseases (eg, diabetes, gout, Alzheimer disease) and autoinflammatory disorders (eg, osteomyelitis and cryopyrin-associated periodic syndrome) result from uncontrolled IL-1β production. IL-1 receptor antagonist protein (IL-1ra) has been used to treat these inflammatory disorders, with great success.81 Recent studies, however, have shown nonredundant roles for IL-1α and IL-1β in contributing to disease outcomes,31,82 and because both IL-1α and IL-1β signal through IL-1R, a more specific targeting of the IL-1 pathways is required. Specific ability to block caspase-8–mediated IL-1β can be expected to ensure the release of IL-1β (which might still be important for combating infection) through other pathways that do not require caspase-8. For instance, specific blockade caspase-8 will leave caspase-1 intact, which can process and release IL-1β and IL-1α, critical for fighting infections.
Conclusion
The role of caspase-8 apart from its function in apoptosis is only beginning to unfold. To date, most studies have examined the role of caspase-8 only in myeloid cells (specifically, macrophages and dendritic cells). Because caspase-8 can play such diverse roles and can engage multiple proteins depending on the stimuli present, it is conceivable that caspase-8 could have completely different functions depending on the cell types involved. Further studies and identification of novel pathways regulated by caspase-8 in regulating IL-1β production in various cell types will undoubtedly be important in the discovery of novel therapeutics to treat the related autoimmune and autoinflammatory diseases.
Acknowledgments
We thank John R. Lukens, Si Ming Man, and Ricardo Weinlich for critical review of the manuscript. We attempted a comprehensive literature review but sincerely apologize to authors whose work is not cited.
Footnotes
Supported by NIH grants R01 AR056296, R01 CA163507, and R01 AI101935 and the American Lebanese Syrian Associated Charities (ALSAC) (T.-D.K.). P.G. is a postdoctoral fellow supported by a Paul Barrett Endowed Fellowship from St. Jude Children’s Research Hospital.
Disclosures: None declared.
References
- 1.Boldin M.P., Goncharov T.M., Goltsev Y.V., Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 2.Muzio M., Chinnaiyan A.M., Kischkel F.C., O’Rourke K., Shevchenko A., Ni J., Scaffidi C., Bretz J.D., Zhang M., Gentz R., Mann M., Krammer P.H., Peter M.E., Dixit V.M. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death–inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 3.Irmler M., Thome M., Hahne M., Schneider P., Hofmann K., Steiner V., Bodmer J.L., Schröter M., Burns K., Mattmann C., Rimoldi D., French L.E., Tschopp J. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 4.Ferreira K.S., Kreutz C., Macnelly S., Neubert K., Haber A., Bogyo M., Timmer J., Borner C. Caspase-3 feeds back on caspase-8, Bid and XIAP in type I Fas signaling in primary mouse hepatocytes. Apoptosis. 2012;17:503–515. doi: 10.1007/s10495-011-0691-0. [DOI] [PubMed] [Google Scholar]
- 5.Sohn D., Schulze-Osthoff K., Jänicke R.U. Caspase-8 can be activated by interchain proteolysis without receptor-triggered dimerization during drug-induced apoptosis. J Biol Chem. 2005;280:5267–5273. doi: 10.1074/jbc.M408585200. [DOI] [PubMed] [Google Scholar]
- 6.Cowling V., Downward J. Caspase-6 is the direct activator of caspase-8 in the cytochrome c-induced apoptosis pathway: absolute requirement for removal of caspase-6 prodomain. Cell Death Differ. 2002;9:1046–1056. doi: 10.1038/sj.cdd.4401065. [DOI] [PubMed] [Google Scholar]
