Abstract
Esophageal squamous cell carcinoma (ESCC) is an aggressive malignancy with a poor prognosis due to its highly invasive and metastatic potential. The molecular pathogenesis underlying the invasive mechanism of ESCC is not well known because of the lack of existing models to study this disease. p120-Catenin (p120ctn) and the epidermal growth factor receptor (EGFR) have each been implicated in several cancers, including ESCC. p120ctn is down-regulated in 60% of ESCC tumors, whereas EGFR is the most commonly overexpressed oncogene in ESCC. For these reasons, we investigated the cooperation between p120ctn and EGFR and its effect on ESCC invasion. We show that p120ctn down-regulation is commonly associated with EGFR overexpression. By using a three-dimensional culture system, we demonstrate that the inverse relationship between p120ctn and EGFR has biological implications. Specifically, p120ctn down-regulation coupled with EGFR overexpression in human esophageal keratinocytes (EPC1-PE) was required to promote invasion. Morphological comparison of EPC1-PE cells grown in three-dimensional culture and human ESCC revealed identical features, including significantly increased cellularity, nuclear grade, and proliferation. Molecular characteristics were measured by keratin expression patterns, which were nearly identical between EPC1-PE cells in three-dimensional culture and ESCC samples. Altogether, our analyses have demonstrated that p120ctn down-regulation and EGFR overexpression are able to mimic human ESCC in a relevant three-dimensional culture model.
Esophageal cancer is the eighth most common cancer type1 in the United States, ranking as the seventh leading cause of cancer-related mortality in the United States2 and fifth worldwide.3 Esophageal cancers are classified into two distinct histological subtypes with unique clinical behaviors: esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma. ESCC is a highly aggressive malignancy that is typically diagnosed at an advanced tumor stage.3–5 The disease develops as the result of a multistep process, arising as squamous dysplasia in the stratified squamous epithelium of the esophageal mucosa and subsequently invading through the submucosa and muscle layers of the esophagus.6 ESCC is aggressive, with a high rate of direct local invasion to adjacent organs, such as the aorta, respiratory tract, and lungs.7 The underlying molecular pathogenesis and biological features of this invasive mechanism in ESCC are not known and are understudied because of the lack of existing models available.
p120-Catenin (p120ctn; alias CTNND1) is a tumor suppressor gene whose down-regulation is correlated with poorly differentiated tumors and a metastatic phenotype in several cancers, including prostate, lung, and adenocarcinoma of the gastroesophageal junction.8–10 It has been shown previously that p120ctn is either down-regulated or lost in 35% to 60% of ESCC tumors.11,12 Epidermal growth factor receptor (EGFR) is the most commonly overexpressed oncogene in many cancer types, including ESCC,13 with the ability to modulate signal transduction pathways involved in tumor cell migration, proliferation, angiogenesis, and inhibition of apoptosis.14,15 EGFR is overexpressed in 43% to 97% of ESCC patients,15–18 and its overexpression is significantly correlated with the depth of tumor invasion.15
Because p120ctn is so often down-regulated or lost and EGFR is so often overexpressed in ESCC, we focused on investigating the cooperation between these pathways in ESCC. In addition, we used a novel and relevant three-dimensional (3D) tissue culture model using immortalized human esophageal keratinocytes (EPC1-hTERT) grown to form an epithelium on an extracellular matrix embedded with human esophageal fibroblasts19 with which to study the disease.
This model constitutes a complete stratified squamous epithelium, histologically resembles in vivo esophageal epithelium, and recreates the normal differentiation program of the esophagus.19–21 We assessed the intersection of two genetic events (namely, down-regulation of the tumor suppressor p120ctn and overexpression of the oncogene EGFR), and its ability to lead to an invasive ESCC phenotype. Our data suggest that p120ctn down-regulation and EGFR overexpression in our 3D model are able to mimic human ESCC.
Materials and Methods
Cell Lines
EPC1-hTERT and EPC2-hTERT cells were a gracious gift from Anil K. Rustgi (University of Pennsylvania, Philadelphia, PA).20 EPC1-hTERT and EPC2-hTERT cells were grown in keratinocyte serum-free medium (Invitrogen, Carlsbad, CA) supplemented with 40 μg/mL bovine pituitary extract (Invitrogen), 1.0 ng/mL EGF (Invitrogen), 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen) and maintained at 37°C and 5% CO2. Modifications of EPC1-hTERT and EPC2-hTERT cells were made with TRIPZ (Open Biosystems, Waltham, MA) and pLVX (Clontech, Mountain View, CA) vectors. All derivatives of parental EPC1-hTERT and EPC2-hTERT cells will be termed EPC1-(genetic modification) or EPC2-(genetic modification), respectively. EPC1-hTERT cells with down-regulation of p120ctn (EPC1-P cells) were generated by infection of EPC1-hTERT cells with a TRIPZ inducible lentiviral p120ctn shRNA purchased from Open Biosystems (V2THS_113295). Down-regulation of p120ctn was induced through 1 μg/mL doxycycline treatment of cells for 72 hours. The pLVX-IRES-Neo vector purchased from Clontech was used to express EGFRdel (E746-A750). EPC1-hTERT cells with overexpression of EGFR (EPC1-E cells) were generated by infection of EPC1-hTERT cells with a pLVX-IRES-Neo-EGFRdel (E746-A750) vector. EPC1-hTERT cells with down-regulated p120ctn and overexpressed EGFR (EPC1-PE cells) were generated by infection of EPC1-hTERT cells with both a TRIPZ inducible lentiviral p120ctn shRNA and a pLVX-IRES-Neo-EGFRdel (746-750) vector. EPC2-hTERT cells were modified in an identical manner as described for EPC1-hTERT cells to generate EPC2-P, EPC2-E, and EPC2-PE cells. Activated human fetal esophageal fibroblasts (FEF3) and quiescent human adult esophageal fibroblasts (HEFs) were gracious gifts from Anil K. Rustgi20 and were grown in Dulbecco's modified Eagle's medium (Mediatech, Inc., Manassas, VA) supplemented with 10% fetal bovine serum (HyClone; Thermo Scientific, Waltham, MA), 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen).
Western Blot Analysis
EPC1-hTERT cells were harvested and incubated in lysis buffer [1% Triton X-100, 1% Nonidet P-40, 150 mmol/L NaCl, 50 mmol/L Tris (pH 8.0), and protease inhibitors (Roche, Indianapolis, IN) and phosphatase inhibitors (10 mmol/L Na, 2 mmol/L Na3VO4, and 5 mmol/L Na-pyrophosphate)]. Denatured proteins were resolved by SDS-PAGE and transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore, Billerica, MA). Membranes were incubated overnight at 4°C with primary antibodies, followed by appropriate horseradish peroxidase (HRP)–conjugated secondary antibodies for 1 hour at room temperature. Proteins were visualized using ECL Prime chemiluminescence (GE Healthcare, Piscataway, NJ), according to the manufacturer's instructions.
Migration and Invasion Assays
Migration assays were performed using Boyden transwell chambers with 8-μm pore filters (BD Biosciences, San Jose, CA). Invasion assays were performed using BD Biocoat Matrigel Invasion Chambers with 8-μm pore filters (BD Biosciences). For both assays, the bottom wells of the companion plate were filled with serum-containing medium to act as a chemoattractant for the cells. Cells (2.5 × 104) were seeded in keratinocyte serum-free medium in each insert. Migration assays were incubated for 8 hours and invasion assays were incubated for 12 hours at 37°C in a humidified incubator with 5% CO2. Migratory and invasive cells were stained with a Diff-Quick stain set (Fisher Scientific, Waltham, MA). Migrated or invaded cells were imaged with an Olympus BX53 light microscope (Olympus America, Center Valley, PA) and counted in five random fields.
3D Organotypic Culture
EPC1-hTERT human esophageal keratinocytes were grown in organotypic culture on a 3D matrix, as previously described.19 An acellular collagen layer was applied to a transwell insert, followed by a collagen/Matrigel layer with 7.5 × 104 human fetal or adult esophageal fibroblasts. Matrices were allowed to contract for 7 days, at which point 5 × 105 epithelial cells were seeded on top of the matrix. Cultures were grown for an additional 8 days and then harvested. Half of the culture was fixed in 10% neutral-buffered formalin (Fisher Scientific) and paraffin embedded, whereas epithelium was peeled from the top of the other half of the culture and lysed for protein.
Patients and Specimens
Approval was obtained from the Institutional Review Board of Pennsylvania State Hershey Medical Center for all human sample experiments performed in this study. The Surgical Pathology Archive of the Pennsylvania State Hershey Medical Center was searched for ESCC cases. A total of 24 paraffin-embedded human ESCC cases with normal-appearing adjacent epithelium between 1978 and 2013 were selected for inclusion in this study. A hematoxylin and eosin (H&E)–stained section of each case was examined by an anatomical pathologist (X.Y.) to confirm the diagnosis. All of the selected ESCC cases were moderately well differentiated. Half of the specimens were resections, and the other half were biopsy specimens. Staging information was unavailable for biopsy patients. The 12 surgical resection specimens varied in size and stage, although no specimens were stage IV. Ten human normal esophageal epithelium samples used as controls were also selected for inclusion in this study. Handling of tissue specimens and protection of patient privacy followed strict policies of the Institutional Review Board.
