Abstract
Wnt glycoproteins control key processes during development and disease by activating various downstream pathways. Wnt secretion requires post-translational modification mediated by the O-acyltransferase encoded by the Drosophila porcupine homolog gene (PORCN). In humans, PORCN mutations cause focal dermal hypoplasia (FDH, or Goltz syndrome), an X-linked dominant multisystem birth defect that is frequently accompanied by ocular abnormalities such as coloboma, microphthalmia, or even anophthalmia. Although genetic ablation of Porcn in mouse has provided insight into the etiology of defects caused by ectomesodermal dysplasia in FDH, the requirement for Porcn and the actual Wnt ligands during eye development have been unknown. In this study, Porcn hemizygosity occasionally caused ocular defects reminiscent of FDH. Conditional inactivation of Porcn in periocular mesenchyme led to defects in mid- and hindbrain and in craniofacial development, but was insufficient to cause ocular abnormalities. However, a combination of conditional Porcn depletion in optic vesicle neuroectoderm, lens, and neural crest–derived periocular mesenchyme induced severe eye abnormalities with high penetrance. In particular, we observed coloboma, transdifferentiation of the dorsal and ventral retinal pigment epithelium, defective optic cup periphery, and closure defects of the eyelid, as well as defective corneal morphogenesis. Thus, Porcn is required in both extraocular and neuroectodermal tissues to regulate distinct Wnt-dependent processes during morphogenesis of the posterior and anterior segments of the eye.
Focal dermal hypoplasia (FDH) or Goltz syndrome (OMIM #305600) is an X-linked dominant syndrome resulting from defective development and interaction of ectodermal and mesodermal tissues.1,2 FDH patients exhibit variable manifestations of skin hypoplasia, hypodontia, skeletal abnormalities (including limb and digit defects, as well as reduced bone density), and defects in ocular, kidney, and abdominal wall development. FDH is caused by mutations in the porcupine homolog (Drosophila) gene (PORCN), which encodes for a highly conserved transmembrane O-acyltransferase localized to the endoplasmic reticulum.3–5 In mouse, PORCN-mediated palmitoylation is critical for trafficking and signaling activity of Wnt proteins, a family of highly conserved cysteine-rich glycoproteins.6,7 Although Wnt-independent activity of PORCN has been reported in some cases,8 a systematic analysis revealed that all Wnt proteins require palmitoylation by PORCN for their secretion.9 Several human developmental disorders have been linked to mutations in Wnt pathway components.10
Most FDH patients are heterozygous female with mosaic PORCN function, and the variable phenotypes are possibly due to individual X-chromosome inactivation. Approximately 10% of FDH patients are male, with postzygotic mosaic mutations. Studies in mouse revealed that PORCN is strictly required during gastrulation; thus, zygotic Porcn mutations are most likely lethal.11–14 Furthermore, zygotic deletion of the paternal Porcn allele in mouse accurately recapitulates the phenotypic mosaicism observed in female patients and results usually in perinatal lethality.11,15 Although FDH is considered a rare disease, with a prevalence of 1:1,000,000 based on the number of observed live births, studies in mouse suggest that prenatal lethality may affect up to 98% of PORCN mutant individuals, implying a prevalence of 1:25,000.15 Thus, FDH may affect embryonic survival much more significantly than has been acknowledged.
PORCN is expressed in the developing mouse eye and surrounding tissues, and FDH patients frequently exhibit congenital eye defects, including microphthalmia, anophthalmia, colobomata (iris, choroid, retina, and optic nerve), aniridia, and pigment abnormalities.1,3,12,16 During normal eye development, morphogenesis of the optic cup is a critical step that involves invagination of the distal optic vesicle and overlying surface ectoderm. The resulting inner layer of the optic cup develops into the neural retina, whereas the outer layer gives rise to the retinal pigment epithelium (RPE). The ventral optic cup is connected to the forebrain by the optic stalk, and both the ventral optic cup and the stalk invaginate, resulting in formation of the optic fissure. The peripheral rim of the optic cup differentiates into the ciliary body and iris in the postnatal mouse. The surrounding periocular mesenchyme consists of multiple cell lineages, both neural crest–derived and mesoderm-derived, and its interaction with the adjacent neuroepithelium and lens ectoderm is critical for differentiation of the anterior segment, patterning of the RPE, and optic stalk. Developmental problems during these processes are likely to cause the severe congenital ocular abnormalities observed in FDH.
Although the specific role of Porcn during eye development is unknown, interference with downstream components of Wnt pathways in mouse, zebrafish, chick, and frog has revealed that Wnt signaling is critical for diverse processes during eye development. Wnt proteins bind to several surface receptors, including the Frizzled family of transmembrane proteins, and activate several different pathways. In mice and humans, 19 Wnt ligands and 10 Frizzled receptors have been identified. The best characterized is the canonical Wnt–β-catenin pathway, which functions through stabilization of β--catenin, its translocation into the nucleus, and activation of TCF/LEF transcription factors. The role of the Wnt–β-catenin pathway during eye development in vertebrates is often context- and species-dependent, with functions in coordinating retinal progenitor proliferation and differentiation; development of the RPE, lens, ciliary body, and iris; and ocular angiogenesis.17–38 For example, we and others have shown that Wnt–β-catenin signaling is required for differentiation of the RPE in the mouse optic cup, most likely by direct interaction of TCF/LEF with enhancers of the key regulatory genes Mitf and Otx2.20,21,25
In noncanonical Wnt signaling, activation of Frizzled receptors leads to an increase in intracellular calcium and activation of PKC and CaMKII (Wnt/Ca2+ pathway) or activation of small GTPases (RHO, RAC1, CDC42) and JNK, with participation of VANGL and DAAM (PCP pathway).39 In addition, noncanonical Wnt proteins such as WNT4, WNT5A, and WNT11 can activate receptors other than Frizzled (eg, ROR, RYK).40 Studies in frog and zebrafish indicate that noncanonical Wnt signaling is essential for formation and/or maintenance of the eye field.38,41–44 In mouse, disruption of PCP effectors encoded by the Fuz, Wdpcp, and Int genes cause anophthalmia (Fuz, Wdpcp) and coloboma (Int), respectively; however, the underlying cellular defects are unknown.45–47
Several Wnt proteins are robustly expressed in ocular and periocular tissues, such as WNT2B, WNT3, WNT4, WNT5A, WNT5B, WNT7B, and WNT11 in the optic cup, lens, or surface ectoderm and in the periocular mesenchyme.48,49 Recent observations in chick, frog, and zebrafish demonstrate that WNT2B, WNT3A, WNT4, and WNT11 regulate eye field formation and development of the RPE and the lens.37,41,42,44,50,51 Interestingly, TGF-β signaling can act cooperatively or adversely with Wnt proteins to regulate different processes of eye development, as recently shown in chick, suggesting that interference with Wnt signaling may also affect other pathways.37,50,51 To date, however, no ocular defects resulting from deficiency of particular Wnt proteins have been described in mammals.
Redundancy among Wnt ligands, crossregulation between noncanonical and canonical Wnt pathways, and overlap of downstream components with other pathways (and the lack of appropriate tools) have made it difficult to analyze the role of Wnt proteins, particularly those acting via the noncanonical pathway in the developing mouse eye.52 To gain insight into how ocular development is affected in FDH and to understand the role of Porcn during eye development in mammals, we disrupted Porcn in ocular and extraocular tissues in mouse. Our results demonstrate that PORCN is expressed in neuroectoderm, lens, and neural crest–derived periocular mesenchyme and that it regulates closure of the optic fissure and eyelid, RPE differentiation, and corneal morphogenesis.
Materials and Methods
Mice
Animal handling and procedures were approved by the University of Utah Institutional Animal Care and Use Committee. The generation of mice carrying the floxed Porcn allele [Porcnlox/lox; kindly provided by L. Charles Murtaugh (University of Utah, Salt Lake City, UT)] has been described recently.13 For the purpose of distinction, male mice harboring the floxed Porcn allele are referred to as Porcnlox/Y. In all crosses, we maintained a mixed genetic background with C57BL/6 and CD-1 mice (Charles River Laboratories International, Hollister, CA). Porcnlox/lox mice were crossed with Six3-Cre mice (kindly provided by Yasuhide Furuta, Riken Center for Developmental Biology, Kobe, Japan),53 Wnt1-Cre mice (Jackson Laboratory, Bar Harbor, ME),54 Rx3-Cre mice [kindly provided by Milan Jamrich (Baylor College of Medicine, Houston, TX)],55 ROSA26RLacZ mice (Jackson Laboratory),56 and Axin2lacZ mice (Jackson Laboratory).57 Except as otherwise indicated, wild-type (WT) littermates without a Cre allele were used as controls.