- 7.Medema J.P., Toes R.E., Scaffidi C., Zheng T.S., Flavell R.A., Melief C.J., Peter M.E., Offringa R., Krammer P.H. Cleavage of FLICE (caspase-8) by granzyme B during cytotoxic T lymphocyte-induced apoptosis. Eur J Immunol. 1997;27:3492–3498. doi: 10.1002/eji.1830271250. [DOI] [PubMed] [Google Scholar]
- 8.Conus S., Pop C., Snipas S.J., Salvesen G.S., Simon H.U. Cathepsin D primes caspase-8 activation by multiple intra-chain proteolysis. J Biol Chem. 2012;287:21142–21151. doi: 10.1074/jbc.M111.306399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varfolomeev E.E., Schuchmann M., Luria V., Chiannilkulchai N., Beckmann J.S., Mett I.L., Rebrikov D., Brodianski V.M., Kemper O.C., Kollet O., Lapidot T., Soffer D., Sobe T., Avraham K.B., Goncharov T., Holtmann H., Lonai P., Wallach D. Targeted disruption of the mouse caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9:267–276. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- 10.Kaiser W.J., Upton J.W., Long A.B., Livingston-Rosanoff D., Daley-Bauer L.P., Hakem R., Caspary T., Mocarski E.S. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oberst A., Dillon C.P., Weinlich R., McCormick L.L., Fitzgerald P., Pop C., Hakem R., Salvesen G.S., Green D.R. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinon F., Burns K., Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 13.Agostini L., Martinon F., Burns K., McDermott M.F., Hawkins P.N., Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 14.Mariathasan S., Weiss D.S., Newton K., McBride J., O’Rourke K., Roose-Girma M., Lee W.P., Weinrauch Y., Monack D.M., Dixit V.M. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 15.Kanneganti T.D., Ozören N., Body-Malapel M., Amer A., Park J.H., Franchi L., Whitfield J., Barchet W., Colonna M., Vandenabeele P., Bertin J., Coyle A., Grant E.P., Akira S., Núñez G. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 16.Mariathasan S., Newton K., Monack D.M., Vucic D., French D.M., Lee W.P., Roose-Girma M., Erickson S., Dixit V.M. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 17.Rathinam V.A., Jiang Z., Waggoner S.N., Sharma S., Cole L.E., Waggoner L., Vanaja S.K., Monks B.G., Ganesan S., Latz E., Hornung V., Vogel S.N., Szomolanyi-Tsuda E., Fitzgerald K.A. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandes-Alnemri T., Yu J.W., Juliana C., Solorzano L., Kang S., Wu J., Datta P., McCormick M., Huang L., McDermott E., Eisenlohr L., Landel C.P., Alnemri E.S. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat Immunol. 2010;11:385–393. doi: 10.1038/ni.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vladimer G.I., Weng D., Paquette S.W., Vanaja S.K., Rathinam V.A., Aune M.H., Conlon J.E., Burbage J.J., Proulx M.K., Liu Q., Reed G., Mecsas J.C., Iwakura Y., Bertin J., Goguen J.D., Fitzgerald K.A., Lien E. The NLRP12 inflammasome recognizes Yersinia pestis. Immunity. 2012;37:96–107. doi: 10.1016/j.immuni.2012.07.006. [Erratum appeared in Immunity 2012, 37:588] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elinav E., Strowig T., Kau A.L., Henao-Mejia J., Thaiss C.A., Booth C.J., Peaper D.R., Bertin J., Eisenbarth S.C., Gordon J.I., Flavell R.A. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu H., Yang J., Gao W., Li L., Li P., Zhang L., Gong Y.N., Peng X., Xi J.J., Chen S., Wang F., Shao F. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature. 2014;513:237–241. doi: 10.1038/nature13449. [DOI] [PubMed] [Google Scholar]