IHC Data
Formalin-fixed, paraffin-embedded sections were divided into sections (5 μm thick). Tissue sections were baked for 1 hour at 60°C and deparaffinized in xylene. Immunohistochemistry (IHC) on human tissues was performed by The Pennsylvania State University College of Medicine (Hershey, PA) Morphological and Molecular Pathology Core Research Lab on a Dako Autostainer Plus (Dako Cytomation, Carpinteria, CA). For 3D organotypic culture sections, antigen unmasking was performed with the Retriever (Electron Microscopy Sciences, Hatfield, PA) in either 10 mmol/L sodium citrate buffer, pH 6.0, or EDTA buffer, pH 8.0. Endogenous peroxidase activity was blocked by incubation with 3% hydrogen peroxide for 6 minutes. Sections were incubated with primary antibodies overnight at 4°C, followed by 30 minutes with the appropriate ImmPRESS reagent, either HRP anti-mouse Ig or HRP anti-rabbit Ig (Vector Laboratories, Burlingame, CA), according to the manufacturer's protocol. HRP activity was then detected using diaminobenzidene substrate (Thermo Scientific) for 10 minutes before coverslipping with Permount (Fisher Scientific). The sections were evaluated on an Olympus BX53 light microscope at ×100, ×200, and ×400 magnifications, and images were captured by an Olympus DP25 camera (Olympus America) and Olympus CellSens Dimension software version 1.11.
Antibodies
The antibodies against p120ctn (number 610134; BD Transduction Laboratories, San Jose, CA) and EGFR (number 4267; Cell Signaling Technology, Beverly, MA) were used for immunoblotting (p120ctn, 1:10,000; EGFR, 1:5000) and IHC (p120ctn, 1:200; EGFR, 1:75). Phosphorylated EGFR (Y1173) was also probed with a Cell Signaling Technology antibody (1:5000) (number 4407; Beverly, MA). Antibodies for keratin (KRT) 1 (1:200) (ab11471), KRT4 (1:400) (ab51599), KRT5 (1:200) (ab75869), KRT8 (1:400) (ab49778), and KRT10 (1:400) (ab20208) were obtained from Abcam (Cambridge, MA). KRT15 (1:300) antibody (number GTX61551) was purchased from GeneTex, Inc. (Irvine, CA). The antibody for Ki-67 (1:100; number M7240) was obtained from Dako Cytomation. β-Actin (1:5000; number A5316), used as a loading control for immunoblotting, was purchased from Sigma-Aldrich Corp. (St. Louis, MO).
Scoring and Morphological Analysis
All morphological and molecular analysis and scoring was performed by an anatomical pathologist (X.Y.). A semiquantitative score system (H-score) was used in scoring IHC.22 IHC immunoreactivity was graded as follows: 0 indicates no staining; 1 indicates mild staining; 2 indicates moderate staining; and 3 indicates strong staining. A semiquantitative H-score was obtained by multiplying 3× percentage of strong staining, 2× percentage of moderate staining, and 1× percentage of weak staining, giving a range of 0 to 300. Normal-appearing adjacent epithelia were also scored. Samples with a score <200 were classified as negative (underexpression). Samples with a score >200 were classified as positive (overexpression).
Cellularity (number of nuclei in the epithelium) and nuclear size of 3D culture sections were quantitated by digital imaging of the H&E-stained sections. Five images of each section at ×200 magnification were taken. The cellularity and nuclear size were analyzed by using ImageJ software version 1.46r (NIH, Bethesda, MD). Nuclear grade was scored on a 1 to 4 scale, measured by size, shape, chromatin, and nucleolus. The proliferation index (Ki-67%) of 3D culture sections was also measured by using ImageJ software.
Statistical Analysis
A two-tailed Student's t-test was used for determining significant differences between groups. The correlation of p120ctn and EGFR expression was performed using χ2 analysis. P ≤ 0.05 was considered statistically significant.
Results
p120ctn Down-Regulation and EGFR Overexpression in Human Esophageal Keratinocytes Result in Invasion
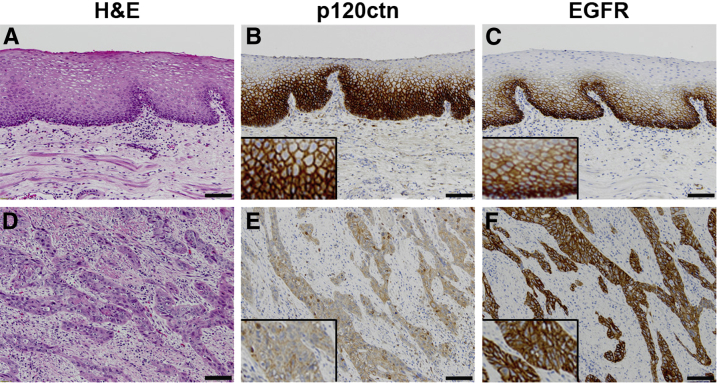
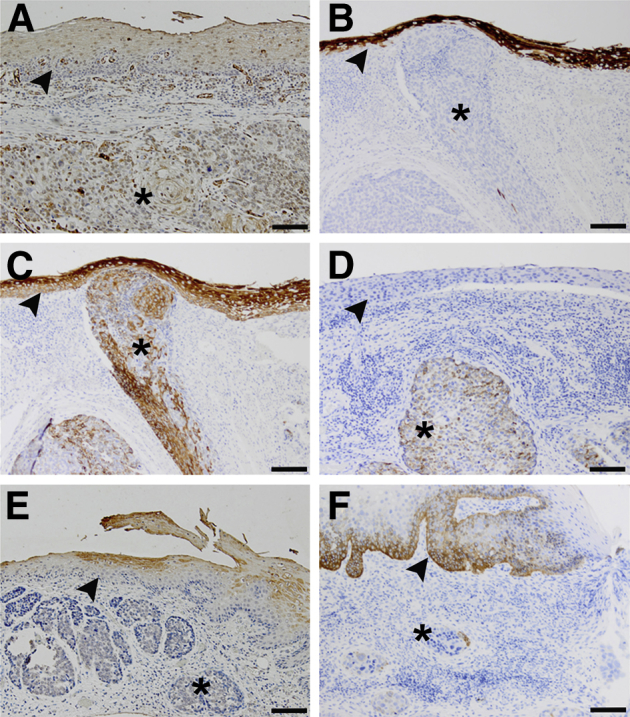
As mentioned previously, p120ctn down-regulation and EGFR overexpression are each frequently occurring events in ESCC and other cancers.8–15 IHC analysis was performed on 10 human normal esophageal epithelia samples with no evidence of dysplasia or tumor (Figure 1, A–C) and 24 human ESCC samples containing histologically normal-appearing adjacent (control) esophageal epithelia (control epithelia not shown) (Figure 1, D–F, and Supplemental Figure S1). We identified an inverse relationship between p120ctn and EGFR, with decreased expression of p120ctn in ESCC compared with control epithelium (Figure 1E and Supplemental Figure S1, B, E, and H) and increased expression of EGFR in ESCC compared with control epithelium (Figure 1F and Supplemental Figure S1, C, F, and I). In fact, p120ctn was down-regulated and EGFR was overexpressed concurrently in 67% of human ESCC samples (Table 1), making this the most abundant condition in our sample set. These data demonstrate that p120ctn down-regulation, together with EGFR overexpression, is a condition that is represented in most ESCCs analyzed; therefore, this study is focused on a significant intersection between two prominent genes in ESCC.
Figure 1.
p120ctn Down-regulation and epidermal growth factor receptor (EGFR) overexpression in human ESCC. A: H&E staining of human normal esophageal epithelium. B: p120ctn Expression in normal esophageal epithelium. C: EGFR expression in normal esophageal epithelium. D: H&E staining of human esophageal squamous cell carcinoma (ESCC). E: p120ctn Expression is decreased in human ESCC tissue. F: EGFR expression is decreased in human ESCC tissue. Insets (B, C, E, and F): Protein localization at a higher magnification. Scale bar = 100 μm.
Table 1.
Down-Regulation of p120ctn with Overexpression of EGFR Is Overrepresented in ESCC Human Samples
| Variable | p120ctn Decrease (H-score = 158) | p120ctn No change/increase (H-score = 226) |
|---|---|---|
| EGFR no change/decrease (H-score = 138) | 1/24 (4) | 3/24 (12) |
| EGFR increase (H-score = 250) | 16/24 (67) | 4/24 (17) |
Data are given as number/total (percentage).