Counting for timed pregnancies was started at embryonic day 0.5 (E0.5), the day a vaginal plug was detected. Embryos were genotyped by PCR using limb or tail DNA with primer combinations as follows. The Porcn forward primer 5′-TGAGTGCTCAAATCCCAACC-3′ and reverse primer 5′-CCAGCATGTGAAAATGTCAAC-3′ generate Porcnwt (685 bp) and Porcnlox (762 bp) amplicons, and the reverse primer 5′-GTGTCCACCATGTGCATCTC-3′ combines with the same forward primer to produce the Porcn delta (PorcnΔ) amplicon (485 bp). Primers for Six3-(generic-)Cre were forward 5′-TCGATGCACGAGTGATGAG-3′ and reverse 5′-TTCGGCTATACGTAACAGGG-3′; for Six3-(specific-)Cre, forward 5′-CCCTTACGTCCTTCCTCCTC-3′ and reverse 5′-ATGTTTAGCTGGCCCAAATG-3′; for Wnt1-Cre, forward 5′-TAAGAGGCCTATAAGAGGCGG-3′ and reverse 5′-ATCAGTCTCCACTGAAGC-3′; for Rx3-Cre, forward 5′-GTTGGGAGAATGCTCCGTAA-3′ and reverse 5′-GTATCCCACAATTCCTTGCG-3′; for SRY, forward 5′-GCTGGGATGCAGGTGGAAAA-3′ and reverse 5′-CCCTCCGATGAGGCTGATATT-3′; for ROSAR26LacZ, forward 5′-GGAGCGGGAGAAATGGATATG-3′, reverse 5′-GCGAAGAGTTTGTCCTCAACC-3′, and reverse 5′-AAAGTCGCTCTGAGTTGTTAT-3′.
Histology, Immunohistochemistry, Quantitative Analysis, and in Situ Hybridization
For histology, embryos were fixed in 4% formaldehyde, embedded in paraffin, sectioned at 5 μm and stained with hematoxylin and eosin according to standard procedures. For immunohistochemical analysis, embryo heads were fixed in 4% paraformaldehyde, cryoembedded, and sectioned (usually at 12 μm). If necessary, cryostat sections were treated for antigen retrieval with hot citrate buffer (pH 6) or 1% Triton X-100. The following primary antibodies or markers were used for immunohistochemistry: BRN-3 (dilution 1:50; sc-6026; Santa Cruz Biotechnology, Dallas, TX), caspase-3 (dilution 1:200; 559565; BD Pharmingen, San Jose, CA), CDC42 (dilution 1:150; 2462; Cell Signaling Technology, Danvers, MA), cytokeratin 12 (dilution 1:50; sc-17101; Santa Cruz Biotechnology), F-actin/phalloidin (dilution 1:500; A12379; Life Technologies, Carlsbad, CA), β-galactosidase (β-gal) (dilution 1:5000; Cappel 855976; MP Biomedicals, Aurora, OH), β-gal [dilution 1:750; a generous gift from Nadean Brown (University of California, Davis)], HES1 (dilution 1:1000; a generous gift from Nadean Brown), phospho-histone H3 (dilution 1:1000; Upstate 06-570; EMD Millipore, Billerica, MA), phospho-c-JUN (dilution 1:500; 3270; Cell Signaling Technology), laminin (dilution 1:2000; ab30320; Abcam, Cambridge, MA), LEF1 (dilution 1:100; C12A5; Cell Signaling Technology), MITF (dilution 1:400; X1405M; Exalpha Biologicals, Shirley, MA), OTX2 (dilution 1:1500; AB9566; EMD Millipore), PAX2 (dilution 1:100; Covance PRB-276P; BioLegend, Dedham, MA), PAX6 (dilution 1:300; AB2237; EMD Millipore), PITX2 (dilution 1:1000; PA1020-100; Capra Science, Angelholm, Sweden), β-tubulin class III (dilution 1:2000; Covance PRB-435P; BioLegend), and VSX2 (dilution 1:300; X1180P; Exalpha Biologicals). These were used in combination with the following secondary antibodies: goat anti-rabbit, goat anti-mouse, or goat anti-rat, conjugated with Alexa Fluor 488, 568, or 647 (dilution 1:1000; Life Technologies), donkey anti-goat conjugated with tetramethylrhodamine isothiocyanate (dilution 1:500; 705-025-147; Jackson ImmunoResearch, West Grove, PA), and donkey anti-sheep conjugated with tetramethylrhodamine isothiocyanate (dilution 1:500; 713-165-003; Jackson ImmunoResearch). For quantitative analysis, the number of caspase-3 and p-histone H3–labeled cells in the central and posterior optic cup was counted in alternating sagittal sections for each eye at E11.5, and counts were analyzed using Student's t-test. Whole-mount in situ hybridization using digoxigenin-labeled Tbx5 and Vax2 riboprobes was performed as described previously.18 Except as otherwise indicated, at least three embryos were analyzed per genotype, time point, and marker.
Epifluorescence images were captured using an Olympus (Tokyo, Japan) XM10 camera on an upright Olympus BX51 microscope and were processed using Adobe Photoshop CS3 software. Confocal images were captured using an Olympus FV1000 system and were processed using ImageJ version 1.43u (NIH, Bethesda, MD) and Adobe Photoshop CS3 software. All other images were captured using an Olympus MicroFire digital microscope camera U-CMAD3 mounted on the aforementioned BX51 microscope or on an Olympus SZX12 stereomicroscope.
Results
Porcn Hemizygosity Can Result in Diverse Ocular Defects during Embryonic Development
In mouse, male Porcn-mutant embryos do not survive beyond gastrulation, because of a failure in mesoderm formation, and female heterozygous mutants typically die perinatally.11–13 To investigate the role of Porcn during ocular development, we performed tissue-specific and temporally controlled inactivation, using females homozygous for a floxed Porcn allele and males heterozygous for Six3-Cre.13,53 Six3-Cre is activated in the retina and ventral optic stalk at E9.0.53 Genotyping of extraocular tissues revealed that this cross generated male conditional mutant embryos with two distinct genotypes: Porcn mutants harboring a floxed Porcn allele that developed normally (Porcnlox/Y;Six3-Cre; n = 19) (Figure 1B and Table 1), and, unexpectedly, Porcn-mutant embryos with a combination of floxed and delta alleles, which in rare cases exhibited some developmental abnormalities (PorcnΔ/lox/Y;Six3-Cre in Table 1). Because Six3-Cre can be expressed ectopically,58 this suggests postzygotic mosaic recombination of the floxed Porcn allele.
Figure 1.