- 22.Van Opdenbosch N., Gurung P., Vande Walle L., Fossoul A., Kanneganti T.D., Lamkanfi M. Activation of the NLRP1b inflammasome independently of ASC-mediated caspase-1 autoproteolysis and speck formation. Nat Commun. 2014;5:3209. doi: 10.1038/ncomms4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gurung P., Malireddi R.K., Anand P.K., Demon D., Vande Walle L., Liu Z., Vogel P., Lamkanfi M., Kanneganti T.D. Toll or interleukin-1 receptor (TIR) domain-containing adaptor inducing interferon-β (TRIF)-mediated caspase-11 protease production integrates Toll-like receptor 4 (TLR4) protein- and Nlrp3 inflammasome-mediated host defense against enteropathogens. J Biol Chem. 2012;287:34474–34483. doi: 10.1074/jbc.M112.401406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Z., Zaki M.H., Vogel P., Gurung P., Finlay B.B., Deng W., Lamkanfi M., Kanneganti T.D. Role of inflammasomes in host defense against Citrobacter rodentium infection. J Biol Chem. 2012;287:16955–16964. doi: 10.1074/jbc.M112.358705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amer A., Franchi L., Kanneganti T.D., Body-Malapel M., Ozören N., Brady G., Meshinchi S., Jagirdar R., Gewirtz A., Akira S., Núñez G. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J Biol Chem. 2006;281:35217–35223. doi: 10.1074/jbc.M604933200. [DOI] [PubMed] [Google Scholar]
- 26.Hazuda D., Webb R.L., Simon P., Young P. Purification and characterization of human recombinant precursor interleukin 1 beta. J Biol Chem. 1989;264:1689–1693. [PubMed] [Google Scholar]
- 27.Provoost S., Maes T., Pauwels N.S., Vanden Berghe T., Vandenabeele P., Lambrecht B.N., Joos G.F., Tournoy K.G. NLRP3/caspase-1-independent IL-1beta production mediates diesel exhaust particle-induced pulmonary inflammation. J Immunol. 2011;187:3331–3337. doi: 10.4049/jimmunol.1004062. [DOI] [PubMed] [Google Scholar]
- 28.Kono H., Orlowski G.M., Patel Z., Rock K.L. The IL-1-dependent sterile inflammatory response has a substantial caspase-1-independent component that requires cathepsin C. J Immunol. 2012;189:3734–3740. doi: 10.4049/jimmunol.1200136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karmakar M., Sun Y., Hise A.G., Rietsch A., Pearlman E. Cutting edge: IL-1beta processing during Pseudomonas aeruginosa infection is mediated by neutrophil serine proteases and is independent of NLRC4 and caspase-1. J Immunol. 2012;189:4231–4235. doi: 10.4049/jimmunol.1201447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edye M.E., Lopez-Castejon G., Allan S.M., Brough D. Acidosis drives damage-associated molecular pattern (DAMP)-induced interleukin-1 secretion via a caspase-1-independent pathway. J Biol Chem. 2013;288:30485–30494. doi: 10.1074/jbc.M113.478941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lukens J.R., Gross J.M., Calabrese C., Iwakura Y., Lamkanfi M., Vogel P., Kanneganti T.D. Critical role for inflammasome-independent IL-1beta production in osteomyelitis. Proc Natl Acad Sci U S A. 2014;111:1066–1071. doi: 10.1073/pnas.1318688111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cassel S.L., Janczy J.R., Bing X., Wilson S.P., Olivier A.K., Otero J.E., Iwakura Y., Shayakhmetov D.M., Bassuk A.G., Abu-Amer Y., Brogden K.A., Burns T.L., Sutterwala F.S., Ferguson P.J. Inflammasome-independent IL-1beta mediates autoinflammatory disease in Pstpip2-deficient mice. Proc Natl Acad Sci U S A. 2014;111:1072–1077. doi: 10.1073/pnas.1318685111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shenderov K., Riteau N., Yip R., Mayer-Barber K.D., Oland S., Hieny S., Fitzgerald P., Oberst A., Dillon C.P., Green D.R., Cerundolo V., Sher A. Cutting edge: endoplasmic reticulum stress licenses macrophages to produce mature IL-1beta in response to TLR4 stimulation through a caspase-8- and TRIF-dependent pathway. J Immunol. 