Abstract
Serological diagnosis of West Nile virus (WNV) infection is complicated by extensive antigenic cross-reactivity with other closely related flaviviruses, such as St. Louis encephalitis virus. Here we describe a recombinant, bacterially expressed antigen equivalent to structural domain III of the WNV envelope protein that has allowed clear discrimination of antibody responses to WNV from those against other related flaviviruses in indirect enzyme-linked immunosorbent assays using standardized control antisera and field-collected samples.
Since 1999, the United States has experienced annual epidemics of disease in humans and animals caused by West Nile virus (WNV) over an expanding geographical range. To date during 2003, WNV has been isolated in 46 states and the District of Columbia, and more than 8,500 cases of human disease, resulting in 199 deaths, have been reported (1). Outbreaks of WNV disease with neurological manifestations have also been reported in Eastern Europe, North Africa, and Israel since the mid-1990s (reviewed in reference 17). WNV is clearly an emerging and significant public health problem. Research priorities to limit the impact of WNV include the development of more-specific rapid diagnostic assays (5, 18).
WNV is a member of the Japanese encephalitis (JE) virus group of the genus Flavivirus, family Flaviviridae; other members include Japanese encephalitis virus (JEV), found throughout Asia, St. Louis encephalitis virus (SLEV), found in the Americas, and Murray Valley encephalitis virus (MVEV), found in Australia and New Guinea. These viruses have a similar ecology and are antigenically related to WNV, and their cocirculation in several regions of the world has complicated the specific diagnosis of infections by these viruses in humans and other vertebrate hosts (10, 15). Cross-reactions in patients ultimately diagnosed with probable dengue virus infections have also been reported in evaluations of WNV testing assays (19).
Previously we have reported the identification of WNV-specific neutralizing epitopes within structural domain III of the WNV envelope (E) protein (2). Earlier investigations with other flaviviruses have also reported the presence of virus-specific epitopes within this region of the E protein (e.g., see references 6, 22, and 25), and other authors have suggested the utility of domain III from dengue virus types 1 to 4 or JEV as antigens for specific serological diagnosis of infections with those flaviviruses (10, 24). These observations led us to investigate the utility of a recombinant, bacterially expressed domain III (r-EIII) antigen derived from the envelope protein of a North American WNV strain (385-99) for discrimination of WNV from other JE virus group infections.
MATERIALS AND METHODS
Expression and purification of recombinant WNV E protein domain III.
Regions corresponding to structural domain III of the WNV strain 385-99 E protein were reverse transcription-PCR amplified for cloning and expression either as a glutathione S-transferase (GST) fusion using the pGEX-2T system (Amersham Pharmacia Biotech, Piscataway, N.J.) or as a maltose binding protein (MBP) fusion using the pMAL-c2x/p2x system (New England Biolabs, Beverly Mass.). RNA extractions, reverse transcription-PCR and cloning of the domain III coding region for construction of expression plasmids were performed as described previously (2). For the GST fusion, the amplicon comprised the region encoding amino acids 295 to 410, while the MBP amplicon encoded amino acids 295 to 395.
Protocols for expression and purification of the WNV r-EIII GST fusion protein, followed by cleavage of the fusion protein and purification of r-EIII away from the GST fusion partner, were based on those described by Bhardwaj et al. (4). After induction and sonication, the MBP fusion protein was purified from contaminating bacterial proteins on an amylose resin column (New England Biolabs), concentrated using Amicon Ultra 10-kDa cutoff spin columns (Millipore, Bedford, Mass.), and then cleaved with Factor Xa (Novagen, Madison, Wis.). WNV r-EIII was purified from the MBP partner and remaining Factor Xa on a sephadex G75 size exclusion column (Amersham Pharmacia Biotech), and homogeneity of the purified protein was confirmed by mass spectroscopy (data not shown).
Antigens and antisera.
Suckling mouse brain (smb), sucrose-acetone-extracted antigen preparations (2) for WNV (strain 385-99), JEV (strain Nakayama), SLEV (strain Parton), and MVEV (prototype strain) viruses were obtained from the World Arbovirus Reference Collection at the University of Texas Medical Branch. Titers of each antigen were determined by hemagglutination (HA) assay.
WNV r-EIII expressed and purified using the GST system was sent to Harlan Bioproducts for Science (Indianapolis, Ind.) to be used as an antigen for the preparation of a polyclonal rabbit serum using Harlan's standard immunization protocol in New Zealand White Rabbits (details available at http://www.hbps.com). In addition, polyclonal mouse immune ascitic fluids (MIAF) against WNV strains 385-99 (genetic lineage 1) and B956 (lineage 2), as well as JEV, SLEV, MVEV, dengue virus type 2, and yellow fever virus, were also obtained from the Arbovirus Reference Collection.