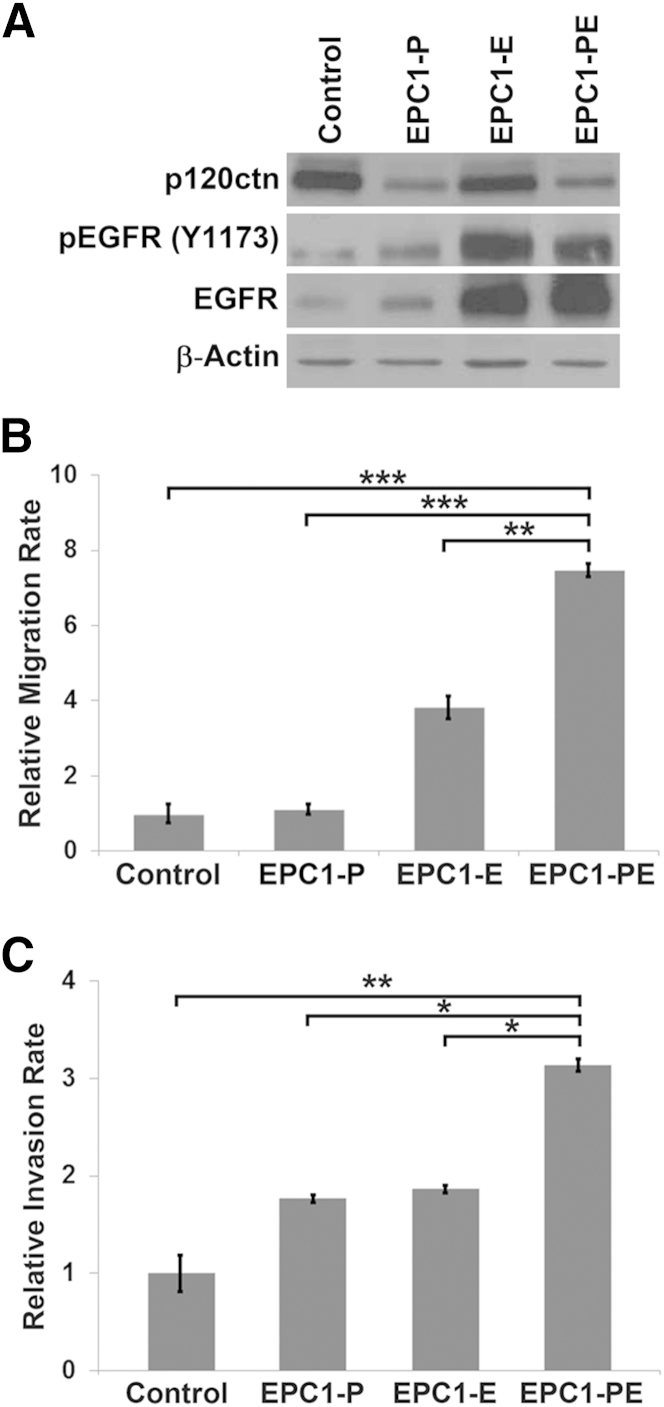
Western blot analyses show that parental EPC1-hTERT cells exhibit high expression levels of p120ctn and low levels of EGFR, making this cell line ideal to investigate the role of these genes in ESCC. Therefore, we knocked down p120ctn and overexpressed EGFR individually and in combination in EPC1-hTERT cells. The p120ctn knockdown was performed using an inducible lentiviral p120ctn shRNA in EPC1-hTERT cells, whereas EGFR overexpression was achieved by transducing cells with an EGFR del (E746-A750) mutant. This mutant form of EGFR was used to mimic the levels of EGFR activation seen in human ESCC. Western blot analysis shows that p120ctn was successfully down-regulated in EPC1-P cells and EGFR was overexpressed in EPC1-E cells (Figure 2A). We were also able to establish EPC1-PE cells, which exhibit both decreased p120ctn and increased EGFR (Figure 2A). Importantly, activated EGFR levels were increased in both EPC1-E and EPC1-PE cells, as measured by phosphorylation of EGFR at tyrosine 1173 (Figure 2A).
Figure 2.
In vitro characterization of EPC1 human esophageal keratinocytes. A: Western blot analysis of EPC1-hTERT cells demonstrates down-regulation of p120ctn in EPC1-P and EPC1-PE cells. Epidermal growth factor receptor (EGFR) is constitutively overexpressed in EPC1-E and EPC1-PE cells, and activation of EGFR, as measured by phosphorylation at Y1173, is increased in these cells. β-Actin is used as a loading control. B:In vitro Boyden chamber migration assays were performed and demonstrate significantly increased migratory capabilities of EPC1-PE cells compared with EPC1 control cells. C:In vitro invasion assays demonstrate a significantly increased ability of EPC1-PE cells to invade through Matrigel compared with EPC1 control cells. B and C: Data are presented as means ± SEM. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
The in vitro migratory and invasive capabilities of EPC1-PE cells were then assessed using Boyden chamber migration assays and Matrigel invasion assays, respectively. Results of these experiments demonstrated a significantly increased ability of EPC1-PE cells to migrate compared with EPC1-hTERT control (P < 0.001), EPC1-P (P < 0.001), or EPC1-E cells (P < 0.01) (Figure 2B). Moreover, the invasive capabilities of the EPC1-PE cells were significantly increased in comparison to EPC1-hTERT control cells (P < 0.01) and EPC1-P (P < 0.05) or EPC1-E cells (P < 0.05) (Figure 2C). These experiments were performed using EPC2-hTERT cells and yielded identical results. These data suggest that the effects seen as a result of p120ctn down-regulation and EGFR overexpression together are greater than p120ctn or EGFR modification alone.
p120ctn Down-Regulation and EGFR Overexpression Result in an Invasive Phenotype in a 3D Culture Model System That Is Independent of Fibroblast Activation
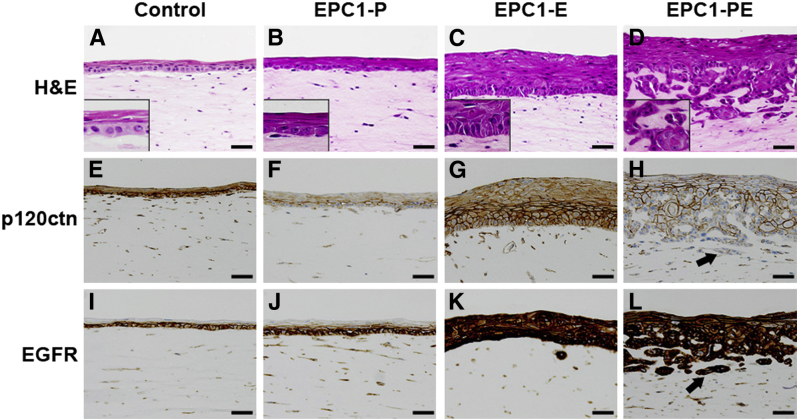
Given the increased invasive capabilities of EPC1-PE cells, we wanted to further study these esophageal keratinocytes in a more biologically relevant 3D model of ESCC. Our 3D model of the stratified squamous esophageal epithelium incorporates the interaction between genetically modified epithelial cells and fibroblasts that exist in the stromal compartment, because this interaction is vital for cancer cell invasion.20,21 Herein, activated fibroblasts (FEF3) were embedded within the matrix beneath EPC1-hTERT cells. H&E staining of 3D cultures with EPC1-hTERT control cells showed an organized epithelium (Figure 3A), whereas mild disorganization of the basal layer was seen in EPC1-P cell cultures (Figure 3B). EPC1-E cultures showed moderate disorganization with increased epithelial thickness (Figure 3C). Interestingly, only in EPC1-PE cultures did the epithelium appear to be transformed, resulting in cellular invasion into the matrix (Figure 3D). IHC staining was performed to examine the expression of p120ctn and EGFR in 3D cultures. Down-regulation of p120ctn was seen in most EPC1-P (Figure 3F) and EPC1-PE cells (Figure 3H) in 3D culture, with strong knockdown in approximately 50% of cells, compared with EPC1-hTERT control cells (Figure 3E) and EPC1-E cells (Figure 3G) in 3D culture. Interestingly, there was nearly complete loss of p120ctn in the most invasive components of the EPC1-PE cultures (Figure 3H). IHC also demonstrated overexpression of EGFR in EPC1-E (Figure 3K) and EPC1-PE cultures (Figure 3L) compared with the EPC1-hTERT control (Figure 3I) and EPC1-P cultures (Figure 3J).
Figure 3.
p120ctn and epidermal growth factor receptor (EGFR) expression in a 3D culture model system using activated esophageal fibroblasts. A: H&E staining of 3D organotypic cultures with EPC1-hTERT control cells demonstrates an organized epithelial layer. Mild disorganization occurs with EPC1-P cells in 3D culture (B) and disorganization and increased epithelial thickness occurs with EPC1-E cells (C). D: EPC1-PE cells in 3D culture result in a highly invasive phenotype. Insets (A–D): epithelial morphological features at a higher magnification. IHC staining of 3D cultures for p120ctn displays wild-type levels of p120ctn in EPC1-hTERT controls (E) and EPC1-E cultures (G). Expression of p120ctn is decreased in EPC1-P (F) and EPC1-PE 3D cultures (H). Arrow in H denotes the loss of p120ctn expression in the most invasive cells. IHC staining of 3D cultures for EGFR displays wild-type levels of EGFR in EPC1-hTERT controls (I) and EPC1-P cultures (J), but increased expression of EGFR in EPC1-E (K) and EPC1-PE cultures (L). Arrow in L denotes EGFR overexpression in the most invasive cells. Scale bar = 50 μm.