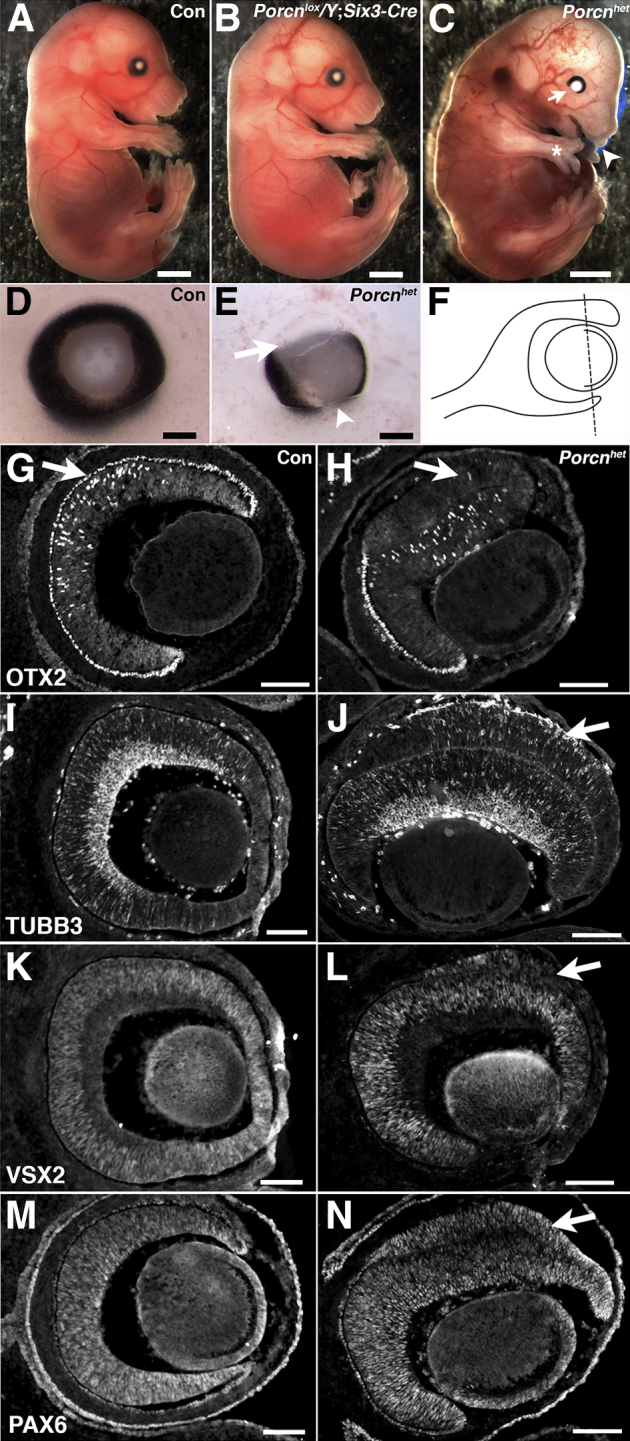
Retinal pigment epithelium (RPE) to retina transdifferentiation and closure defect of the optic fissure in heterozygous Porcn female embryos. A: Wild-type (WT) embryo at E15.5. B: Male embryo with Six3-Cre–mediated deletion of Porcn (Porcnlox/Y;Six3-Cre). C: Female embryo with heterozygous deletion of Porcn in the absence of Six3-Cre [genotype: PorcnΔ/lox (Porcnhet)] exhibiting syndactyly and absence of digits in the forelimb (asterisk), abnormal craniofacial development (arrowhead), and coloboma (arrow). D: WT eye at E13.5. E:Porcnhet embryonic eye at E13.5 with coloboma (arrowhead) and severe pigment loss in the dorsal RPE (arrow) (genotype: PorcnΔ/lox;Six3-Cre). F: Location of the sagittal sections in the remaining panels. G–N: Immmunohistological analysis of embryos at E13.5. G: OTX2 is expressed in the RPE (arrow) and, at this age, becomes normally expressed in progenitor cells and photoreceptor precursors in WT retina. H: In Porcnhet embryos, the dorsal RPE has lost widespread expression of OTX2 (arrow), but starts to acquire the retina-specific, dispersed pattern of OTX2 expression (genotype: PorcnΔ/lox;Six3-Cre). I: Expression of the retina-specific marker TUBB3 in differentiating neurons in WT retina. J: TUBB3 is misexpressed in the dorsal RPE of Porcnhet eyes (arrow) (genotype: PorcnΔ/lox). K: Expression of VSX2 in retinal progenitor cells in a control eye. L: In Porcnhet eyes, the dorsal RPE is multilayered and VSX2 is ectopically up-regulated (arrow) (genotype: PorcnΔ/lox;Six3-Cre). M: In control eyes, PAX6 expression is present in the retina, in the optic cup margins, RPE, anterior epithelium of the lens, and surface ectoderm. N: In Porcnhet eyes, PAX6 is expressed in all ocular tissues, including the dorsal RPE (arrow) (genotype: PorcnΔ/lox;Six3-Cre). Scale bars: 1 mm (A–C); 100 μm (D, E, G–N). Con, wild-type control.
Table 1.
Ocular and Extraocular Abnormalities in Porcn-Mutant Mice at Embryonic Age E12.5 to E18.0
| Genotype | OFCD, % | RPE defects,∗ % |
Open eyelid,† % | Cranio-facial defects, % | MHB defects, % | Normal, % | Total embryos, No. | ||
|---|---|---|---|---|---|---|---|---|---|
| Severe | Moderate | Mild | |||||||
| Porcnlox/Y; Six3-Cre | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 19 |
| PorcnΔ/lox/Y; Six3-Cre | 0 | 0 | 0 | 7 | 13 | 3 | 3 | 90 | 29 |
| Porcnhet‡ | 10 | 5 | 18 | 42 | 43 | 28 | 3 | 42 | 60 |
| PorcnΔ/lox | 8 | 4 | 31 | 22 | 20 | 35 | 4 | 31 | 26 |
| PorcnΔ/lox/+; Six3-Cre | 12 | 6 | 9 | 53 | 56 | 24 | 3 | 50 | 34 |
| Porcnlox/Y; Wnt1-Cre | 2 | 0 | 0 | 0 | 0 | 100 | 100 | 0 | 61 |
| Porcnlox/Y; Rx3-Cre | 13 | 0 | 20 | 50 | 0 | 95 | 17 | 0 | 30 |
| Porcnlox/Y; Wnt1-Cre; Rx3-Cre | 72 | 52 | 69 | 18 | 100 | 100 | 100 | 0 | 29 |
| Porcnlox/+; Wnt1-Cre; Rx3-Cre | 9 | 0 | 9 | 82 | 0 | 0 | 0 | 22 | 23 |
| WT | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 109 |
MHB, Mid- and hindbrain; NE, normal; OFCD, optic fissure closure defect; WT, wild-type.
Severe RPE defects: embryos with transdifferentiated RPE or almost complete loss of pigment (eg, Figure 1E). Moderate RPE defects: eyes with severe pigment gaps or reduction of pigment circumferentially (eg, Figure 2D). Mild RPE defects: eyes with small gaps or a subtle reduction of pigment circumferentially (eg, Figure 2A), analyzed between E15.5 and E18.0.
The eyelid closure defect was determined at E16.5 to E18.0.
PorcnΔ/lox and PorcnΔ/lox;Six3-Cre combined.
Most PorcnΔ/lox/Y;Six3-Cre embryos seemed unaffected (Table 1) and when born showed normal life expectancy. However, when PorcnΔ/lox/Y;Six3-Cre were used as breeders, they recurrently generated female embryos exhibiting variable ocular and extraocular defects and harboring a combination of recombined and unrecombined Porcn alleles (Porcnhet) (Figures 1 and 2 and Table 1). The observed abnormalities can occur in the absence of Six3-Cre (Figures 1C and 2A, and PorcnΔ/lox in Table 1), most likely because of prezygotic deletion of the paternal Porcn allele by ectopic CRE activity.15,58 In accord with previous studies,11,13,15 we observed extraocular abnormalities with variable frequency and severity, including abnormal craniofacial development and limbs with digit loss and/or fusion (Figure 1C), as well as skin hypoplasia, open ventral body wall, limb atrophy, tail defects (curly or short), and posterior truncation (not shown). Female embryos harboring Six3-Cre in addition to recombined and unrecombined Porcn alleles exhibited similar defects; however, we could not distinguish between pre- or postzygotic deletion of Porcn (Figures 1E and 2C, and PorcnΔ/lox/+;Six3-Cre in Table 1). Because the developmental defects are consistent across all genotypes, we show here representative examples of female offspring harboring a deleted Porcn allele with or without Six3-Cre, referred to hereafter as Porcnhet.
Figure 2.