Panels of monkey, horse, and human sera were also tested. These included 19 monkey sera obtained from a serosurvey of rhesus monkeys (Macaca mulatta) housed in outdoor corrals at the Tulane National Primate Research Center in Covington, La. (20), 57 sera obtained from horses in Mexico (9), and convalescent-phase sera from 11 confirmed WNV encephalitis patients and 1 SLEV encephalitis patient (all collected between July and August 2003 from patients infected during the summer of 2002 in Houston, Tex.), as well as four control sera from flavivirus naive donors. As part of the serosurveys, these sera had been screened for the presence of anti-WNV antibody by comparative hemagglutination inhibition (HI) testing against WNV and SLEV antigens (results for monkey and human sera are summarized in Tables 1 and 2, respectively). WNV-positive samples typically had HI titers against WNV antigen that were fourfold higher than against SLEV antigen. Negative samples did not inhibit hemagglutination by WNV antigen (titers of <20). For confirmation, sera were also tested for their ability to neutralize WNV strain 385-99 in 90% plaque reduction neutralization titer (PRNT90) assays.
TABLE 1.
Reactivity of a panel of rhesus monkey sera with WNV, SLEV, and/or recombinant WNV E protein domain-III antigens in an indirect IgG ELISA, HI test, and PRNT90 test
| Serum | Absorbance in IgG indirect ELISAa
|
Titer in HI
|
WNV PRNT90
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| WNV Ag | SLEV Ag | r-EIII | BSA | r-EIII titerb | WNV | SLEV | No pretreatment | + β-MEd | |
| CL35 | 0.452 | 0.574 | 1.374 | 0.121 | 6,400 | 320 | 80 | ≥320 | ≥320 |
| CL07 | 0.620 | 0.588 | 1.346 | 0.156 | 3,200 | 320 | 160 | ≥320 | 160 |
| CK62 | 0.452 | 0.424 | 1.251 | 0.073 | NTc | 320 | 160 | 80 | NT |
| CP50 | 0.341 | 0.431 | 0.974 | 0.049 | 1,600 | 80 | 20 | 40 | 40 |
| CL93 | 0.341 | 0.643 | 0.763 | 0.094 | 1,600 | 640 | 160 | 80 | NT |
| CL80 | 0.495 | 0.591 | 0.745 | 0.061 | 1,600 | 640 | 160 | ≥320 | 160 |
| CJ13 | 0.424 | 0.512 | 0.650 | 0.073 | 400 | 40 | <20 | 40 | NT |
| CP51 | 0.271 | 0.439 | 0.326 | 0.069 | 400 | 320 | 80 | 40 | NT |
| CV38 | 0.136 | 0.322 | 0.103 | 0.127 | <100 | 640 | 160 | 80 | <20 |
| CL13 | 0.119 | 0.266 | 0.174 | 0.051 | <100 | 640 | 160 | 40 | <20 |
| CM08 | 0.165 | 0.222 | 0.046 | 0.060 | <100 | <20 | <20 | <20 | NT |
| CP48 | 0.109 | 0.184 | 0.048 | 0.043 | <100 | <20 | <20 | <20 | NT |
| CM13 | 0.117 | 0.251 | 0.095 | 0.075 | <100 | <20 | <20 | <20 | NT |
| CI94 | 0.122 | 0.129 | 0.039 | 0.062 | <100 | <20 | <20 | <20 | NT |
| CL14 | 0.219 | 0.329 | 0.049 | 0.060 | <100 | <20 | <20 | <20 | NT |
| CM07 | 0.143 | 0.241 | 0.070 | 0.094 | <100 | <20 | <20 | <20 | NT |
| CL87 | 0.132 | 0.176 | 0.052 | 0.065 | <100 | <20 | <20 | <20 | NT |
| CK74 | 0.189 | 0.409 | 0.054 | 0.053 | <100 | <20 | <20 | <20 | NT |
| CI72 | 0.196 | 0.281 | 0.065 | 0.059 | <100 | <20 | <20 | <20 | NT |
Absorbance values given are means of triplicate wells. Sera were screened against WNV and SLEV antigens and the r-EIII protein at a 1:100 dilution. BSA-coated wells were used as a control for nonspecific binding of antibodies.
To determine WNV r-EIII IgG ELISA endpoint titers, a positive result was defined as being those wells that had a corrected absorbance (i.e., absorbance versus r-EIII minus absorbance versus the BSA control well) ≥0.2.
NT, not tested.
PRNT90 titer following pretreatment of serum samples with 0.1M β-mercaptoethanol to reduce IgM antibodies.
TABLE 2.