2014;192:2029–2033. doi: 10.4049/jimmunol.1302549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bossaller L., Chiang P.I., Schmidt-Lauber C., Ganesan S., Kaiser W.J., Rathinam V.A., Mocarski E.S., Subramanian D., Green D.R., Silverman N., Fitzgerald K.A., Marshak-Rothstein A., Latz E. Cutting edge: FAS (CD95) mediates noncanonical IL-1beta and IL-18 maturation via caspase-8 in an RIP3-independent manner. J Immunol. 2012;189:5508–5512. doi: 10.4049/jimmunol.1202121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antonopoulos C., El Sanadi C., Kaiser W.J., Mocarski E.S., Dubyak G.R. Proapoptotic chemotherapeutic drugs induce noncanonical processing and release of IL-1beta via caspase-8 in dendritic cells. J Immunol. 2013;191:4789–4803. doi: 10.4049/jimmunol.1300645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gurung P., Anand P.K., Malireddi R.K., Vande Walle L., Van Opdenbosch N., Dillon C.P., Weinlich R., Green D.R., Lamkanfi M., Kanneganti T.D. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J Immunol. 2014;192:1835–1846. doi: 10.4049/jimmunol.1302839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang T.B., Oh G.S., Scandella E., Bolinger B., Ludewig B., Kovalenko A., Wallach D. Mutation of a self-processing site in caspase-8 compromises its apoptotic but not its nonapoptotic functions in bacterial artificial chromosome-transgenic mice. J Immunol. 2008;181:2522–2532. doi: 10.4049/jimmunol.181.4.2522. [DOI] [PubMed] [Google Scholar]
- 38.Chaudhary P.M., Eby M.T., Jasmin A., Kumar A., Liu L., Hood L. Activation of the NF-kappaB pathway by caspase 8 and its homologs. Oncogene. 2000;19:4451–4460. doi: 10.1038/sj.onc.1203812. [DOI] [PubMed] [Google Scholar]
- 39.Hu W.H., Johnson H., Shu H.B. Activation of NF-kappaB by FADD, Casper, and caspase-8. J Biol Chem. 2000;275:10838–10844. doi: 10.1074/jbc.275.15.10838. [DOI] [PubMed] [Google Scholar]
- 40.Su H., Bidère N., Zheng L., Cubre A., Sakai K., Dale J., Salmena L., Hakem R., Straus S., Lenardo M. Requirement for caspase-8 in NF-kappaB activation by antigen receptor. Science. 2005;307:1465–1468. doi: 10.1126/science.1104765. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi K., Kawai T., Kumar H., Sato S., Yonehara S., Akira S. Roles of caspase-8 and caspase-10 in innate immune responses to double-stranded RNA. J Immunol. 2006;176:4520–4524. doi: 10.4049/jimmunol.176.8.4520. [DOI] [PubMed] [Google Scholar]
- 42.Rajput A., Kovalenko A., Bogdanov K., Yang S.H., Kang T.B., Kim J.C., Du J., Wallach D. RIG-I RNA helicase activation of IRF3 transcription factor is negatively regulated by caspase-8-mediated cleavage of the RIP1 protein. Immunity. 2011;34:340–351. doi: 10.1016/j.immuni.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 43.Masumoto J., Dowds T.A., Schaner P., Chen F.F., Ogura Y., Li M., Zhu L., Katsuyama T., Sagara J., Taniguchi S., Gumucio D.L., Núñez G., Inohara N. ASC is an activating adaptor for NF-kappa B and caspase-8-dependent apoptosis. Biochem Biophys Res Commun. 2003;303:69–73. doi: 10.1016/s0006-291x(03)00309-7. [DOI] [PubMed] [Google Scholar]