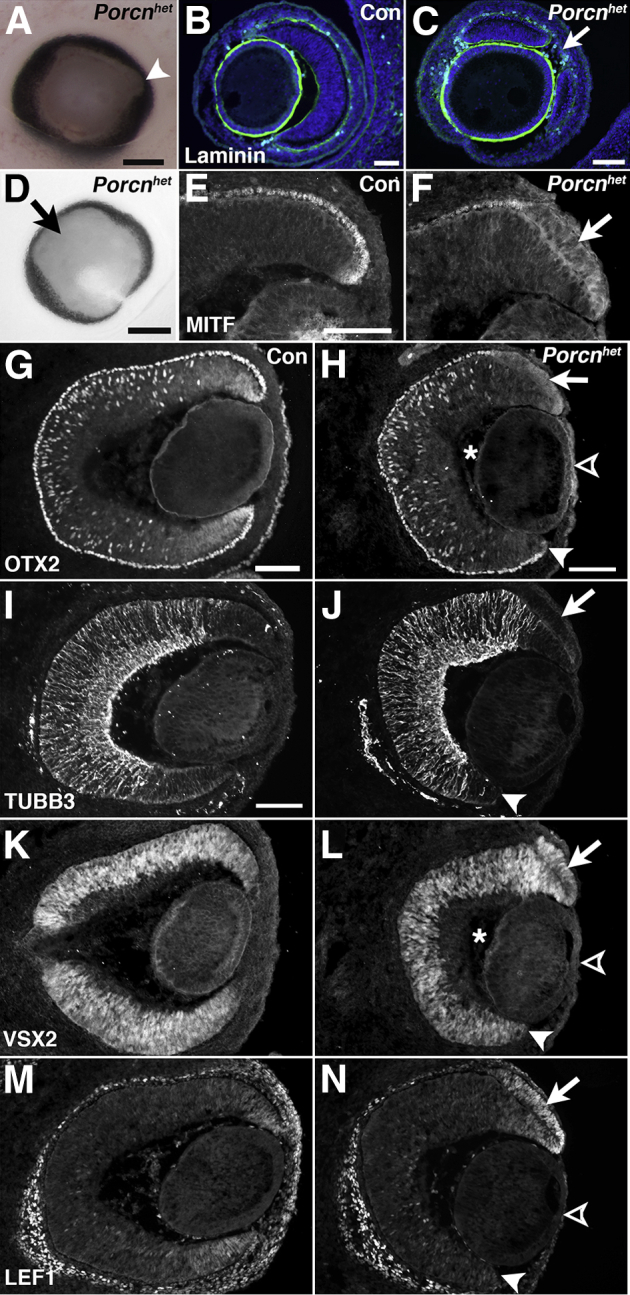
Porcnhet embryos exhibit pigment defects in the optic cup periphery at E13.5. A:Porcnhet eyes exhibit patches of depigmentation, often found temporally (arrowhead) (genotype: PorcnΔ/lox). B: Sagittal view of the ocular periphery shows continuous, basal laminin expression surrounding the lens and the retina and RPE in WT embryo. C: In Porcnhet embryos, unpigmented patches arise from tissue gaps evident by interrupted laminin expression (arrow) (same eye as in A). D: Colobomatous Porcnhet eye with circumferential decrease in pigment (arrow) (genotype: PorcnΔ/lox;Six3-Cre). E: Coronal view of expression of the RPE-specific protein MITF in WT dorsal RPE. F: In Porcnhet embryos, MITF expression is decreased in the RPE of the optic cup periphery (arrow) (same eye as in D). G: Coronal view of OTX2 expression in WT eye. H: OTX2 expression is slightly decreased in the dorsal RPE of Porcnhet embryos (arrow; same eye as in D). I: Expression of the retina-specific marker TUBB3 in WT retina. J: In Porcnhet eyes, TUBB3 is normally expressed in the retina and absent in the dorsal RPE periphery (arrow) (same eye as in D). K: Coronal expression of the retina-specific transcription factor VSX2 in WT retina. L: VSX2 is ectopically up-regulated in the dorsal RPE of Porcnhet embryos (arrow) (same eye as in D). M: In WT embryos at E13.5, LEF1 is expressed in the periphery of the retina and RPE, and in periocular mesenchyme. N: In Porcnhet embryos, LEF1 expression in the ventral optic cup (arrowhead) and in corneal mesenchyme (open arrowhead) is absent but robustly up-regulated in the dorsal RPE (arrow; same eye as in D). H, J, L, and N: Note the shortening of the ventral optic cup (arrowhead), reduction of the vitreous (asterisks), and thinning of the cornea (open arrowhead) in Porcnhet embryonic eyes. Scale bars: 200 μm (A and D); 100 μm (B, C, E, G, H, and I).
Notably, we detected ocular abnormalities in Porcnhet embryos that have not been described previously and that may reflect, at least in part, extraocular Porcn deletion by ectopic CRE activity. Colobomata were detected in 10% of the embryos (n = 60) (Figure 1, C and E and Table 1) and pigment defects of the optic cup with varying severity in up to 42% (Figure 1E and Table 1). To examine the pigment defects in more detail, we investigated whether differentiation of the RPE is disturbed. Normally, RPE specification and differentiation are regulated by the key regulatory transcription factors MITF and OTX2.59–61 Interference with MITF or OTX2 expression during early eye development results in microphthalmia and coloboma; the RPE transdifferentiates into retina because of a loss of RPE-specific morphology and gene expression, hyperproliferation, and ectopic up-regulation of retina-specific genes (reviewed by Fuhrmann et al62). In the developing eye, OTX2 is normally expressed in both the RPE and retina; however, the expression pattern differs, reflecting different requirements in each tissue.61,63 In Porcnhet embryos exhibiting severe pigment defects in the dorsal optic cup, RPE-specific expression of OTX2 is down-regulated (Figure 1, G and H). The affected RPE acquires a retinal fate by up-regulation of the retina-specific genes Tubb3 and Vsx2 (Figure 1, I–L), whereas Pax6 is robustly expressed (Figure 1, M and N). These results indicate that large regions of the RPE in Porcnhet eyes can transdifferentiate into neural retina.
Porcnhet embryos also exhibited a range of less severe pigment abnormalities in the optic cup periphery between E13.5 and E15.5, particularly as unpigmented patches or circumferential pigmentation loss (Figure 2 and Table 1). Unpigmented patches can arise from missing tissue, as shown by interrupted laminin expression, separating two distinct areas of retinal tissue adjacent to the gap (Figure 2, A–C). These gaps may be caused by asymmetrical growth and defective fusion of the optic cup margins. Circumferential pigment loss ranges in severity, resulting from pigmentation defects of the dorsal peripheral RPE (Figure 2D) and absence of tissue in the ventral optic cup periphery (see Anterior Segment Abnormalities). Loss of dorsal pigmentation is accompanied by a decrease in expression of the key regulatory genes Mitf and Otx2 (Figure 2, E–H). Interestingly, the retina-specific gene Tubb3 is not ectopically up-regulated (Figure 2, I and J), in contrast to Vsx2 (Figure 2, K and L). The HMG box transcription factor Lef1 can be a target and readout for active canonical Wnt signaling in many tissues, including the developing eye. Normally, LEF1 is expressed in very few cells in the embryonic retina but is robustly present in the peripheral RPE, corneal mesenchyme, and optic cup periphery (Figure 2M).64 In Porcnhet embryos, LEF1 is strongly expressed in the dorsal, abnormal RPE periphery suggesting that Wnt–β-catenin signaling is still active (Figure 2N). These observations suggest that the moderately affected RPE periphery does not undergo a complete transdifferentiation into retina, but rather acquires an intermediate fate. The overall morphology of Porcnhet eyes exhibits other abnormalities; the vitreous is reduced (Figure 2, H and L), and the developing cornea appears thinner (Figure 2, H, L, and N). Furthermore, the ventral optic cup in Porcnhet embryos can exhibit hypoplasia in the periphery (Figure 2, H, J, L, and N), evident as loss of LEF1 expression (Figure 2N).
Porcn Deletion in Multiple Tissues Is Required to Cause Ocular Defects
So far, our results showed that hemizygosity of Porcn can result in variable developmental ocular abnormalities with low penetrance. We hypothesized that a further decrease in PORCN expression would increase the incidence and consistency of ocular defects. In addition, several Wnt proteins are expressed in ocular tissues (optic cup, lens) and surrounding periocular mesenchyme, but it is unclear in which tissue Wnt expression is required.48,49 Because the periocular mesenchyme provides important factors for optic fissure closure and RPE differentiation,65–67 we inactivated Porcn in neural crest–derived periocular mesenchyme using Wnt1-Cre mice (Porcnlox/Y;Wnt1-Cre54) (Supplemental Figure S1A). Porcnlox/Y;Wnt1-Cre embryos showed no ocular abnormalities (n = 61); however, we observed severe, fully penetrant defects in the mid- and hindbrain region (Figure 3, A and B, and Table 1). This is consistent with deletion of other Wnt pathway components, including WLS (alias GPR177), Wnt1 and Wnt3, Wnt5a, and β-catenin.68–72 In particular, the integral membrane protein GPR177 could be considered the closest functional Wnt pathway component to PORCN; it is required for proper trafficking and secretion of Wnt proteins and requires PORCN-mediated palmitoylation of Wnt proteins for recognition.9,73,74 Mutant embryos with Wnt1-Cre–mediated disruption of Wls (alias Gpr177) fail to form the isthmic organizer, resulting in abnormal mid- and hindbrain development.68 We therefore hypothesize that the isthmic organizer may not be established properly in Porcnlox/Y;Wnt1-Cre embryos; further studies are needed for confirmation. In addition, Porcnlox/Y;Wnt1-Cre embryos show abnormal development of facial primordia, cleft palate, and a mild, fully penetrant median cleft lip (Figures 3B, 4B, and 4G).13 Previous studies have demonstrated that inappropriate levels of Wnt–β-catenin and noncanonical Wnt signaling are associated with cleft lip and palate in both humans and mice. Specifically, Wnt proteins are expressed in the mesenchyme of the facial prominences, in the oral ectoderm, and in the palatal shelf mesenchyme and regulate proliferation, cell death, differentiation, and cell migration75 (reviewed by He and Chen76).