Reactivity of convalescent-phase sera from WNV encephalitis patients, one SLEV encephalitis patient, and normal controls with WNV, SLEV, and/or recombinant WNV E protein domain III antigens in indirect IgG ELISA, HI test, and PRNT90 test
| Serumb | Absorbance in IgG indirect ELISAa
|
Titer in HI
|
WNV PRNT90 | ||||
|---|---|---|---|---|---|---|---|
| WNV Ag | SLEV Ag | r-EIII Ag | BSA | WNV | SLEV | ||
| 026 | 1.772 | 0.301 | 2.486 | 0.034 | 5,120 | 320 | ≥2,560 |
| 022 | 1.644 | 0.387 | 0.751 | 0.024 | 1,280 | 20 | 1,280 |
| 071 | 1.885 | 0.388 | 1.822 | 0.021 | 640 | 160 | 640 |
| 028 | 0.876 | 0.216 | 0.831 | 0.023 | 640 | <20 | 160 |
| 034 | 1.187 | 0.252 | 0.683 | 0.031 | 320 | 40 | 160 |
| 017 | 0.847 | 0.175 | 0.396 | 0.017 | 640 | <20 | 80 |
| 008 | 0.685 | 0.235 | 0.328 | 0.030 | 160 | <20 | 80 |
| 016 | 0.790 | 0.172 | 0.333 | 0.029 | 40 | <20 | 80 |
| 025 | 1.099 | 0.238 | 0.162 | 0.055 | 40 | <20 | 40 |
| 015 | 0.572 | 0.205 | 0.146 | 0.026 | 40 | <20 | 40 |
| 031 | 0.545 | 0.231 | 0.084 | 0.022 | 40 | <20 | 20 |
| 050 | 0.156 | 0.280 | 0.026 | 0.025 | <20 | 40 | <20 |
| C1 | 0.117 | 0.140 | 0.020 | 0.022 | <20 | <20 | <20 |
| C2 | 0.093 | 0.121 | 0.020 | 0.020 | <20 | <20 | <20 |
| C3 | 0.086 | 0.130 | 0.054 | 0.056 | <20 | <20 | <20 |
| C4 | 0.125 | 0.153 | 0.046 | 0.033 | <20 | <20 | <20 |
Absorbance values given are means of triplicate wells. Sera were screened against WNV and SLEV antigens and the r-EIII protein at a 1:100 dilution. BSA-coated wells were used as a control for nonspecific binding of antibodies.
Samples C1 to C4 were obtained from flavivirus naive donors; sample 050 was obtained from an SLEV encephalitis patient.
Indirect enzyme-linked immunosorbent assays (ELISAs).
The wells of 96-well microtiter plates (Corning, Inc., Corning, N.Y.) were coated overnight at 4°C with either WNV, JEV, MVEV, or SLEV smb-derived antigen or WNV r-EIII protein or bovine serum albumin (BSA) (Sigma, St. Louis, Mo.) (25 ng/well of r-EIII or BSA), diluted in borate saline (pH 9.0). Dilutions of whole virus and recombinant antigens for coating had been determined previously by titration against specific antisera (e.g., JEV antigen versus anti-JEV MIAF; data not shown). Wells were blocked for 60 min at room temperature with a solution of 3% bovine serum albumin in phosphate-buffered saline (PBS) containing 5% Tween 20 (PBS/Tween) and then washed with PBS/Tween.
As outlined above, block titrations of each specific antigen-antiserum combination had been performed by using the MIAF to determine the appropriate serum dilution ranges to yield comparable absorbance values for ELISAs performed with each antigen. For the diagnostic assays using monkey and horse sera, samples were diluted 1:100 in PBS/Tween and tested in triplicate. Human sera were initially tested at a 1:100 dilution, but due to high levels of nonspecific binding (data not shown) they were subsequently screened at 1:400. This dilution was equivalent to that used in earlier studies (15, 19). After addition of diluted sera, plates were incubated at room temperature for 45 min and then washed four times with PBS/Tween. Horseradish peroxidase-labeled antisera (either anti-mouse immunoglobulin (Ig) [Sigma], anti-rabbit immunoglobulin G (IgG) [Sigma], anti-monkey IgG [Rockland, Gilbertsville, Pa.], anti-human IgG [Sigma], or anti-horse IgG [Sigma]) that had been diluted in PBS/Tween according to the supplier's recommendations was added to each well, plates were again incubated and washed (four times with PBS/Tween, twice with PBS), and antibody binding was visualized by addition of 3,3′,5,5′-tetramethylbenzidene substrate (Sigma). After incubation for 10 min at room temperature, reactions were stopped by addition of 3 M HCl, and absorbances were read at 450 nm with a reference wavelength of 595 nm on a model 3550-UV plate reader (Bio-Rad, Hercules, Calif.).
RESULTS
Specificity of rabbit anti-WNV domain III serum for WNV.