- 44.Hasegawa M., Imamura R., Motani K., Nishiuchi T., Matsumoto N., Kinoshita T., Suda T. Mechanism and repertoire of ASC-mediated gene expression. J Immunol. 2009;182:7655–7662. doi: 10.4049/jimmunol.0800448. [DOI] [PubMed] [Google Scholar]
- 45.Man S.M., Tourlomousis P., Hopkins L., Monie T.P., Fitzgerald K.A., Bryant C.E. Salmonella infection induces recruitment of caspase-8 to the inflammasome to modulate IL-1β production. J Immunol. 2013;191:5239–5246. doi: 10.4049/jimmunol.1301581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaiser W.J., Offermann M.K. Apoptosis induced by the Toll-like receptor adaptor TRIF is dependent on its receptor interacting protein homotypic interaction motif. J Immunol. 2005;174:4942–4952. doi: 10.4049/jimmunol.174.8.4942. [DOI] [PubMed] [Google Scholar]
- 47.Weber A., Kirejczyk Z., Besch R., Potthoff S., Leverkus M., Häcker G. Proapoptotic signalling through Toll-like receptor-3 involves TRIF-dependent activation of caspase-8 and is under the control of inhibitor of apoptosis proteins in melanoma cells. Cell Death Differ. 2010;17:942–951. doi: 10.1038/cdd.2009.190. [DOI] [PubMed] [Google Scholar]
- 48.Gringhuis S.I., Kaptein T.M., Wevers B.A., Theelen B., van der Vlist M., Boekhout T., Geijtenbeek T.B. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1beta via a noncanonical caspase-8 inflammasome. Nat Immunol. 2012;13:246–254. doi: 10.1038/ni.2222. [DOI] [PubMed] [Google Scholar]
- 49.Maelfait J., Vercammen E., Janssens S., Schotte P., Haegman M., Magez S., Beyaert R. Stimulation of Toll-like receptor 3 and 4 induces interleukin-1beta maturation by caspase-8. J Exp Med. 2008;205:1967–1973. doi: 10.1084/jem.20071632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaufman R.J. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002;110:1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li J., Lee B., Lee A.S. Endoplasmic reticulum stress-induced apoptosis: multiple pathways and activation of p53-up-regulated modulator of apoptosis (PUMA) and NOXA by p53. J Biol Chem. 2006;281:7260–7270. doi: 10.1074/jbc.M509868200. [DOI] [PubMed] [Google Scholar]
- 52.Anand P.K., Malireddi R.K., Kanneganti T.D. Role of the Nlrp3 inflammasome in microbial infection. Front Microbiol. 2011;2:12. doi: 10.3389/fmicb.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kayagaki N., Warming S., Lamkanfi M., Vande Walle L., Louie S., Dong J., Newton K., Qu Y., Liu J., Heldens S., Zhang J., Lee W.P., Roose-Girma M., Dixit V.M. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 54.Muñoz-Planillo R., Kuffa P., Martínez-Colón G., Smith B.L., Rajendiran T.M., Núñez G. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee G.S., Subramanian N., Kim A.I., Aksentijevich I., Goldbach-Mansky R., Sacks D.B., Germain R.N., Kastner D.L., Chae J.J. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012;492:123–127. doi: 10.1038/nature11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoffman H.M., Mueller J.L., Broide D.H., Wanderer A.A., Kolodner R.D. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29:301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hull K.M., Shoham N., Chae J.J., Aksentijevich I., Kastner D.L. The expanding spectrum of systemic autoinflammatory disorders and their rheumatic manifestations. Curr Opin Rheumatol. 2003;15:61–69. doi: 10.1097/00002281-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 58.McDermott M.F. Genetic clues to understanding periodic fevers, and possible therapies. Trends Mol Med. 2002;8:550–554. doi: 10.1016/s1471-4914(02)02425-5. [DOI] [PubMed] [Google Scholar]