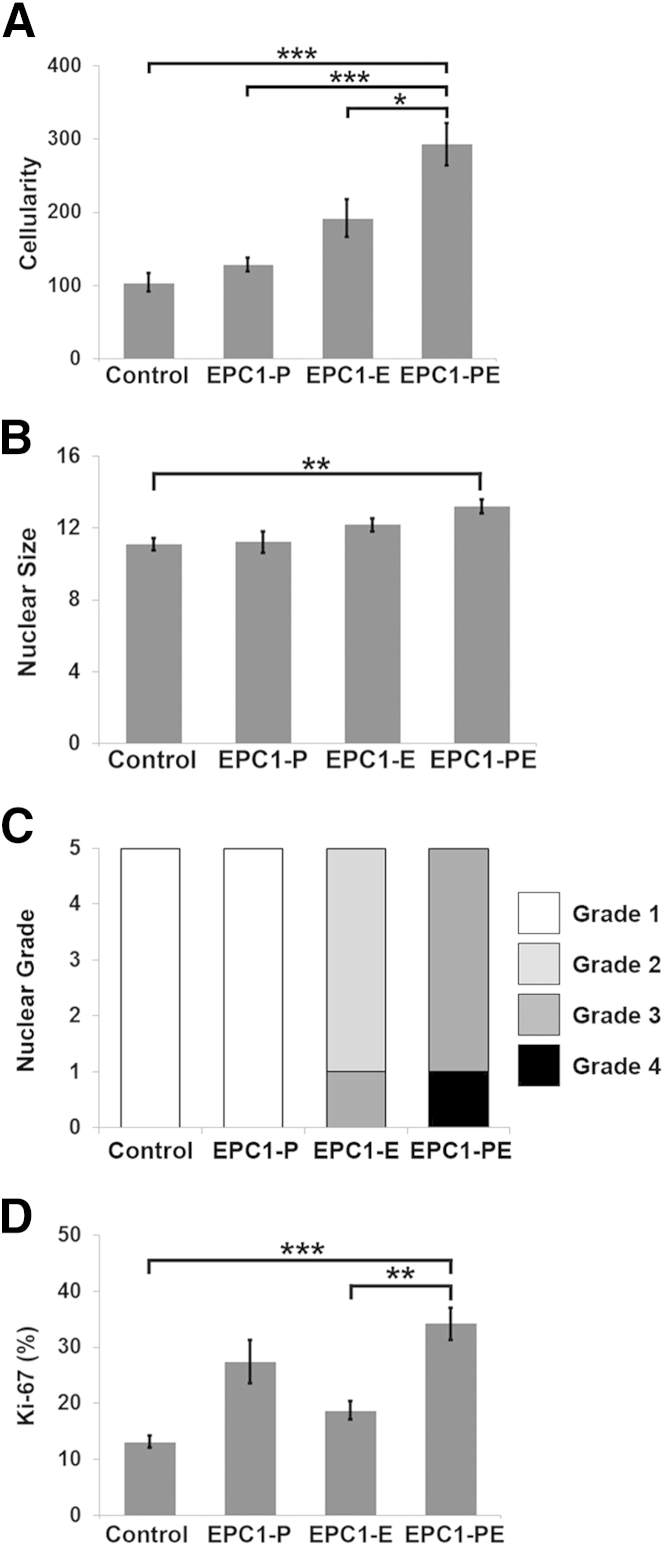
Morphological features were assessed in 3D culture samples as a measure of similarity between the EPC1-PE cells in 3D culture and human ESCC. Cellularity was significantly increased in EPC1-PE cultures compared with the EPC1-hTERT control (P < 0.001), EPC1-P (P < 0.001), and EPC1-E cultures (P < 0.05) (Figure 4A). Measurements of nuclear size demonstrated a significant increase in the nuclear size of EPC1-PE cells in 3D culture compared with EPC1-hTERT control cells in culture (P < 0.01) (Figure 4B). With nuclear grade scored on a 1 to 4 scale, EPC1-hTERT control and EPC1-P cultures had a nuclear grade of 1, whereas EPC1-E cultures were grades 2 to 3 (Figure 4C). EPC1-PE cultures were grades 3 to 4, similar to ESCC, which typically has a nuclear grade of 323 (Figure 4C). Proliferation was then measured in 3D cultures by Ki-67 staining. The percentage of proliferating cells was significantly increased in EPC1-PE cultures compared with EPC1-hTERT control (P < 0.001) and EPC1-E cultures (P < 0.01) (Figure 4D). The results of the morphological analysis of 3D cultures showed that the morphological characteristics of our 3D model when p120ctn is down-regulated and EGFR is overexpressed resembled those of human ESCC.23,24
Figure 4.
Morphological analysis of 3D cultures. A: Cellularity is significantly increased in EPC1-PE human esophageal keratinocyte 3D cultures. B: Nuclear size is significantly increased in EPC1-PE cultures compared with the control. C: Nuclear grades of EPC1-hTERT control and EPC1-P 3D cultures were considered to be grade 1, whereas EPC1-E cultures were grades 2 and 3, and EPC1-PE cultures were grades 3 and 4. D: Ki-67 staining demonstrates a significant increase in the percentage of proliferating cells in EPC1-PE cultures compared with EPC1-hTERT control and EPC1-E cultures. A, B, and D: Data are presented as means ± SEM. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
In agreement with the 3D culture studies described above, previous work reported that the source and activity levels of fibroblasts in the 3D culture model are critical in affecting the extent of tumor cell invasion and degree of tumor differentiation.20 Because our original experiments used FEF3 fibroblasts as a model of a reactive stromal component, we next investigated the extent to which altered p120ctn and EGFR expression cooperated with a normal stromal component incorporated into the matrix of our 3D system. For this, we used quiescent fibroblasts (HEFs). Histological assessment of the 3D cultures showed that EPC1-hTERT control cells formed an organized epithelium on top of the HEF-containing matrix (Figure 5A), whereas mild disorganization of the basal layer was seen in EPC1-P cultures (Figure 5B). EPC1-E cultures showed disorganization, increased epithelial thickness, and the formation of keratin pearls in the epithelium, but contained no invasion (Figure 5C). As seen in cultures with activated fibroblasts, only in EPC1-PE cultures did invasion into the quiescent HEF-containing extracellular matrix occur (Figure 5D). Activation of the quiescent HEFs was tested as a possible explanation for the apparent fibroblast-independent invasion of the EPC1-PE cells. Immunofluorescence was performed on HEF 3D cultures to examine α-smooth muscle actin, a common marker of fibroblast activation. α-Smooth muscle actin staining was negative in all HEF 3D cultures (data not shown). These data suggest that in the 3D culture system, p120ctn down-regulation and EGFR overexpression result in increased aggressive behavior of squamous epithelium in a relatively cell-autonomous manner and are not dependent on fibroblast activation state in this culture system.
Figure 5.
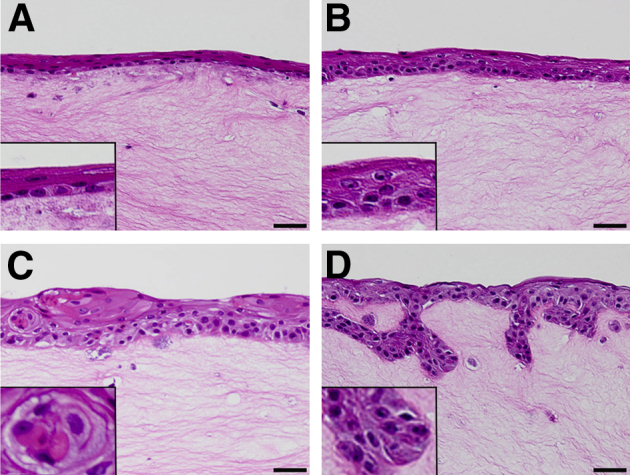
Characterization of 3D culture model system with human adult esophageal fibroblasts. A: H&E staining of 3D cultures with HEF and EPC1-hTERT cells demonstrates an organized epithelial layer. B: Mild disorganization occurs with EPC1-P cells in 3D culture. C: Cultures with EPC1-E cells display epithelial disorganization and the formation of keratin pearls. D: Cultures seeded with EPC1-PE cells that have down-regulated p120ctn and overexpression of epidermal growth factor receptor display an invasive phenotype. Insets: Epithelial morphological features at a higher magnification. Scale bar = 50 μm.