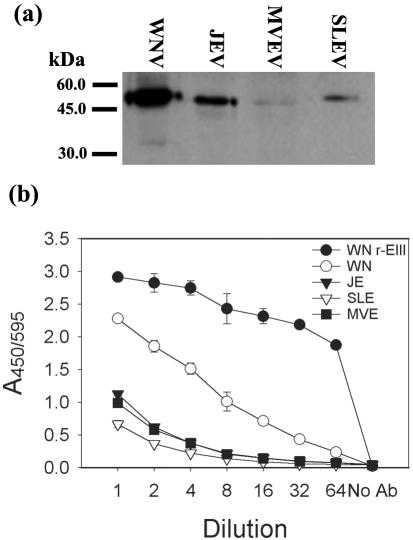
In Western blotting and indirect ELISAs with JEV, MVEV, and SLEV smb-derived antigen preparations, some weak cross-reactivity of the anti-WNV r-EIII rabbit serum was observed with the envelope proteins of those viruses (Fig. 1). In PRNT assays, the rabbit antiserum neutralized the infectivity titer of WNV strain 385-99 by more than 5,000-fold, while less than 10-fold reductions in titer were observed in assays with JEV or SLEV (data not shown).
FIG. 1.
Comparative reactivity of rabbit anti-WNV r-EIII serum with selected mosquito-borne flaviviruses in Western blotting (a) and indirect ELISA assays (b). For Western blotting, the wells were loaded with equal quantities of each antigen, determined by hemagglutination titer. For indirect ELISA, wells were coated with one pH 6.2 HA unit of each viral antigen, and the starting dilution of the antiserum was 1:1,000.
WNV r-EIII antigen is more specific than whole virus antigens in indirect ELISA using polyclonal MIAFs raised against JE serocomplex viruses.
The apparent specificity of epitopes in domain III (as evidenced by the limited cross-reactivity of the anti-WNV domain III serum against other JE virus group antigens) pointed to the potential utility of r-EIII as an antigen for serological assays. Comparative indirect ELISAs were performed with WNV, JEV, SLEV, or MVEV smb-derived antigens or with WNV r-EIII antigen using a panel of MIAFs raised against several mosquito-borne flaviviruses.
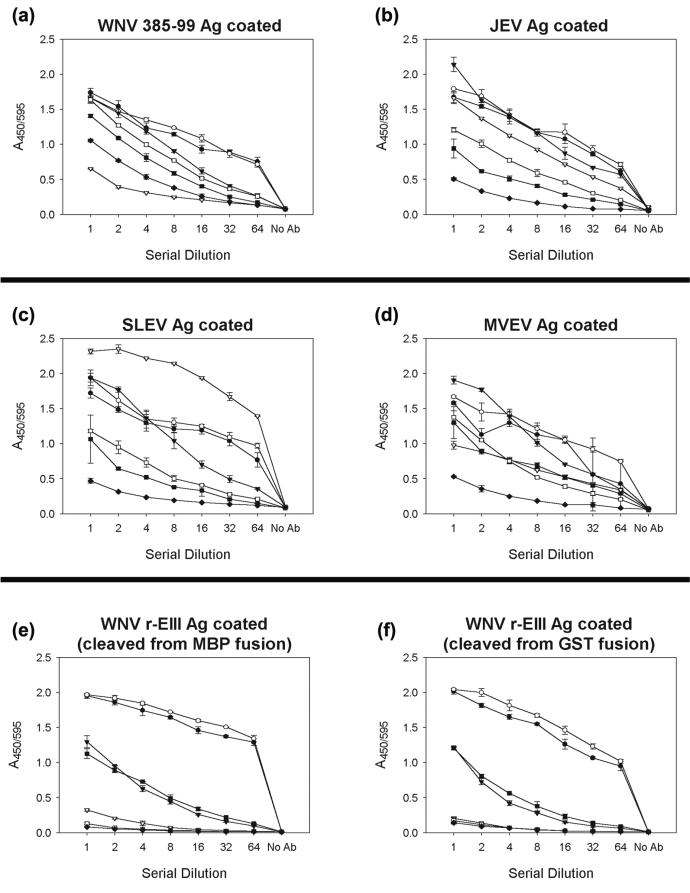
In assays where plates were coated with whole-virus antigens, extensive cross-reactivity was observed with most MIAF antisera (Fig. 2a to d). In general, the strongest reactions were observed between specific antigen-antiserum combinations (e.g., anti-WNV 385-99 [genetic lineage 1] or B956 [genetic lineage 2] serum with WNV antigen). However, in each case, at least two other antisera also reacted strongly (absorbance values at least 75% of those for the homologous serum) at most of the dilutions tested. The binding activity of the anti-MVEV MIAF was lower than that of the other JE virus group antisera in each assay; however, its cross-reactive binding to WNV, JEV, or SLEV antigen was at least 60% of its binding to the MVEV antigen.
FIG. 2.