Figure 3.
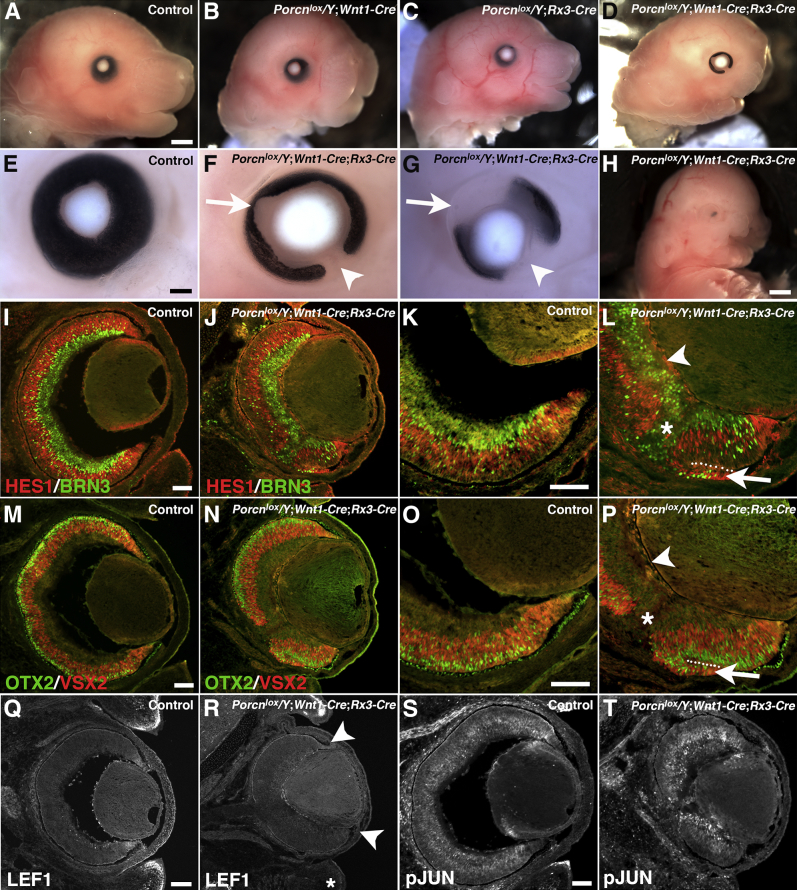
Conditional Porcn inactivation in ocular and neural crest–derived mesenchyme induces pigment abnormalities and transdifferentiation of the retinal pigment epithelium (RPE) at E15.5. A–D: Lateral views of WT (A), Porcnlox/Y;Wnt1-Cre (B), Porcnlox/Y;Rx3-Cre (C), and Porcnlox/Y;Wnt1-Cre;Rx3-Cre embryos (D). E: WT eye. F–H: Range of severities of ocular phenotypes found in Porcnlox/Y;Wnt1-Cre;Rx3-Cre embryos: colobomata (arrowhead) with largely circumferential pigment loss (F, arrow); more severe pigment loss dorsally (G); or, in rare cases, completely absent pigment (H). I–T: Coronal views. I and K: Expression of HES1 (red) in retinal progenitor cells and BRN-3 (green) in differentiating ganglion cells in WT retina, with WT ventral optic cup shown at higher magnification (K). J: In Porcnlox/Y;Wnt1-Cre;Rx3-Cre retina, expression of HES1 and BRN-3 shows a normal distribution. L: Higher magnification of the Porcnlox/Y;Wnt1-Cre;Rx3-Cre ventral optic cup reveals ectopic up-regulation of HES1 and BRN-3 in the RPE (arrow). The dotted line marks the apical borders of retina and transdifferentiated RPE; note optic stalk (asterisk) and reduced vitreous (arrowhead). M and O: Expression of OTX2 (green) in retinal progenitors and photoreceptor precursors and of VSX2 (red) in retinal progenitors in WT retina, with ventral optic cup shown at higher magnification (O). N: Expression of OTX2 and VSX2 appears normal in Porcnlox/Y;Wnt1-Cre;Rx3-Cre retina. P: Higher magnification of the Porcnlox/Y;Wnt1-Cre;Rx3-Cre ventral optic cup reveals ectopic up-regulation of VSX2 and retinal OTX2 expression in the RPE (arrow); the dotted line marks the apical borders of retina and transdifferentiated RPE; note optic stalk (asterisk) and reduced vitreous (arrowhead). Q: LEF1 expression in WT retina. R: In Porcnlox/Y;Wnt1-Cre;Rx3-Cre embryos, LEF1 is down-regulated in the dorsal and ventral optic cup periphery (arrowheads) and in the ventral eyelid mesenchyme (asterisk). S: p-JUN expression in WT retina and lens. T: p-JUN in Porcnlox/Y;Wnt1-Cre;Rx3-Cre embryos appears normal. Scale bars: 1 mm (A and H); 200 μm (E); 100 μm (I, K, M, O, Q, and S).
Figure 4.
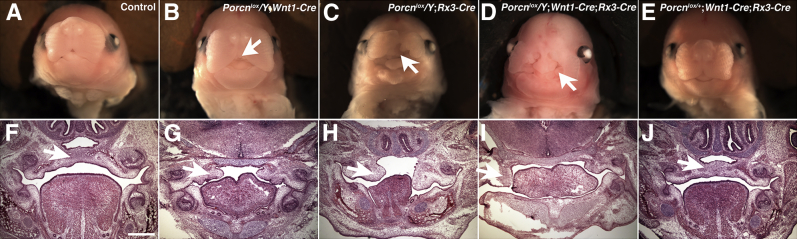
Craniofacial defects in conditional Porcn-mutant embryos. Gross (A–E) and histological (F–J) images of control and mutant embryos at E15.5. Hematoxylin and eosin stain. A: Frontal view of WT embryo. B:Porcnlox/Y;Wnt1-Cre embryos have a mild cleft in the medial region of the upper lip (arrow). C: Porcnlox/Y;Rx3-Cre embryos have cleft lips (arrow). D:Porcnlox/Y;Wnt1-Cre;Rx3-Cre embryos exhibit craniofacial abnormalities including severe cleft lips (arrow). E: Normal craniofacial development in Porcnlox/+;Wnt1-Cre;Rx3-Cre embryos. F: In control embryos, a uniform palate is formed (arrow). G: In Porcnlox/Y;Wnt1-Cre embryos, the palatal shelves rise horizontally but do not fuse (arrow). H:Porcnlox/Y;Rx3-Cre embryos have cleft palate (arrow). I: In Porcnlox/Y;Wnt1-Cre;Rx3-Cre embryos, the palatal shelves do not rise above the tongue and they fail to fuse (arrow). J: Palatogenesis is normal in Porcnlox/+;Wnt1-Cre;Rx3-Cre embryos (arrow). Scale bar = 500 μm.