- 59.Allam R., Lawlor K.E., Yu E.C., Mildenhall A.L., Moujalled D.M., Lewis R.S., Ke F., Mason K.D., White M.J., Stacey K.J., Strasser A., O’Reilly L.A., Alexander W., Kile B.T., Vaux D.L., Vince J.E. Mitochondrial apoptosis is dispensable for NLRP3 inflammasome activation but non-apoptotic caspase-8 is required for inflammasome priming. EMBO Rep. 2014;15:982–990. doi: 10.15252/embr.201438463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vince J.E., Wong W.W., Gentle I., Lawlor K.E., Allam R., O’Reilly L., Mason K., Gross O., Ma S., Guarda G., Anderton H., Castillo R., Häcker G., Silke J., Tschopp J. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity. 2012;36:215–227. doi: 10.1016/j.immuni.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 61.Weng D., Marty-Roix R., Ganesan S., Proulx M.K., Vladimer G.I., Kaiser W.J., Mocarski E.S., Pouliot K., Chan F.K., Kelliher M.A., Harris P.A., Bertin J., Gough P.J., Shayakhmetov D.M., Goguen J.D., Fitzgerald K.A., Silverman N., Lien E. Caspase-8 and RIP kinases regulate bacteria-induced innate immune responses and cell death. Proc Natl Acad Sci U S A. 2014;111:7391–7396. doi: 10.1073/pnas.1403477111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Philip N.H., Dillon C.P., Snyder A.G., Fitzgerald P., Wynosky-Dolfi M.A., Zwack E.E., Hu B., Fitzgerald L., Mauldin E.A., Copenhaver A.M., Shin S., Wei L., Parker M., Zhang J., Oberst A., Green D.R., Brodsky I.E. Caspase-8 mediates caspase-1 processing and innate immune defense in response to bacterial blockade of NF-kappaB and MAPK signaling. Proc Natl Acad Sci U S A. 2014;111:7385–7390. doi: 10.1073/pnas.1403252111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Uchiyama R., Yonehara S., Tsutsui H. Fas-mediated inflammatory response in Listeria monocytogenes infection. J Immunol. 2013;190:4245–4254. doi: 10.4049/jimmunol.1203059. [DOI] [PubMed] [Google Scholar]
- 64.Kang T.B., Yang S.H., Toth B., Kovalenko A., Wallach D. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity. 2013;38:27–40. doi: 10.1016/j.immuni.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 65.Cuda C.M., Misharin A.V., Gierut A.K., Saber R., Haines G.K., 3rd, Hutcheson J., Hedrick S.M., Mohan C., Budinger G.S., Stehlik C., Perlman H. Caspase-8 acts as a molecular rheostat to limit RIPK1- and MyD88-mediated dendritic cell activation. J Immunol. 2014;192:5548–5560. doi: 10.4049/jimmunol.1400122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ben Moshe T., Barash H., Kang T.B., Kim J.C., Kovalenko A., Gross E., Schuchmann M., Abramovitch R., Galun E., Wallach D. Role of caspase-8 in hepatocyte response to infection and injury in mice. Hepatology. 2007;45:1014–1024. doi: 10.1002/hep.21495. [DOI] [PubMed] [Google Scholar]
- 67.Kovalenko A., Kim J.C., Kang T.B., Rajput A., Bogdanov K., Dittrich-Breiholz O., Kracht M., Brenner O., Wallach D. Caspase-8 deficiency in epidermal keratinocytes triggers an inflammatory skin disease. J Exp Med. 2009;206:2161–2177. doi: 10.1084/jem.20090616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weinlich R., Oberst A., Dillon C.P., Janke L.J., Milasta S., Lukens J.R., Rodriguez D.A., Gurung P., Savage C., Kanneganti T.D., Green D.R. Protective roles for caspase-8 and cFLIP in adult homeostasis. Cell Rep. 2013;5:340–348. doi: 10.1016/j.celrep.2013.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Panayotova-Dimitrova D., Feoktistova M., Ploesser M., Kellert B., Hupe M., Horn S., Makarov R., Jensen F., Porubsky S., Schmieder A., Zenclussen A.C., Marx A., Kerstan A., Geserick P., He Y.W., Leverkus M. cFLIP regulates skin homeostasis and protects against TNF-induced keratinocyte apoptosis. Cell Rep. 2013;5:397–408. doi: 10.1016/j.celrep.2013.09.035. [DOI] [PubMed] [Google Scholar]