Reactions of polyclonal mouse immune ascitic fluids raised against selected mosquito-borne flaviviruses with either whole-virus JE serocomplex antigens (WNV, JEV, SLEV, or MVEV; wells were coated with one pH 6.2 HA unit of virus; panels a to d, respectively) or WNV r-EIII antigens derived from either GST or MBP fusions (25 ng/well) (e and f). Antisera used were anti-WNV 385-99 (solid circle; starting dilution, 1:20), anti-WNV B956 (open circle; starting dilution, 1:20), anti-JEV (solid triangle; starting dilution, 1:20), anti-SLEV (open triangle; starting dilution, 1:6,000), anti-MVEV (solid square; starting dilution, 1:20), anti-dengue virus type 2 (open square; starting dilution, 1:20), anti-yellow fever virus (solid diamond; starting dilution, 1:20). Data points are mean absorbances of duplicate wells; error bars are one standard deviation.
In contrast, the binding of anti-WNV 385-99 or B956 MIAF to WNV r-EIII antigen, derived from either the GST or MBP system, was clearly discriminated from that of the other antisera; absorbance values were approximately two- to threefold higher than those of sera raised against other mosquito-borne flavivirus antigens across the range of dilutions tested, and the differences in titration endpoints were also dramatically increased (Fig. 2e and f).
WNV r-EIII antigen can be used to detect anti-WNV IgG antibody in diagnostic specimens.
Having demonstrated the improved specificity of the WNV r-EIII antigen compared with whole virus antigen preparations in assays with control antisera, it was then used to screen for anti-WNV IgG antibodies in field-collected monkey and horse sera. Nineteen monkey sera and sixteen human sera were screened by indirect IgG ELISA, using plates coated with the WNV r-EIII antigen, WNV or SLEV smb-derived antigens, or bovine serum albumin (included as a control for nonspecific antibody binding), and the results were then compared with the results of prior HI and PRNT testing.
In general, we observed a good correlation between IgG antibody detection by ELISA with r-EIII antigen and the presence of neutralizing and HI antibody against WNV. All of the monkey and human serum samples that were classified as WNV negative by HI/PRNT testing (n = 14) were also clearly negative in the ELISA assay (absorbance values of <0.1 against the r-EIII antigen and <0.2 against smb-derived WNV antigen [Tables 1 and 2]).
For the monkey sera, eight WNV-positive samples reacted strongly with r-EIII, yielding absorbance values ranging between 0.326 and 1.374, compared with their background absorbances against BSA, which were between 0.049 and 0.156. Two samples, CL13 and CV38, that were strongly positive by HI and PRNT (Table 1) reacted very weakly against r-EIII and smb-derived WNV/SLEV antigens as determined by ELISA (absorbances of 0.103 and 0.174, respectively, against the r-EIII antigen). We suspected that these samples contained primarily IgM antibody to WNV, and to confirm this, PRNT assays were repeated after treating the sera with 0.1 M β-mercaptoethanol to denature IgM antibodies (Table 1). The neutralizing activity of samples CL13 and CV38 was abolished, whereas the activity of IgG ELISA positive samples was largely unaffected, with twofold or less changes in PRNT90 titer observed. Attempts at performing an indirect IgM ELISA were complicated by high levels of nonspecific antibody binding for all samples (data not shown).
Endpoint titers for the IgG ELISA positive samples were also determined for the monkey sera, using a corrected absorbance (i.e., after subtracting the background absorbance in BSA-coated wells) of 0.2 as the positive cutoff (Table 1). This value was chosen since it was >3-fold higher than the absorbance values observed in BSA-coated control wells (and absorbance values obtained with WNV-negative samples), which is comparable to “positive to negative” cutoff ratios used in other WNV diagnostic assays (8, 14). Using corrected absorbance values, ELISA titers for the IgG-positive sera ranged between 400 and 6,400, and higher endpoint titers correlated with higher absorbance values in the initial 1:100 screening ELISA (Table 1).
During screening of the convalescent-phase and control human sera, we also observed an association between increasing absorbance against the r-EIII antigen and higher PRNT90 titers (Table 2). All WNV-negative samples, including one sample from an SLEV encephalitis patient (sample 050), had mean absorbance values against the r-EIII Ag that were equivalent to background (<0.055). Eight positive samples had absorbance values that were 10- to 70-fold greater than their background absorbance (against BSA) or the negative control samples. Three weakly positive samples (025, 015, and 031; HI/PRNT90 titers of ≤40 against WNV) each had absorbance values against the r-EIII antigen that were approximately threefold higher than the BSA background.
A panel of horse sera from several regions of Mexico was also made available for testing (9). Fifty-seven of these sera were screened for this study, using the indirect IgG ELISA with WNV r-EIII antigen, and the results were compared with results of HI and PRNT testing (Table 3). Based on the results of the monkey serum testing, horse serum samples were classified as WNV IgG positive if the corrected absorbance was ≥0.2, equivocal if absorbance was between 0.1 and 0.2, and negative if absorbance was <0.1.
TABLE 3.