Porcnlox/Y;Six3-Cre embryos did not exhibit any obvious ocular abnormalities (Figure 1B), and it is possible that the timing of Porcn inactivation in ocular tissues may be critical. To examine the role of Porcn in ocular tissues earlier, we used another eye-specific Cre line, Rx3-Cre, that becomes active in the presumptive retina and RPE of the optic vesicle at E8.75 and can be ectopically expressed in the lens vesicle (Supplemental Figure S1B).55 Rx3-Cre is also expressed in the epithelium of the medial nasal prominence.55 In the present study, Porcnlox/Y;Rx3-Cre mutants (Figure 3C) occasionally exhibited ocular phenotypes such as mild circumferential loss of pigment and coloboma (Table 1). The penetrance was similar to that of Porcnhet embryos, suggesting that extraocular PORCN expression may be sufficient to prevent a higher incidence of ocular defects. In addition, most of the mutant embryos developed bilateral fusion defects in the upper lip and cleft palate, possibly because of Cre activity in the oral ectoderm (Figures 3C, 4C, and 4H and Table 1).
So far, our observations suggested that PORCN may be required in multiple ocular and extraocular tissues. We therefore performed Porcn inactivation using a combination of Wnt1-Cre and Rx3-Cre lines (Supplemental Figure S1C). Indeed, Porcnlox/Y;Wnt1-Cre;Rx3-Cre embryos showed a much higher penetrance of ocular defects and, similar to Porcnlox/Y;Wnt1-Cre embryos, had full penetrance of abnormal mid- and hindbrain development, as well as cleft lip and palate (Figures 3D, 4D, 4I, and 5B and Table 1). In rare, extreme cases, the eyes were almost completely devoid of pigmentation (Figure 3H). Female embryos heterozygous for conditional Porcn inactivation using both Cre lines (Porcnlox/+;Wnt1-Cre;Rx3-Cre) consistently and more frequently (82%) exhibited mild RPE defects (Table 1). Overall, our results demonstrate that the incidence and severity of pigment defects are markedly increased when the level of PORCN expression in retina, RPE, lens, and neural crest–derived mesenchyme is further reduced. (Figure 3, F–H, and Table 1). In addition, affected eyes can appear slightly microphthalmic (Figure 3E). Analysis of the eye perimeter revealed that mutant eyes are 12% smaller than control eyes: 87.84 ± 5.74% in mutants (n = 18 eyes from 9 embryos) versus 100 ± 7.98% in controls (n = 30 eyes from 16 embryos) (P < 0.0001). Further analysis of Porcnlox/Y;Wnt1-Cre;Rx3-Cre embryos revealed transdifferentiation of the dorsal RPE into retina, acquisition of intermediate fate or local loss of tissue (n = 6 embryos at E15.5) (not shown), which is similar to Porcnhet eyes and results in a failure to form the ciliary body and iris epithelium (iris hypoplasia; see Anterior Segment Abnormalities).
Figure 5.
Patterning of the ventral optic cup in Porcnlox/Y;Wnt1-Cre;Rx3-Cre eyes. A–J: Lateral (A and B) and sagittal (C–J) views at E12.5. A: Control embryo. B:Porcnlox/Y;Wnt1-Cre;Rx3-Cre embryo shows coloboma as a narrow gap in the ventral optic cup. In addition, defects in craniofacial (arrow) and mid- and hindbrain development (arrowhead) are detectable. C: Control eye. D:Porcnlox/Y;Wnt1-Cre;Rx3-Cre eye with open optic fissure (arrow). E: Laminin (green) expression in the basement membrane surrounds ocular tissues in control eyes. Nuclei are counterstained with DAPI (blue). F: Persistent laminin expression in the ventral optic cup of Porcnlox/Y;Wnt1-Cre;Rx3-Cre eyes (arrow). G: Colabeling of control ventral optic cup with VSX2 (red) and MITF (green). H:Porcnlox/Y;Wnt1-Cre;Rx3-Cre ventral optic cup shows normal expression of VSX2 in retina and MITF in RPE. I: PAX2 expression (red) in ventral optic cup of control embryos. J: Porcnlox/Y;Wnt1-Cre;Rx3-Cre ventral optic cup exhibits normal PAX2 expression. K–N: Optic cups at E10.5. K: Lateral view of Vax2 mRNA expression in ventral optic cup of control embryos. L: Vax2 mRNA is expressed in the Porcnlox/Y;Wnt1-Cre;Rx3-Cre ventral optic cup (arrow). M: Tbx5 mRNA is expressed in the dorsal optic cup of control eyes. N: In Porcnlox/Y;Wnt1-Cre;Rx3-Cre eyes, Tbx5 mRNA expression is slightly reduced in the dorsal optic cup (arrow). Scale bar = 100 μm.
Recently, it has been shown that Fz5, Fz8, and Sfrp1/2 compound mutants exhibit accelerated generation of early-born ganglion cells and misregulation of HES1 expression, which is required for progenitor expansion during neurogenesis in the embryonic mouse retina.77,78 We therefore performed immunohistochemistry for markers labeling different populations of retinal progenitors (HES1, VSX2, OTX2) and early differentiating retinal cell types (BRN-3 for ganglion cells and OTX2 for photoreceptor precursors) in Porcnlox/Y;Wnt1-Cre;Rx3-Cre eyes. We observed no obvious difference in the developing central retina at E15.5 (Figure 3, J and N, and Supplemental Figure S2), which suggests that retinal neurogenesis at this stage proceeds largely normally, consistent with typical retinal expression of OTX2 and TUBB3 in Porcnhet eyes (Figure 2, H and J). However, the ventral RPE ectopically up-regulated the retina-specific expression of HES1, VSX2, OTX2, and BRN-3, indicating that it undergoes transdifferentiation into retina (Figure 3, K, L, O, and P). Furthermore, similar to Porcnhet eyes, the vitreous is strongly reduced in Porcnlox/Y;Wnt1-Cre;Rx3-Cre eyes, and lens and retina are tightly attached to each other (Figure 3, L and P). Consistent with an effect of Porcn disruption on Wnt–β-catenin signaling, we observed a down-regulation of LEF1 in the optic cup periphery and ventral eyelid mesenchyme (Figure 3, Q and R). p-JUN, the putative downstream effector of several signaling pathways (including noncanonical Wnt), appears to be unaffected in ocular and extraocular tissues in Porcnlox/Y;Wnt1-Cre;Rx3-Cre embryos (Figure 3, S and T).
The Defect in Closure of the Optic Fissure Is Associated with Reduced Wnt–β-Catenin Activity in the Underlying Periocular Mesenchyme
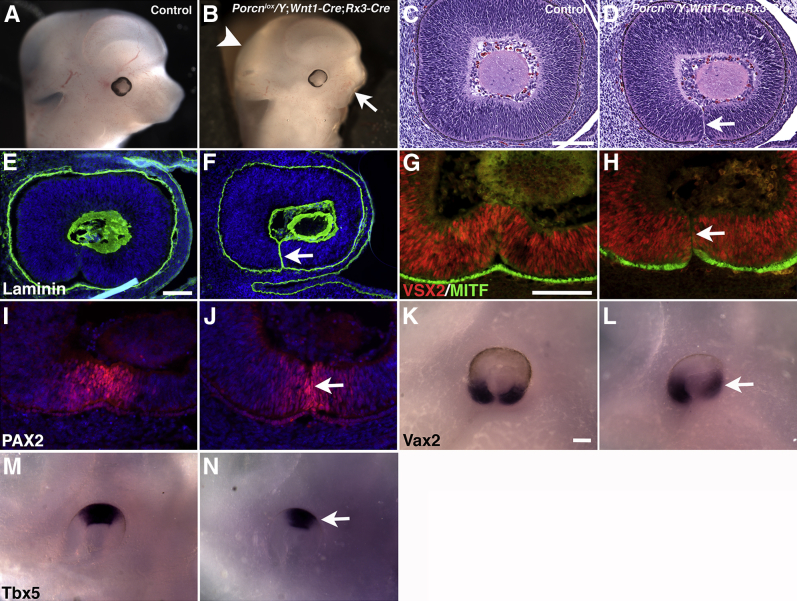
Porcnlox/Y;Wnt1-Cre;Rx3-Cre embryos showed a high incidence of colobomata (72%) (Figure 3, F and G, and Table 1). During normal optic cup morphogenesis, the laterally growing edges of the RPE and retina at the margin of the optic fissure align against each other to fuse and form a continuous optic cup.79,80 The optic fissure forms as a ventral groove by asymmetric invagination extending from the vitreal side to the proximal junction with the forebrain, which allows mesenchymal cells to migrate inward and form the hyaloid vasculature. Closure of the optic fissure in mouse starts at approximately E10.5 and is complete by E12.5. The etiology of coloboma is complex. Coloboma can result from genetic, extracellular signaling, or environmental perturbations within the optic cup neuroepithelium and periocular mesenchyme. Many genes critical for closure of the optic fissure have been identified in humans and in animal models, including Pax2, Pax6, Vax2, Pitx2, JNK1/2, and multiple Wnt pathway components21,77,81,82 (reviewed by Gregory-Evans et al83 and Chang et al84); however, the cellular mechanisms of the closure defects remain largely unresolved. To gain more insight into the possible mechanisms causing coloboma in Porcnlox/Y;Wnt1-Cre;Rx3-Cre embryos, we examined eyes at E12.5, right after completion of optic fissure closure and when any closure defect first becomes obvious. Besides ocular defects, abnormalities in hindbrain and craniofacial development are detectable at this stage (Figure 5, A and B). The most common type of coloboma in Porcnlox/Y;Wnt1-Cre;Rx3-Cre mutants was characterized by a narrow gap in the ventral optic cup, suggesting that the defect occurs late during the closure process [9/11 (82%) embryos] (Figure 5, B–D). The closure defect in Porcnlox/Y;Wnt1-Cre;Rx3-Cre mutants is confirmed by persistent expression of the basement membrane component laminin (Figure 5, E and F).