Keratin Expression Patterns of EPC1-PE 3D Cultures Show a High Level of Similarity to Human ESCC
IHC for keratins is used extensively as diagnostic tumor markers and prognostic markers, because the expression of specific keratins may correlate with epithelial differentiation states.25–27 Six keratins commonly used as molecular markers were selected for IHC analysis of ESCC and 3D culture samples, including KRT1, KRT4, KRT5, KRT8, KRT10, and KRT15.25,28,29 These keratins have been characterized as having differential expression patterns in squamous cell carcinomas, although less is known about KRT15. Specifically, KRT5 and KRT8 are both typically expressed in squamous cell carcinomas, particularly poorly differentiated cases.27,29 Likewise, KRT1 and KRT10 have differential expression in poorly differentiated squamous cell carcinomas, often with decreased and/or focal expression.27,29 Keratin 4 is used as a marker of squamous cell carcinoma and is frequently lost in ESCC compared with normal esophageal epithelia, indicative of malignant transformation.30–32 Although there is little known about the differential expression of KRT15 in tumors and, thus, its use as a diagnostic marker, a recent study demonstrated that KRT15 was generally down-regulated in moderately or poorly differentiated oral squamous cell carcinoma.27,33
IHC analysis was performed on human ESCC containing histologically normal-appearing adjacent (control) esophageal epithelia (Figure 6) and normal esophageal epithelia with no evidence of dysplasia or tumor (data not shown) to further assess the relevance of the 3D system to human ESCC. Expression levels of keratins were evaluated in human samples, with KRT1 (Figure 6A) showing positive staining with no change in expression between control epithelium and ESCC. Keratin 4 expression was lost in ESCC (Figure 6B), whereas KRT5 (Figure 6C) staining was positive with no change in expression between control epithelium and ESCC. Keratin 8 expression was gained in ESCC with weak staining (Figure 6D). Expression of KRT10 (Figure 6E) and KRT15 (Figure 6F) was decreased in ESCC compared with control epithelium.
Figure 6.
Keratin expression profiles of esophageal squamous cell carcinoma (ESCC) human samples. A: IHC staining of ESCC tumor excisions demonstrates that KRT1 expression does not change in ESCC. B: Expression of KRT4 is lost in ESCC compared with control epithelium. C: KRT5 expression does not change in ESCC. D: Control esophageal epithelium is negative for KRT8 staining, but focal and weak expression is gained in ESCC. KRT10 expression is decreased in ESCC (E), as is KRT15 (F), compared with expression levels in control epithelium. Arrowhead denotes normal-appearing adjacent epithelium (control epithelium); asterisk, ESCC. Scale bar = 100 μm.
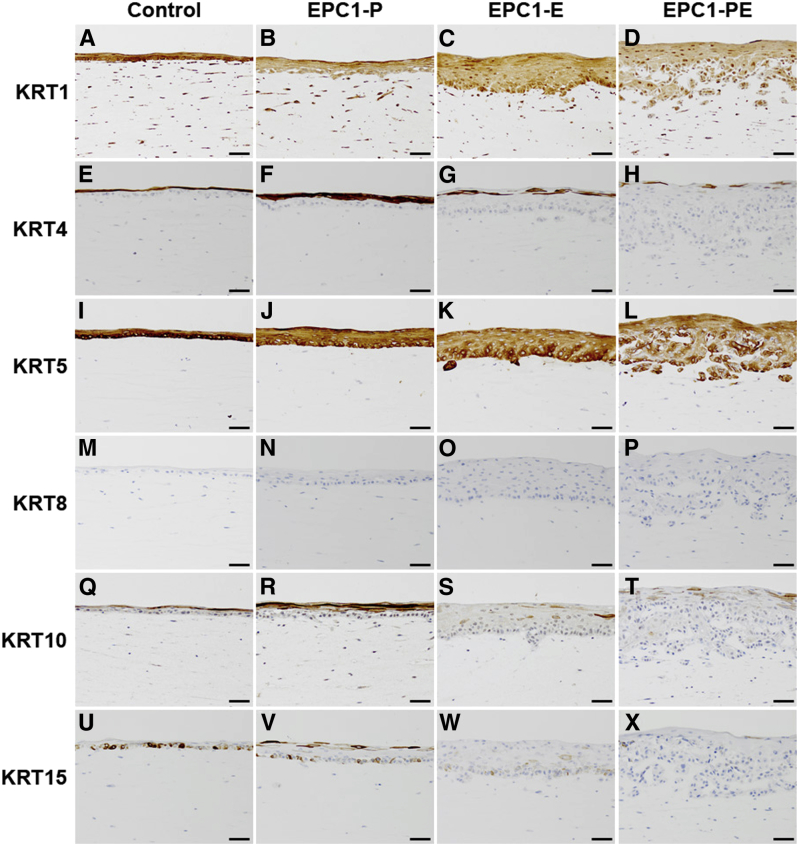
Keratin expression profiles in EPC1-PE 3D cultures were nearly identical to those seen in ESCC human samples. IHC of 3D cultures with activated fetal esophageal fibroblasts revealed that KRT1 was expressed in all 3D cultures and expression levels did not change between EPC1-hTERT control, EPC1-P, EPC1-E, and EPC1-PE cultures (Figure 7, A–D). The same was seen for KRT5, with no change in expression between EPC1-hTERT control, EPC1-P, EPC1-E, and EPC1-PE cultures (Figure 7, I–L). IHC of KRT8 showed no staining in 3D cultures (Figure 7, M–P). Keratin 4 was decreased in expression in EPC1-PE 3D cultures compared with EPC1-hTERT control, EPC1-P, and EPC1-E 3D cultures (Figure 7, E–H). Likewise, KRT10 was decreased in expression in EPC1-PE 3D cultures compared with EPC1-hTERT control, EPC1-P, and EPC1-E 3D cultures (Figure 7, Q–T). Finally, expression of KRT15 was found to be decreased in EPC1-PE 3D cultures compared with all other conditions (Figure 7, U–X).
Figure 7.
Keratin expression profiles of EPC1 3D cultures with activated fetal esophageal fibroblasts. IHC staining of 3D cultures demonstrates no change in staining for KRT1 (A–D), KRT5 (I–L), or KRT8 (M–P) between any conditions. EPC1-PE cultures have decreased expression levels of KRT4 (E–H) and KRT10 (Q–T) and loss of KRT15 (U–X), compared with EPC1-hTERT control 3D cultures. Scale bar = 50 μm.
IHC was also performed on 3D cultures with quiescent human esophageal fibroblasts to determine whether expression of keratins changed with a different stromal source. Expression of KRT10 and KRT15 was examined, both of which were decreased in expression in ESCC samples and EPC1-PE cells grown on FEF3 3D cultures. Results demonstrated that in comparison to EPC1-hTERT control cultures (Figure 8A), EPC1-P cultures (Figure 8B), and EPC1-E cultures (Figure 8C), KRT10 decreased in expression in EPC1-PE cells in 3D culture with quiescent fibroblasts (Figure 8D). Likewise, KRT15 expression was decreased in EPC1-PE cultures compared with the EPC1-hTERT, EPC1-P, or EPC1-E cells in 3D culture with quiescent fibroblasts (Figure 8, E–H). These data demonstrate that the expression patterns of KRT10 and KRT15 in EPC1-PE cells in 3D culture with HEFs are identical to those of both FEF3 3D cultures and human ESCC.
Figure 8.
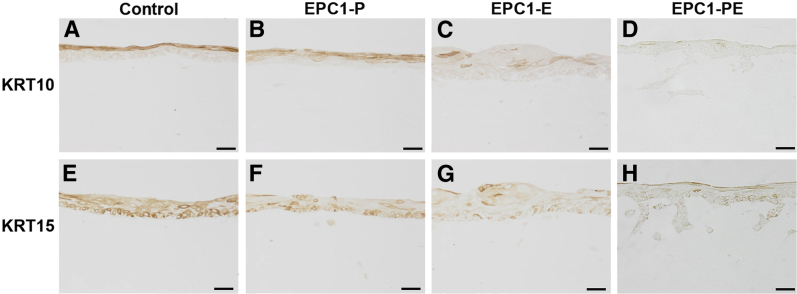
Keratin expression profiles of EPC1 3D cultures with quiescent adult human esophageal fibroblasts. IHC staining of 3D cultures demonstrates decreased expression of KRT10 in EPC1-PE cultures (D) compared with EPC1-hTERT controls (A), EPC1-P (B), or EPC1-E (C) cells in 3D culture. Expression of KRT15 is decreased in EPC1-PE 3D cultures (H) compared with EPC1-hTERT controls (E), EPC1-P (F), or EPC1-E (G) cells in 3D culture. Scale bar = 50 μm.
Together, the results of IHC analysis of keratin expression in our 3D culture model system and human ESCC showed that EPC1-hTERT control cells in 3D culture mimic control epithelium and EPC1-PE cells are able to recapitulate human ESCC in a relevant 3D culture model (Table 2).
Table 2.