- 70.Soung Y.H., Lee J.W., Kim S.Y., Sung Y.J., Park W.S., Nam S.W., Kim S.H., Lee J.Y., Yoo N.J., Lee S.H. Caspase-8 gene is frequently inactivated by the frameshift somatic mutation 1225_1226delTG in hepatocellular carcinomas. Oncogene. 2005;24:141–147. doi: 10.1038/sj.onc.1208244. [DOI] [PubMed] [Google Scholar]
- 71.Kim H.S., Lee J.W., Soung Y.H., Park W.S., Kim S.Y., Lee J.H., Park J.Y., Cho Y.G., Kim C.J., Jeong S.W., Nam S.W., Kim S.H., Lee J.Y., Yoo N.J., Lee S.H. Inactivating mutations of caspase-8 gene in colorectal carcinomas. Gastroenterology. 2003;125:708–715. doi: 10.1016/s0016-5085(03)01059-x. [DOI] [PubMed] [Google Scholar]
- 72.Soung Y.H., Lee J.W., Kim S.Y., Jang J., Park Y.G., Park W.S., Nam S.W., Lee J.Y., Yoo N.J., Lee S.H. CASPASE-8 gene is inactivated by somatic mutations in gastric carcinomas. Cancer Res. 2005;65:815–821. [PubMed] [Google Scholar]
- 73.Ando M., Kawazu M., Ueno T., Fukumura K., Yamato A., Soda M., Yamashita Y., Choi Y.L., Yamasoba T., Mano H. Cancer-associated missense mutations of caspase-8 activate nuclear factor-κB signaling. Cancer Sci. 2013;104:1002–1008. doi: 10.1111/cas.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yin M., Yan J., Wei S., Wei Q. CASP8 polymorphisms contribute to cancer susceptibility: evidence from a meta-analysis of 23 publications with 55 individual studies. Carcinogenesis. 2010;31:850–857. doi: 10.1093/carcin/bgq047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.MacPherson G., Healey C.S., Teare M.D., Balasubramanian S.P., Reed M.W., Pharoah P.D., Ponder B.A., Meuth M., Bhattacharyya N.P., Cox A. Association of a common variant of the CASP8 gene with reduced risk of breast cancer. J Natl Cancer Inst. 2004;96:1866–1869. doi: 10.1093/jnci/dji001. [DOI] [PubMed] [Google Scholar]
- 76.Chun H.J., Zheng L., Ahmad M., Wang J., Speirs C.K., Siegel R.M., Dale J.K., Puck J., Davis J., Hall C.G., Skoda-Smith S., Atkinson T.P., Straus S.E., Lenardo M.J. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature. 2002;419:395–399. doi: 10.1038/nature01063. [DOI] [PubMed] [Google Scholar]
- 77.Dinarello C.A. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 78.Dinarello C.A. IL-1: discoveries, controversies and future directions. Eur J Immunol. 2010;40:599–606. doi: 10.1002/eji.201040319. [DOI] [PubMed] [Google Scholar]
- 79.Weber A., Wasiliew P., Kracht M. Interleukin-1beta (IL-1beta) processing pathway. Sci Signal. 2010;3:cm2. doi: 10.1126/scisignal.3105cm2. [DOI] [PubMed] [Google Scholar]
- 80.Weber A., Wasiliew P., Kracht M. Interleukin-1 (IL-1) pathway. Sci Signal. 2010;3:cm1. doi: 10.1126/scisignal.3105cm1. [DOI] [PubMed] [Google Scholar]
- 81.Dinarello C.A., Thompson R.C. Blocking IL-1: interleukin 1 receptor antagonist in vivo and in vitro. Immunol Today. 1991;12:404–410. doi: 10.1016/0167-5699(91)90142-G. [DOI] [PubMed] [Google Scholar]
- 82.Lukens J.R., Vogel P., Johnson G.R., Kelliher M.A., Iwakura Y., Lamkanfi M., Kanneganti T.D. RIP1-driven autoinflammation targets IL-1alpha independently of inflammasomes and RIP3. Nature. 2013;498:224–227. doi: 10.1038/nature12174. [DOI] [PMC free article] [PubMed] [Google Scholar]