Correlation of WNV r-EIII indirect IgG ELISA testing of a panel of equine sera with results of HI/PRNT testing for anti-WNV antibody
| IgG indirect WNV r-EIII ELISA resulta | No. HI/PRNT positive | No. HI/PRNT negative |
|---|---|---|
| Positive | 31 | 0 |
| Equivocal | 8b | 2 |
| Negative | 2 | 14 |
Classifications for ELISA results with horse sera were based on results of testing with monkey sera (see Table 1) and assigned as follows: positive, corrected absorbance (minus absorbance in BSA control) ≥ 0.2; equivocal, absorbance of 0.1 to 0.2; negative, absorbance of <0.1.
For the ELISA equivocal samples, 6 of the 8 HI/PRNT positive samples were subsequently shown to be strongly IgM positive by independent IgM/IgG ELISA testing (see Results).
Again, a strong correlation was observed between the IgG ELISA and detection of WNV-specific antibody by HI/PRNT (Table 3). Thirty-one samples (54.4%) were positive by all assays, and 14 samples (24.6%) were negative in all assays. Two samples (3.5%) gave discordant negative results in the IgG ELISA compared with results of HI/PRNT testing. Ten samples (17.5%) were classified as equivocal by the IgG ELISA, of which eight were HI/PRNT positive to WNV and two were negative. Further testing of these samples using WNV IgM and IgG capture ELISAs at the U.S. Army Medical Research Institute of Infectious Diseases showed that six of the eight equivocal positive samples contained primarily IgM antibody to WNV.
DISCUSSION
The specific serological diagnosis of mosquito-borne flavivirus infections in areas where more than one virus type circulates is complicated by the relatively high degree of antigenic cross-reactivity between these viruses. Such a situation now exists in the United States, where WNV and SLEV, both members of the JE virus group, are endemic in many areas of the country and where dengue viruses may circulate in some southern border regions (21, 23). Using control MIAFs raised against several JE virus group and other mosquito-borne flaviviruses, we have shown that a recombinant subunit antigen equivalent to structural domain III of the WNV E protein is superior to whole-virus antigens in discriminating specific antibody responses to WNV. Antisera raised against either a lineage 1 (385-99) or lineage 2 (B956) WNV strain reacted equally well with the r-EIII antigen (Fig. 2e and f).
During our evaluation of the WNV r-EIII antigen in an indirect IgG ELISA assay, using panels of monkey, human, and horse sera (92 samples in total), we observed a strong correlation between the presence of antibody that reacted with the r-EIII antigen and detection of WNV neutralizing or HI antibody in the same sample. In general, higher absorbance values against the WNV r-EIII antigen corresponded with increasing PRNT90 titers. We identified no false-positive reactions with the r-EIII antigen, and 75 samples (82%) yielded clearly positive or negative results that were concordant with results of HI and PRNT testing. Of the 17 samples that were classified as equivocal or that yielded discordant negative results in the IgG ELISA, 8 were shown by subsequent testing to be IgM positive to WNV. Three equivocal (or weakly positive) human sera were also weakly positive by HI/PRNT90 (titers of ≤40). Overall, the results of screening for IgG antibody in sera of human and other animal origin, using the WNV r-EIII antigen, were >90% concordant with HI and PRNT testing.
The absorbance values of all WNV-positive monkey and human serum samples in the WNV r-EIII ELISA were approximately 6- to 40-fold higher than those of the negative samples (Tables 1 and 2), suggesting that although these assays utilized a subunit antigen they retained a high level of sensitivity. The correlation between increasing strength of reactivity with the r-EIII antigen and higher PRNT90 titers observed during the testing of convalescent-phase human sera (Table 2) is consistent with the importance of domain III as a target for virus-neutralizing antibodies. Absorbance values of negative samples were not significantly different from the background binding in albumin-coated wells, highlighting another advantage of using a highly purified recombinant antigen. A similar level of discrimination between positive and negative results was obtained during screening of the panel of horse sera (data not shown).
Screening for recent WNV human infections currently relies primarily on an IgM capture ELISA protocol developed by the Centers for Disease Control that utilizes inactivated, whole-virus antigens or, more recently, recombinant subviral particulate antigen (14). Although comparative testing with multiple virus antigens has allowed easier interpretation and greater reliability of diagnosis (15), the assay is ultimately limited by the relative lack of specificity obtained when using whole-virus antigen preparations (e.g., see Fig. 2a to d). Studies with subviral particulate antigens of other flaviviruses have shown them to be antigenically indistinguishable from native virions (11, 13), suggesting that the newer recombinant subviral antigen probably retains this extensive antigenic cross-reactivity.