Keratin Expression Profiles of EPC1-PE Cells in 3D Culture Are Nearly Identical to Human ESCC
| Variable | K1 | K4 | K5 | K8 | K10 | K15 |
|---|---|---|---|---|---|---|
| Normal epithelium | + | + | + | − | + | + |
| Control | + | + | + | − | + | + |
| ESCC | + | − | + | + | − | − |
| EPC1-PE | + | − | + | − | − | − |
+, present; –, absent.
p120ctn and EGFR Cooperate to Affect E-Cadherin Expression
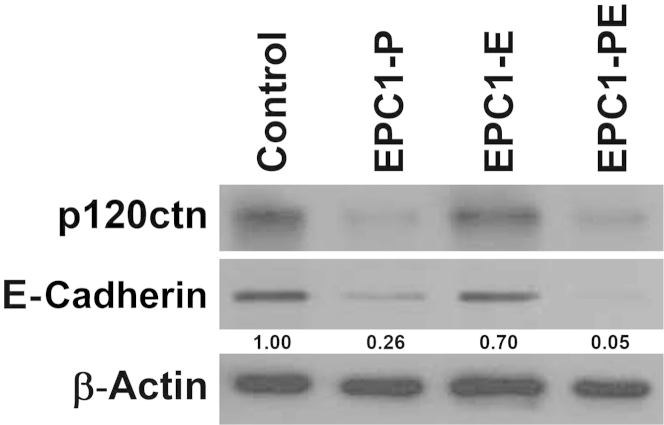
E-cadherin is classically known to be a component of the adherens junction complex and is associated with the epithelial-to-mesenchymal transition (EMT). Interestingly, p120ctn interacts with and stabilizes E-cadherin in the adherens junction complex,34–36 and it has been demonstrated previously that loss or down-regulation of p120ctn correlates with E-cadherin levels in ESCC tumors.11,12 Because our data have established that p120ctn down-regulation and EGFR overexpression induce an invasive phenotype in ESCC, we investigated if alterations in E-cadherin expression could be implicated. Western blot analysis demonstrated that in EPC1-P cells, E-cadherin expression levels were decreased compared with parental cells (Figure 9). Interestingly, in EPC1-PE cells, E-cadherin expression levels were decreased to an even greater degree (5.6-fold compared with EPC1-P cells) (Figure 9), indicating additional suppression of E-cadherin in the context of both p120ctn down-regulation and EGFR overexpression. These results directly implicate changes in p120ctn and EGFR expression in the regulation of E-cadherin expression.
Figure 9.
E-cadherin expression in EPC1-hTERT cells. Western blot analysis demonstrates a decrease in E-cadherin protein levels when p120ctn is down-regulated in EPC1-P cells and a further decrease in E-cadherin expression in EPC1-PE cells. β-Actin is used as a loading control. Band intensity was quantified by densitometry and normalized to EPC1-hTERT β-actin. Values are expressed as relative to the EPC1-hTERT control.
Discussion
Like many malignancies, ESCC develops as a result of multiple genetic alterations. The role of p120ctn as a tumor suppressor in ESCC has been firmly established through previous studies, including the development of a conditional p120ctn knockout mouse model.12 However, p120ctn loss almost certainly coincides with additional genetic event(s) in humans, resulting in the development of ESCC. EGFR overexpression is associated with early events in tumor initiation, including squamous dysplasia.37,38 Such as p120ctn, there exists evidence for a role of EGFR in ESCC. Yet, the potential synergistic relationship between this tumor suppressor and oncogene has not been explored.
We have established p120ctn down-regulation and EGFR overexpression as a common condition in ESCC, reinforcing the relevance and translation of these genes and the 3D model to the human disease. Herein, we demonstrated the development of an invasive ESCC phenotype when p120ctn is down-regulated in the context of EGFR overexpression, but not when either event occurs independently. In the 3D tissue culture model system, this aggressive phenotype persisted regardless of fibroblast activation status. This is contrary to previously published work demonstrating the key dependence of ESCC invasion on fibroblast origin.20 These studies examined EGFR, hTERT, and p53 alterations in the epithelium, and these cells may indeed need to interact with the stroma in a more prevalent way to induce tumor invasion. Because the EPC1-PE cells invade without this dependence on fibroblast activation state, we may be studying a more aggressive epithelial phenotype than has been previously examined,20 although further studies are required. Nonetheless, our data suggest the possibility that p120ctn down-regulation and EGFR overexpression act in an epithelial cell-autonomous manner to drive invasion. The prognosis for patients with ESCC is known to be poor because of the high rate of local invasion and distant metastases at the time of diagnosis. p120ctn down-regulation in squamous cell carcinomas has been correlated with poor patient prognosis,11,39,40 as has EGFR overexpression in ESCC.41,42 Our study demonstrates that the cooperation of p120ctn down-regulation and EGFR overexpression leads to an even more aggressive phenotype than either alteration alone, which may negatively affect the prognosis of ESCC patients even further. Therefore, these genes may have future prognostic and therapeutic value.
The ability of the 3D model of ESCC to lead to an invasive phenotype provides a tool with which to study the biological characteristics behind ESCC invasion. Our 3D model morphologically resembles human ESCC development, including increased cellularity and proliferation, dysplastic changes, and stromal invasion. Indeed, the development and progression of ESCC is multifactorial, and we are taking into account only two genetic events; however, on the basis of morphological and molecular analyses, we are able to mimic human disease. This 3D culture system is a relevant model of ESCC in which we can manipulate the epithelium, allowing us to further investigate the events that affect ESCC invasion. Moreover, once validated using conventional therapeutics, our 3D model has future potential use in testing novel therapeutics in a preclinical setting.
Keratins play important roles in a diverse array of cellular functions.25,27 These data suggest that keratin expression patterns may be related to progressive development of ESCC, and more recent studies even implicate keratin involvement in cancer cell invasion and metastasis.43–46 Keratin expression patterns are typically cell type, differentiation, and functional status dependent in epithelial cells.25 Epithelial tumors, including ESCC, largely maintain the features of specific keratin expression associated with the cell type of origin,25 although tumors may deviate from the patterns of their normal origin by gaining or losing keratins.26,27,29,47,48 Our data suggest specific keratin expression patterns in ESCC, with our 3D culture model displaying loss of some keratins in the most invasive EPC1-PE cells in culture. Further studies with the use of our 3D culture system are required to understand the possible role keratins may be playing in the invasive phenotype of ESCC, particularly in the context of p120ctn down-regulation and EGFR overexpression.
Our studies have demonstrated that p120ctn down-regulation and EGFR overexpression lead to an aggressive and invasive phenotype in ESCC. However, the mechanisms involved in the synergistic relationship between p120ctn down-regulation and EGFR overexpression that leads to ESCC invasion remain unknown. We have shown that E-cadherin levels are decreased with p120ctn down-regulation and even more so when EGFR is concurrently overexpressed. Loss of cell adhesion is vital in cellular migration and invasion and, therefore, E-cadherin may be playing a role in the cooperation between p120ctn and EGFR. E-cadherin is known to be associated with the EMT, and this may be a mechanism by which E-cadherin is involved in the invasive phenotype in our culture system. Further study of the EMT and commonly associated transcription factors involved in the process would be interesting in the context of p120ctn down-regulation and EGFR overexpression. Nonetheless, the interaction of pathways downstream of p120ctn down-regulation and EGFR overexpression has yet to be investigated, and we believe will be key in discovering the mechanisms behind how p120ctn and EGFR lead to an aggressive phenotype in ESCC.
The relationship between p120ctn down-regulation and EGFR overexpression that induces an invasive phenotype will aid us in developing more effective therapeutic drugs for ESCC patients but will also be applicable to many cancer types. Down-regulation or loss of p120ctn has been demonstrated in 11 other cancer types in addition to ESCC, including colon, breast, prostate, and lung.8,9,11,12,39,40,49–53 Likewise, EGFR overexpression has been indicated in these and many other cancers, including melanoma and bladder.54–58 Given the prominence of p120ctn and EGFR in these diseases, it is likely that the intersection of these two genes will be important in not only ESCC, but other cancer types as well. Therefore, our studies to investigate the cooperation between p120ctn down-regulation and EGFR overexpression, with the use of our 3D culture model system, will potentially provide insight into the biological features of invasion in a wide spectrum of diseases.
Acknowledgments
We thank Anil K. Rustgi for graciously providing reagents and cell lines, David J. DeGraff for his thoughtful feedback and input, and The Pennsylvania State University College of Medicine Molecular and Histopathology Core Research Lab for histological technical support.
Footnotes
Supported by the Pennsylvania Department of Health using Tobacco CURE funds (D.S.), NIH grant R00CA138498 (D.S.), and the Penn State College of Medicine Department of Pathology Research Award Program (X.Y. and D.S.).
Disclosures: None declared.
Supplemental Data
p120ctn Down-regulation and epidermal growth factor receptor (EGFR) overexpression in human esophageal squamous cell carcinoma (ESCC). A: H&E staining of human ESCC with normal-appearing adjacent esophageal epithelium. B: p120ctn Expression is decreased in human ESCC tissue compared with normal-appearing adjacent esophageal epithelium. C: EGFR expression is increased in human ESCC tissue compared with normal-appearing adjacent esophageal epithelium. D and G: H&E staining of human ESCC tissue. p120ctn Expression is decreased (E and H) and EGFR expression is increased (F and I) in human ESCC samples. Arrowheads in B and C denotes normal-appearing adjacent epithelium (control epithelium); asterisk in B and C, ESCC. Scale bar = 100 μm.