Previous studies (eg, Westenskow et al,21 Martinez-Morales et al,61 Lee et al,85 and Tang et al86) have shown that genetic models of RPE transdifferentiation can develop colobomata. In Porcnlox/Y;Wnt1-Cre;Rx3-Cre embryos at E15.5, the ventral RPE transdifferentiates (Figure 3, L and P), suggesting that improper RPE differentiation could cause the closure defect in the optic fissure. However, we observed that RPE differentiation appears to be normal at E12.5; MITF is present, and VSX2 expression is confined to the ventral retina in Porcnlox/Y;Wnt1-Cre;Rx3-Cre embryos (Figure 5, G–J). Furthermore, PAX2 and Vax2 are normally expressed in the ventral optic cup of Porcnlox/Y;Wnt1-Cre;Rx3-Cre eyes (Figure 5, I–L). Expression of the dorsal patterning marker Tbx5 is slightly reduced (Figure 5, M and N), which we also observed in Porcnlox/Y;Rx3-Cre mutants (not shown) that exhibit a low penetrance of colobomata (Table 1). Apoptotic cell death and proliferation are important processes during optic cup morphogenesis84,87,88; however, we observed no significant changes in the number of caspase-3 and p-histone H3–labeled cells in Porcnlox/Y;Wnt1-Cre;Rx3-Cre eyes during the closure process (Supplemental Figure S3). Thus, RPE differentiation, dorsoventral patterning, apoptosis, and proliferation in the optic cup in Porcnlox/Y;Wnt1-Cre;Rx3-Cre embryos show minimal changes, if any, and are unlikely to cause a defect in closure of the optic fissure. It is possible, however, that subsequent defects (eg, transdifferentiation of the ventral RPE) could contribute to formation of a wider gap in the optic fissure at E15.5 (Figure 3).
Next, we investigated whether changes in Wnt pathway activity occur in Porcnlox/Y;Wnt1-Cre;Rx3-Cre eyes. The number of LEF1-positive cells was reduced in periocular mesenchyme underlying the ventral optic cup of Porcnlox/Y;Wnt1-Cre;Rx3-Cre embryos (Figure 6, A and B). We also examined expression of Axin-2 in the Axin2lacZ knock-in reporter line57 by detection of β-gal. In Porcnlox/Y;Wnt1-Cre;Rx3-Cre embryos, β-gal expression was reduced in the mesenchyme underlying the optic fissure (n = 5) (Figure 6, C and D). Interestingly, β-gal expression was decreased in the RPE as well, consistent with transdifferentiation into retina at E15.5. Furthermore, Wnt–β-catenin activity is required for maintenance of PITX2, a key transcription factor that is robustly expressed in the periocular mesenchyme (neural crest and mesoderm) and required for anterior segment development.65,89,90 Mutations in Pitx2 cause Axenfeld–Rieger syndrome and can result in coloboma. However, PITX2 appears to be normally expressed in the periocular mesenchyme underneath the optic fissure of Porcnlox/Y;Wnt1-Cre;Rx3-Cre embryos (Figure 6, E and F). Furthermore, we observed no obvious changes in apical distribution of F-actin (Figure 6, G and H) or in expression of p-JUN (Figure 6, I and J). This suggests that apicobasal polarity and some aspects of noncanonical Wnt signaling appear to be normal in Porcnlox/Y;Wnt1-Cre;Rx3-Cre embryos. However, the effects on LEF1 expression and Axin2 reporter activation indicate that Wnt–β-catenin signaling in the ventral optic cup and underlying periocular mesenchyme is compromised.
Figure 6.
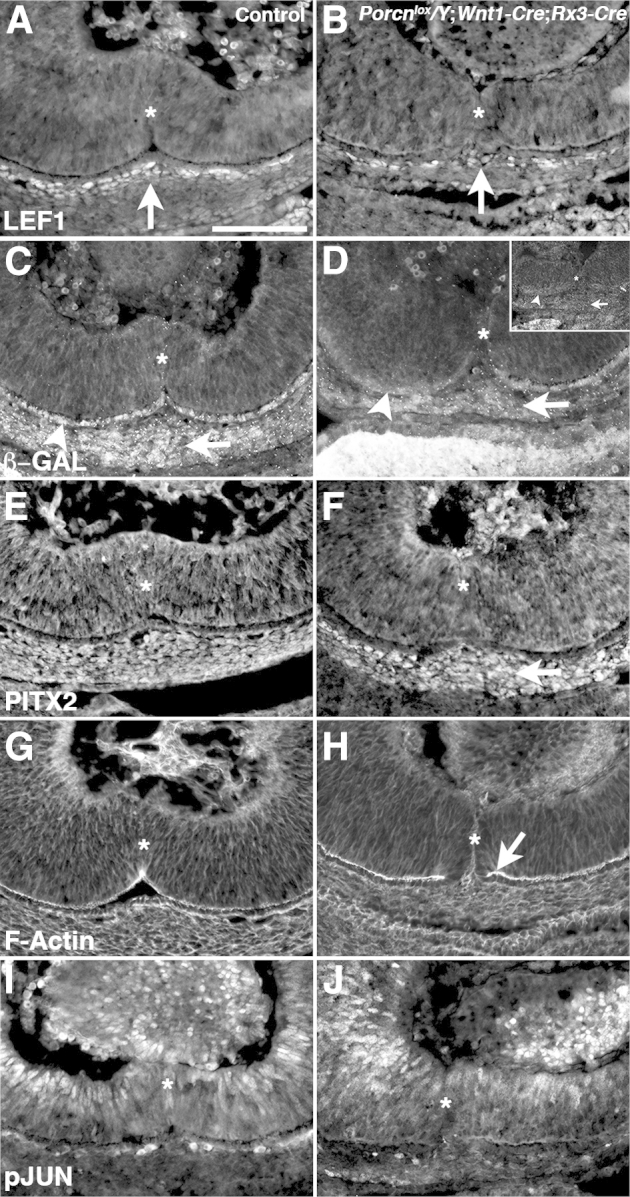
Porcn deficiency affects Wnt–β-catenin activity in the periocular mesenchyme underlying the optic fissure (asterisks), shown in sagittal views at E12.5. A: In control embryos, LEF1 is expressed in the periocular mesenchyme underlying the ventral optic cup, particularly in two to three cell layers underneath the optic fissure (arrow). B: In Porcnlox/Y;Wnt1-Cre;Rx3-Cre embryos, LEF1 is expressed in fewer cells underneath the optic fissure (arrow). C: β-Galactosidase expression in Axin2lacZ knock-in reporter embryos shows Axin-2 activation in the ventral retinal pigment epithelium (RPE) (arrowhead) and underlying mesenchyme (arrow). D: Activation of the Axin2 reporter is reduced (main image) or absent (inset) in the ventral RPE (arrowhead) and mesenchyme (arrow) in mutant embryos. E and F: PITX2 is expressed in the periocular mesenchyme underlying the optic fissure in WT embryos (E) and appears normal in Porcnlox/Y;Wnt1-Cre;Rx3-Cre embryos (F, arrow). G: Apical F-actin expression in the ventral optic cup of control embryos. H: F-actin expression shows a normal, continuous apical distribution in the Porcnlox/Y;Wnt1-Cre;Rx3-Cre ventral retina and RPE (arrow). I and J: Expression of phosphorylated c-JUN (p-JUN) in the ventral optic cup did not differ obviously between control (I) and Porcnlox/Y;Wnt1-Cre;Rx3-Cre (J) embryos. Scale bar = 100 μm.