Preliminary testing of WNV r-EIII in our lab suggests that it is poorly bound by antibodies coated in the wells of ELISA plates or may be captured but not bound by detecting antibody due to steric hindrances associated with the small size of the fragment, and it may not be immediately suitable for a capture assay format. However, it is possible that r-EIII could be chemically coupled to microparticle carriers to allow its utilization in a capture ELISA format (16) or in rapid fluorescence assays, such as the Luminex system (12). In addition, this antigen should be readily adaptable to other rapid diagnostic test formats, including immunochromatographic (dipstick) techniques as described for dengue virus serological testing (7). Using the MBP expression system, we have obtained yields of up to 30 mg of cleaved, purified r-EIII from 1- to 2-liter bacterial cultures (equivalent to ∼12,500 antigen-coated 96-well plates at the coating dilution used in this study), suggesting that r-EIII can be readily expressed and purified in large quantities, making it suitable for commercial batch preparation.
Clearly, more extensive testing and evaluation of this antigen will be needed to develop reliable diagnostic assays and establish parameters for clear discrimination of positive, negative, and equivocal results. Further testing against non-WNV clinical samples (i.e., from humans or animals infected with SLEV or other flaviviruses) and samples from individuals with multiple flavivirus exposures will also be necessary to confirm the specificity of this antigen for WNV diagnostics. However, our initial results using laboratory control and field-collected sera suggest that the WNV r-EIII antigen provides good sensitivity and specificity compared to whole-virus antigens and has potential for applications directed at specific, rapid screening of samples for the presence of anti-WNV antibody.
Acknowledgments
We thank the staff of UTMB's Protein Expression and Purification Laboratory and Xiuzhen Fan for assistance with purification of the WNV r-EIII protein, Tamara Clements for conducting IgM capture assays, and Josh Hellums for assistance with the collection of human sera. We also thank Igor Romero-Sosa and Roberto Navarro-Lopez from the Mexican Agricultural Ministry (CPA) and Leonides Cardena from USDA-APHIS, Mexico, for providing and collecting horse sera.
This study was supported by funding from the Centers for Disease Control (cooperative agreements U50/CCU620538-02 and U90/CCU618754) and the NIH (RO1AI10984, P51RR000164, and NO1-AI-25489). Collection and testing of human sera was approved by the UTH IRB, Committee for the Protection of Human Subjects #HSC-SPH-03-039.
REFERENCES
- 1.Anonymous. 2003. West Nile virus activity—United States, November 20-25, 2003. Morb. Mortal. Wkly. Rep. 52:1160. [PubMed] [Google Scholar]
- 2.Beasley, D. W. C., and A. D. T. Barrett. 2002. Identification of neutralizing epitopes within structural domain III of the West Nile virus envelope protein. J. Virol. 76:13097-13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beaty, B. J., C. H. Calisher, and R. E. Shope. 1995. Arboviruses, p. 189-212. In E. H. Lennette, D. A. Lennette, and E. T. Lennette (ed.), Diagnostic procedures for viral, Rickettsial and chlamydial infections, 7th ed. American Public Health Association, Washington, D.C.
- 4.Bhardwaj, S., M. Holbrook, R. E. Shope, A. D. T. Barrett, and S. J. Watowich. 2001. Biophysical characterization and vector-specific antagonist activity of domain III of the tick-borne flavivirus envelope protein. J. Virol. 75:4002-4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2003. Epidemic/epizootic West Nile virus in the United States: guidelines for surveillance, prevention, and control, 3rd revision. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Center for Infectious Diseases, Division of Vector-Borne Infectious Diseases, Fort Collins, Colo..
- 6.Chambers, T. J., M. Halevy, A. Nestorowicz, C. M. Rice, and S. Lustig. 1998. West Nile virus envelope proteins: nucleotide sequence analysis of strains differing in mouse neuroinvasiveness. J. Gen. Virol. 79:2375-2380. [DOI] [PubMed] [Google Scholar]