References
- 1.Siegel R., Naishadham D., Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Global Cancer Facts & Figures. 2nd edition. Atlanta, GA: American Cancer Society; 2011
- 3.Matuschek C., Bolke E., Zahra T., Knoefel W.T., Peiper M., Budach W., Erhardt A., Scherer A., Baldus S.E., Gerber P.A., Buhren B.A., Schauer M., Hoff N.P., Gattermann N., Orth K. Trimodal therapy in squamous cell carcinoma of the esophagus. Eur J Med Res. 2011;16:437–444. doi: 10.1186/2047-783X-16-10-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nair K.S., Naidoo R., Chetty R. Expression of cell adhesion molecules in oesophageal carcinoma and its prognostic value. J Clin Pathol. 2005;58:343–351. doi: 10.1136/jcp.2004.018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rice T.W., Blackstone E.H., Rusch V.W. 7th Edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17:1721–1724. doi: 10.1245/s10434-010-1024-1. [DOI] [PubMed] [Google Scholar]
- 6.Rubio C.A., Liu F.S., Zhao H.Z. Histological classification of intraepithelial neoplasias and microinvasive squamous carcinoma of the esophagus. Am J Surg Pathol. 1989;13:685–690. doi: 10.1097/00000478-198908000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Rustgi A.K. Models of esophageal carcinogenesis. Semin Oncol. 2006;33:S57–58. doi: 10.1053/j.seminoncol.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Wijnhoven B.P., Pignatelli M., Dinjens W.N., Tilanus H.W. Reduced p120ctn expression correlates with poor survival in patients with adenocarcinoma of the gastroesophageal junction. J Surg Oncol. 2005;92:116–123. doi: 10.1002/jso.20344. [DOI] [PubMed] [Google Scholar]
- 9.Jones J., Wang H., Zhou J., Hardy S., Turner T., Austin D., He Q., Wells A., Grizzle W.E., Yates C. Nuclear Kaiso indicates aggressive prostate cancers and promotes migration and invasiveness of prostate cancer cells. Am J Pathol. 2012;181:1836–1846. doi: 10.1016/j.ajpath.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L., Gallup M., Zlock L., Finkbeiner W., McNamara N.A. p120-Catenin modulates airway epithelial cell migration induced by cigarette smoke. Biochem Biophys Res Commun. 2012;417:49–55. doi: 10.1016/j.bbrc.2011.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung Y., Lam A.K., Luk J.M., Law S., Chan K.W., Lee P.Y., Wong J. Altered E-cadherin expression and p120 catenin localization in esophageal squamous cell carcinoma. Ann Surg Oncol. 2007;14:3260–3267. doi: 10.1245/s10434-007-9511-8. [DOI] [PubMed] [Google Scholar]
- 12.Stairs D.B., Bayne L.J., Rhoades B., Vega M.E., Waldron T.J., Kalabis J., Klein-Szanto A., Lee J.S., Katz J.P., Diehl J.A., Reynolds A.B., Vonderheide R.H., Rustgi A.K. Deletion of p120-catenin results in a tumor microenvironment with inflammation and cancer that establishes it as a tumor suppressor gene. Cancer Cell. 2011;19:470–483. doi: 10.1016/j.ccr.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ozawa S., Ueda M., Ando N., Shimizu N., Abe O. Prognostic significance of epidermal growth factor receptor in esophageal squamous cell carcinomas. Cancer. 1989;63:2169–2173. doi: 10.1002/1097-0142(19890601)63:11<2169::aid-cncr2820631117>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 14.Andl C.D., Mizushima T., Nakagawa H., Oyama K., Harada H., Chruma K., Herlyn M., Rustgi A.K. Epidermal growth factor receptor mediates increased cell proliferation, migration, and aggregation in esophageal keratinocytes in vitro and in vivo. J Biol Chem. 2003;278:1824–1830. doi: 10.1074/jbc.M209148200. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y.L., Xu K.L., Zhou Y., Gao X., Chen L.R. Correlation of epidermal growth factor receptor overexpression with increased epidermal growth factor receptor gene copy number in esophageal squamous cell carcinomas. Chin Med J (Engl) 2012;125:450–454. [PubMed] [Google Scholar]
- 16.Hanawa M., Suzuki S., Dobashi Y., Yamane T., Kono K., Enomoto N., Ooi A. EGFR protein overexpression and gene amplification in squamous cell carcinomas of the esophagus. Int J Cancer. 2006;118:1173–1180. doi: 10.1002/ijc.21454. [DOI] [PubMed] [Google Scholar]
- 17.Abedi-Ardekani B., Dar N.A., Mir M.M., Zargar S.A., Lone M.M., Martel-Planche G., Villar S., Mounawar M., Saidi F., Malekzadeh R., Hainaut P. Epidermal growth factor receptor (EGFR) mutations and expression in squamous cell carcinoma of the esophagus in central Asia. BMC Cancer. 2012;12:602. doi: 10.1186/1471-2407-12-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navarini D., Gurski R.R., Madalosso C.A., Aita L., Meurer L., Fornari F. Epidermal growth factor receptor expression in esophageal adenocarcinoma: relationship with tumor stage and survival after esophagectomy. Gastroenterol Res Pract. 2012;2012:941954. doi: 10.1155/2012/941954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalabis J., Wong G.S., Vega M.E., Natsuizaka M., Robertson E.S., Herlyn M., Nakagawa H., Rustgi A.K. Isolation and characterization of mouse and human esophageal epithelial cells in 3D organotypic culture. Nat Protoc. 2012;7:235–246. doi: 10.1038/nprot.2011.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okawa T., Michaylira C.Z., Kalabis J., Stairs D.B., Nakagawa H., Andl C.D., Johnstone C.N., Klein-Szanto A.J., El-Deiry W.S., Cukierman E., Herlyn M., Rustgi A.K. The functional interplay between EGFR overexpression, hTERT activation, and p53 mutation in esophageal epithelial cells with activation of stromal fibroblasts induces tumor development, invasion, and differentiation. Genes Dev. 2007;21:2788–2803. doi: 10.1101/gad.1544507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Unger M., Weaver V.M. The tissue microenvironment as an epigenetic tumor modifier. Methods Mol Biol. 2003;223:315–347. doi: 10.1385/1-59259-329-1:315. [DOI] [PubMed] [Google Scholar]
- 22.Yildiz-Aktas I.Z., Dabbs D.J., Bhargava R. The effect of cold ischemic time on the immunohistochemical evaluation of estrogen receptor, progesterone receptor, and HER2 expression in invasive breast carcinoma. Mod Pathol. 2012;25:1098–1105. doi: 10.1038/modpathol.2012.59. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton S.R., Aaltonen L.A., editors. Pathology and Genetics of Tumors of the Digestive System. IARC Press; Lyon, France: 2000. [Google Scholar]
- 24.Taylor P.R., Abnet C.C., Dawsey S.M. Squamous dysplasia: the precursor lesion for esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2013;22:540–552. doi: 10.1158/1055-9965.EPI-12-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karantza V. Keratins in health and cancer: more than mere epithelial cell markers. Oncogene. 2011;30:127–138. doi: 10.1038/onc.2010.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moll R. Cytokeratins as markers of differentiation in the diagnosis of epithelial tumors. Subcell Biochem. 1998;31:205–262. [PubMed] [Google Scholar]
- 27.Moll R., Divo M., Langbein L. The human keratins: biology and pathology. Histochem Cell Biol. 2008;129:705–733. doi: 10.1007/s00418-008-0435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi H., Shikata N., Senzaki H., Shintaku M., Tsubura A. Immunohistochemical staining patterns of keratins in normal oesophageal epithelium and carcinoma of the oesophagus. Histopathology. 1995;26:45–50. doi: 10.1111/j.1365-2559.1995.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 29.Singh A., Kapur S., Chattopadhyay I., Purkayastha J., Sharma J., Mishra A., Hewitt S.M., Saxena S. Cytokeratin immunoexpression in esophageal squamous cell carcinoma of high-risk population in Northeast India. Appl Immunohistochem Mol Morphol. 2009;17:419–424. doi: 10.1097/PAI.0b013e31819d3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung J.Y., Braunschweig T., Hu N., Roth M., Traicoff J.L., Wang Q.H., Knezevic V., Taylor P.R., Hewitt S.M. A multiplex tissue immunoblotting assay for proteomic profiling: a pilot study of the normal to tumor transition of esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15:1403–1408. doi: 10.1158/1055-9965.EPI-05-0651. [DOI] [PubMed] [Google Scholar]
- 31.Viaene A.I., Baert J.H. Expression of cytokeratin-mRNAs in squamous-cell carcinoma and balloon-cell formation of human oesophageal epithelium. Histochem J. 1995;27:69–78. doi: 10.1007/BF00164174. [DOI] [PubMed] [Google Scholar]