- 7.Cuzzubbo, A. J., T. P. Endy, A. Nisalak, S. Kalayanrooj, D. W. Vaughn, S. A. Ogata, D. E. Clements, and P. L. Devine. 2001. Use of recombinant envelope proteins for serological diagnosis of Dengue virus infection in an immunochromatographic assay. Clin. Diagn. Lab. Immunol. 8:1150-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebel, G. D., A. P. Dupuis II, D. Nicholas, D. Young, J. Maffei, and L. D. Kramer. 2002. Detection by enzyme-linked immunosorbent assay of antibodies to West Nile virus in birds. Emerg. Infect. Dis. 8:979-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Estrada-Franco, J. G., R. Navarro-Lopez, D. W. C. Beasley, L. Coffey, A.-S. Carrara, A. P. A. Travassos da Rosa, T. Clements, E. Wang, G. V. Ludwig, A. C. Cortes, P. P. Ramirez, R. B. Tesh, A. D. T. Barrett, and S. C. Weaver. 2003. Isolation of West Nile virus in Mexico and serologic evidence of widespread circulation since July, 2002. Emerg. Infect. Dis. 9:1604-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fonseca, B. A., K. Khoshnood, R. E. Shope, and P. W. Mason. 1991. Flavivirus type-specific antigens produced from fusions of a portion of the E protein gene with the Escherichia coli trpE gene. Am. J. Trop. Med. Hyg. 44:500-508. [DOI] [PubMed] [Google Scholar]
- 11.Heinz, F. X., S. L. Allison, K. Stiasny, J. Schalich, H. Holzmann, C. W. Mandl, and C. Kunz. 1995. Recombinant and virion-derived soluble and particulate immunogens for vaccination against tick-borne encephalitis. Vaccine 13:1636-1642. [DOI] [PubMed] [Google Scholar]
- 12.Jones, L. P., H. Q. Zheng, R. A. Karron, T. C. Peret, C. Tsou, and L. J. Anderson. 2002. Multiplex assay for detection of strain-specific antibodies against the two variable regions of the G protein of respiratory syncytial virus. Clin. Diagn. Lab. Immunol. 9:633-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konishi, E., A. Fujii, and P. W. Mason. 2001. Generation and characterization of a mammalian cell line continuously expressing Japanese encephalitis virus subviral particles. J. Virol. 75:2204-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin, D. A., D. T. Muth, T. Brown, A. J. Johnson, N. Karabatsos, and J. T. Roehrig. 2000. Standardization of immunoglobulin M capture enzyme-linked immunosorbent assays for routine diagnosis of arboviral infections. J. Clin. Microbiol. 38:1823-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin, D. A., B. J. Biggerstaff, B. Allen, A. J. Johnson, R. S. Lanciotti, and J. T. Roehrig. 2002. Use of immunoglobulin M cross-reactions in differential diagnosis of human flaviviral encephalitis infections in the United States. Clin. Diagn. Lab. Immunol. 9:544-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matson, R. S. 2000. Solid-phase reagents, p. 129-163. In J. P. Goslin (ed.), Immunoassays. Oxford University Press, Oxford, United Kingdom.
- 17.Murgue, B., H. Zeller, and V. Deubel. 2002. The ecology and epidemiology of West Nile virus in Africa, Europe and Asia. Curr. Top. Microbiol. Immunol. 267:195-221. [DOI] [PubMed] [Google Scholar]
- 18.Petersen, L. R., J. T. Roehrig, and J. M. Hughes. 2002. West Nile virus encephalitis. N. Engl. J. Med. 347:1225-1226. [DOI] [PubMed] [Google Scholar]
- 19.Prince, H. E., and W. R. Hogrefe. 2003. Performance characteristics of an in-house assay system used to detect West Nile virus (WNV)-specific immunoglobulin M during the 2001 WNV season in the United States. Clin. Diagn. Lab. Immunol. 10:177-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ratteree, M. S., A. P. A. Travassos da Rosa, R. P. Bohm, Jr., F. B. Cogswell, K. M. Phillippi, K. Caillouet, S. Schwanberger, R. E. Shope, and R. B. Tesh. 2003. West Nile virus infection in nonhuman primate breeding colony, concurrent with human epidemic, southern Louisiana. Emerg. Infect. Dis. 9:1388-1394. [DOI] [PubMed] [Google Scholar]
- 21.Reiter, P., S. Lathrop, M. Bunning, B. Biggerstaff, D. Singer, T. Tiwari, L. Baber, M. Amador, J. Thirion, J. Hayes, C. Seca, J. Mendez, B. Ramirez, J. Robinson, J. Rawlings, V. Vorndam, S. Waterman, D. Gubler, G. Clarke, and E. Hayes. 2003. Texas lifestyle limits transmission of dengue virus. Emerg. Infect. Dis. 9:86-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roehrig, J. T., R. A. Bolin, and R. G. Kelly. 1998. Monoclonal antibody mapping of the envelope glycoprotein of the dengue 2 virus, Jamaica. Virology 246:317-328. [DOI] [PubMed] [Google Scholar]
- 23.Roehrig, J. T., M. Layton, P. Smith, G. L. Campbell, R. Nasci, and R. S. Lanciotti. 2002. The emergence of West Nile virus in North America: ecology, epidemiology and surveillance. Curr. Top. Microbiol. Immunol. 267:223-240. [DOI] [PubMed] [Google Scholar]
- 24.Simmons, M., K. R. Porter, J. Escamilla, R. Graham, D. M. Watts, K. H. Eckels, and C. G. Hayes. 1998. Evaluation of recombinant dengue viral envelope B domain protein antigens for the detection of dengue complex-specific antibodies. Am. J. Trop. Med. Hyg. 58:144-151. [DOI] [PubMed] [Google Scholar]
- 25.Trirawatanapong, T., B. Chandran, R. Putnak, and R. Padmanabhan. 1992. Mapping of a region of dengue virus type-2 glycoprotein required for binding by a neutralizing monoclonal antibody. Gene 116:139-150. [DOI] [PMC free article] [PubMed] [Google Scholar]