- 32.Xue L.Y., Hu N., Song Y.M., Zou S.M., Shou J.Z., Qian L.X., Ren L.Q., Lin D.M., Tong T., He Z.G., Zhan Q.M., Taylor P.R., Lu N. Tissue microarray analysis reveals a tight correlation between protein expression pattern and progression of esophageal squamous cell carcinoma. BMC Cancer. 2006;6:296. doi: 10.1186/1471-2407-6-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khanom R., Sakamoto K., Pal S.K., Shimada Y., Morita K., Omura K., Miki Y., Yamaguchi A. Expression of basal cell keratin 15 and keratin 19 in oral squamous neoplasms represents diverse pathophysiologies. Histol Histopathol. 2012;27:949–959. doi: 10.14670/HH-27.949. [DOI] [PubMed] [Google Scholar]
- 34.Davis M.A., Ireton R.C., Reynolds A.B. A core function for p120-catenin in cadherin turnover. J Cell Biol. 2003;163:525–534. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ireton R.C., Davis M.A., van Hengel J., Mariner D.J., Barnes K., Thoreson M.A., Anastasiadis P.Z., Matrisian L., Bundy L.M., Sealy L., Gilbert B., van Roy F., Reynolds A.B. A novel role for p120 catenin in E-cadherin function. J Cell Biol. 2002;159:465–476. doi: 10.1083/jcb.200205115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishiyama N., Lee S.H., Liu S., Li G.Y., Smith M.J., Reichardt L.F., Ikura M. Dynamic and static interactions between p120 catenin and E-cadherin regulate the stability of cell-cell adhesion. Cell. 2010;141:117–128. doi: 10.1016/j.cell.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 37.Okano J., Snyder L., Rustgi A.K. Genetic alterations in esophageal cancer. Methods Mol Biol. 2003;222:131–145. doi: 10.1385/1-59259-328-3:131. [DOI] [PubMed] [Google Scholar]
- 38.Mandard A.M., Hainaut P., Hollstein M. Genetic steps in the development of squamous cell carcinoma of the esophagus. Mutat Res. 2000;462:335–342. doi: 10.1016/s1383-5742(00)00019-3. [DOI] [PubMed] [Google Scholar]
- 39.Thoreson M.A., Reynolds A.B. Altered expression of the catenin p120 in human cancer: implications for tumor progression. Differentiation. 2002;70:583–589. doi: 10.1046/j.1432-0436.2002.700911.x. [DOI] [PubMed] [Google Scholar]
- 40.Schackmann R.C., Tenhagen M., van de Ven R.A., Derksen P.W. p120-Catenin in cancer: mechanisms, models and opportunities for intervention. J Cell Sci. 2013;126:3515–3525. doi: 10.1242/jcs.134411. [DOI] [PubMed] [Google Scholar]
- 41.Wang Q., Zhu H., Xiao Z., Zhang W., Liu X., Zhang X., He J., Sun K., Wang L., Xu N. Expression of epidermal growth factor receptor is an independent prognostic factor for esophageal squamous cell carcinoma. World J Surg Oncol. 2013;11:278. doi: 10.1186/1477-7819-11-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu W.W., Guo Y.M., Zhu M., Cai X.W., Zhu Z.F., Zhao W.X., Fu X.L. Clinicopathological and prognostic significance of EGFR over-expression in esophageal squamous cell carcinoma: a meta-analysis. Hepatogastroenterology. 2011;58:426–431. [PubMed] [Google Scholar]
- 43.Obermajer N., Doljak B., Kos J. Cytokeratin 8 ectoplasmic domain binds urokinase-type plasminogen activator to breast tumor cells and modulates their adhesion, growth and invasiveness. Mol Cancer. 2009;8:88. doi: 10.1186/1476-4598-8-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chu Y.W., Seftor E.A., Romer L.H., Hendrix M.J. Experimental coexpression of vimentin and keratin intermediate filaments in human melanoma cells augments motility. Am J Pathol. 1996;148:63–69. [PMC free article] [PubMed] [Google Scholar]
- 45.Hendrix M.J., Seftor E.A., Seftor R.E., Trevor K.T. Experimental co-expression of vimentin and keratin intermediate filaments in human breast cancer cells results in phenotypic interconversion and increased invasive behavior. Am J Pathol. 1997;150:483–495. [PMC free article] [PubMed] [Google Scholar]
- 46.Mizuuchi E., Semba S., Kodama Y., Yokozaki H. Down-modulation of keratin 8 phosphorylation levels by PRL-3 contributes to colorectal carcinoma progression. Int J Cancer. 2009;124:1802–1810. doi: 10.1002/ijc.24111. [DOI] [PubMed] [Google Scholar]
- 47.van Dorst E.B., van Muijen G.N., Litvinov S.V., Fleuren G.J. The limited difference between keratin patterns of squamous cell carcinomas and adenocarcinomas is explicable by both cell lineage and state of differentiation of tumour cells. J Clin Pathol. 1998;51:679–684. doi: 10.1136/jcp.51.9.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Makino T., Yamasaki M., Takeno A., Shirakawa M., Miyata H., Takiguchi S., Nakajima K., Fujiwara Y., Nishida T., Matsuura N., Mori M., Doki Y. Cytokeratins 18 and 8 are poor prognostic markers in patients with squamous cell carcinoma of the oesophagus. Br J Cancer. 2009;101:1298–1306. doi: 10.1038/sj.bjc.6605313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kurley S.J., Bierie B., Carnahan R.H., Lobdell N.A., Davis M.A., Hofmann I., Moses H.L., Muller W.J., Reynolds A.B. p120-Catenin is essential for terminal end bud function and mammary morphogenesis. Development. 2012;139:1754–1764. doi: 10.1242/dev.072769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reynolds A.B., Roczniak-Ferguson A. Emerging roles for p120-catenin in cell adhesion and cancer. Oncogene. 2004;23:7947–7956. doi: 10.1038/sj.onc.1208161. [DOI] [PubMed] [Google Scholar]
- 51.Schackmann R.C., Klarenbeek S., Vlug E.J., Stelloo S., van Amersfoort M., Tenhagen M., Braumuller T.M., Vermeulen J.F., van der Groep P., Peeters T., van der Wall E., van Diest P.J., Jonkers J., Derksen P.W. Loss of p120-catenin induces metastatic progression of breast cancer by inducing anoikis resistance and augmenting growth factor receptor signaling. Cancer Res. 2013;73:4937–4949. doi: 10.1158/0008-5472.CAN-13-0180. [DOI] [PubMed] [Google Scholar]
- 52.Skoudy A., Gomez S., Fabre M., Garcia de Herreros A. p120-Catenin expression in human colorectal cancer. Int J Cancer. 1996;68:14–20. doi: 10.1002/(SICI)1097-0215(19960927)68:1<14::AID-IJC3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 53.Liu Y., Li Q.C., Miao Y., Xu H.T., Dai S.D., Wei Q., Dong Q.Z., Dong X.J., Zhao Y., Zhao C., Wang E.H. Ablation of p120-catenin enhances invasion and metastasis of human lung cancer cells. Cancer Sci. 2009;100:441–448. doi: 10.1111/j.1349-7006.2008.01067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spano J.P., Lagorce C., Atlan D., Milano G., Domont J., Benamouzig R., Attar A., Benichou J., Martin A., Morere J.F., Raphael M., Penault-Llorca F., Breau J.L., Fagard R., Khayat D., Wind P. Impact of EGFR expression on colorectal cancer patient prognosis and survival. Ann Oncol. 2005;16:102–108. doi: 10.1093/annonc/mdi006. [DOI] [PubMed] [Google Scholar]
- 55.Masuda H., Zhang D., Bartholomeusz C., Doihara H., Hortobagyi G.N., Ueno N.T. Role of epidermal growth factor receptor in breast cancer. Breast Cancer Res Treat. 2012;136:331–345. doi: 10.1007/s10549-012-2289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Di Lorenzo G., Tortora G., D'Armiento F.P., De Rosa G., Staibano S., Autorino R., D'Armiento M., De Laurentiis M., De Placido S., Catalano G., Bianco A.R., Ciardiello F. Expression of epidermal growth factor receptor correlates with disease relapse and progression to androgen-independence in human prostate cancer. Clin Cancer Res. 2002;8:3438–3444. [PubMed] [Google Scholar]
- 57.Lipponen P., Eskelinen M. Expression of epidermal growth factor receptor in bladder cancer as related to established prognostic factors, oncoprotein (c-erbB-2, p53) expression and long-term prognosis. Br J Cancer. 1994;69:1120–1125. doi: 10.1038/bjc.1994.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boone B., Jacobs K., Ferdinande L., Taildeman J., Lambert J., Peeters M., Bracke M., Pauwels P., Brochez L. EGFR in melanoma: clinical significance and potential therapeutic target. J Cutan Pathol. 2011;38:492–502. doi: 10.1111/j.1600-0560.2011.01673.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
p120ctn Down-regulation and epidermal growth factor receptor (EGFR) overexpression in human esophageal squamous cell carcinoma (ESCC). A: H&E staining of human ESCC with normal-appearing adjacent esophageal epithelium. B: p120ctn Expression is decreased in human ESCC tissue compared with normal-appearing adjacent esophageal epithelium. C: EGFR expression is increased in human ESCC tissue compared with normal-appearing adjacent esophageal epithelium. D and G: H&E staining of human ESCC tissue. p120ctn Expression is decreased (E and H) and EGFR expression is increased (F and I) in human ESCC samples. Arrowheads in B and C denotes normal-appearing adjacent epithelium (control epithelium); asterisk in B and C, ESCC. Scale bar = 100 μm.