Abnormalities in Anterior Segment Development in Porcnlox/Y;Wnt1-Cre;Rx3-Cre Eyes
Further analysis of Porcnlox/Y;Wnt1-Cre;Rx3-Cre embryos showed that eyelid morphogenesis and some aspects of anterior segment development can be disturbed (Figure 7). Because of perinatal lethality, it is not possible to examine formation of the chamber angle and trabecular meshwork, which develop postnatally (reviewed by Cvekl and Tamm91). Normally, in the optic cup periphery at E17.5, the ciliary body starts to fold and some extension of the iris epithelium is detectable (Figure 7C). In Porcnlox/Y;Wnt1-Cre;Rx3-Cre eyes, pigmentation and early formation of ciliary body and iris are disturbed (Figure 7D), consistent with the patterning abnormalities of the optic cup margins observed at earlier ages (Figures 2 and 3).
Figure 7.
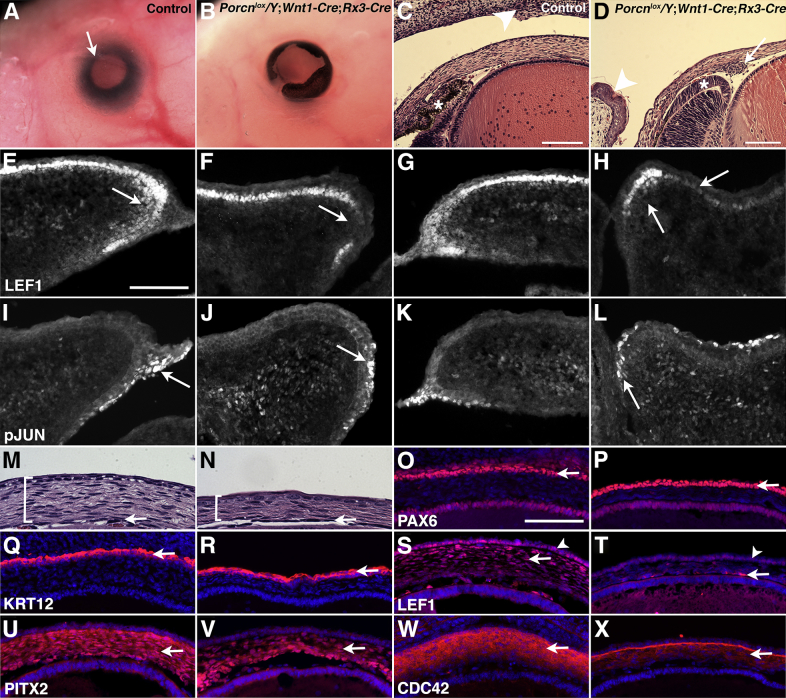
Eyelid closure defect and abnormal corneal development in Porcnlox/Y;Wnt1-Cre;Rx3-Cre mutants. A: Control embryo at E17.5 with closed eyelid (arrow). B:Porcnlox/Y;Wnt1-Cre;Rx3-Cre mutant at E17.5 with open eyelid, coloboma, and pigment abnormalities in the optic cup periphery. C: Frontal section of the chamber angle of control eye at E17.5 with closed eyelid (arrowhead) and developing ciliary body and iris (asterisk). Hematoxylin and eosin stain. D:Porcnlox/Y;Wnt1-Cre;Rx3-Cre anterior segment at E17.5 shows growth-arrested eyelid folds (arrowhead), abnormal development of the peripheral retinal pigment epithelium (RPE) in the absence of ciliary body and iris (asterisk), and ectopic cells in the chamber angle (arrow). E: LEF1 expression in palpebral epidermis, mesenchyme (arrow), and conjunctival epithelium in upper eyelid of control eye at E15.5. F: In Porcnlox/Y;Wnt1-Cre;Rx3-Cre embryos, LEF1 expression in the palpebral epidermis and conjunctival epithelium of the upper eyelid is maintained, but is absent in the mesenchyme (arrow). G: Expression of LEF1 in the lower eyelid in control embryo. H: In the lower eyelid of Porcnlox/Y;Wnt1-Cre;Rx3-Cre embryos, LEF1 expression in the palpebral epidermis is decreased (right arrow), along with decreased expression in the mesenchyme (left arrow). I and K: Detection of p-JUN in the periderm, periderm extension (arrow), and conjunctival epithelium of upper (I) and lower (K) eyelids in control embryo at E15.5. J and L: In Porcnlox/Y;Wnt1-Cre;Rx3-Cre eyelids at E15.5, p-JUN appears to be normal (arrows). M and N: Histological cross section of the cornea at E17.5 in control (M) and Porcnlox/Y;Wnt1-Cre;Rx3-Cre eyes (N). Corneal thickness is indicated by a bracket; the corneal endothelium is marked by an arrow. O–X: Embryos at E15.5. O: PAX6 (red) expression in the corneal epithelium of control eyes (arrow). Nuclei are counterstained with DAPI (blue). P: PAX6 is present in the corneal epithelium in Porcnlox/Y;Wnt1-Cre;Rx3-Cre eyes (arrow). Q and R: Expression of cytokeratin-12 in the corneal epithelium of control (Q) and Porcnlox/Y;Wnt1-Cre;Rx3-Cre (R) embryos shows no difference (arrows). S: In control eyes, LEF1 is expressed in the corneal mesenchyme (arrow) and epithelium (arrowhead). T: LEF1 is decreased in the Porcnlox/Y;Wnt1-Cre;Rx3-Cre corneal mesenchyme, except for some cells between lens and cornea (arrow). Reduced LEF1 expression in the corneal epithelium is variable (arrowhead). U: Robust PITX2 expression in control corneal mesenchyme (arrow). V: In Porcnlox/Y;Wnt1-Cre;Rx3-Cre eyes, PITX2 is present in the hypocellular corneal mesenchyme (arrow). W: The Rho GTPase CDC42 is robustly expressed in the corneal mesenchyme in control eyes (arrow). X: CDC42 expression is severely reduced in Porcnlox/Y;Wnt1-Cre;Rx3-Cre corneal mesenchyme (arrow). Scale bar = 100 μm.
The eyelid closure defect in Porcnlox/Y;Wnt1-Cre;Rx3-Cre embryos is completely penetrant (100%) (Table 1), and is not observed in conditional mutants with a single Cre allele, indicating that Porcn is required in multiple ocular surface tissues. Normally, between E11.5 and E15.5, the dorsal and ventral periocular ectoderm invaginate, and the resulting eyelid folds grow toward each other across the surface of the eye. A projection of the outer, peridermal layer extends from the eyelid margins across the cornea until the periderm extensions meet and fuse. In Porcnlox/Y;Wnt1-Cre;Rx3-Cre mutants, the periocular ectoderm has invaginated at E15.5 (Figure 3); however, growth of the eyelid folds is arrested and periderm extensions are not detectable (Figure 7, D–L). LEF1 is normally present in conjunctival epithelial cells, palpebral epidermal cells, and in mesenchymal cells, consistent with previous studies showing Axin2 or Tcf/Lef reporter expression in developing eyelids (Figure 7, E and G).92–95 In Porcnlox/Y;Wnt1-Cre;Rx3-Cre embryos, mesenchymal expression of LEF1 is reduced or absent in both in upper and lower eyelids (Figure 7, F and H). In addition, LEF1 expression is decreased in palpebral epidermal cells of the lower eyelid (Figure 7H). We also examined p-JUN expression in the periderm and its extension, because it is required for eyelid closure.96 p-JUN is detectable in the periderm of both upper and lower eyelids in Porcnlox/Y;Wnt1-Cre;Rx3-Cre eyes (Figure 7, I–L). Overall, these results indicate that LEF1 expression in the eyelid mesenchyme is significantly affected by disruption of Porcn.
During normal morphogenesis of the cornea (approximately E12 in mouse), the periocular mesenchyme responds to cues from the lens and migrates into the space between lens vesicle and surface ectoderm, which matures into the corneal epithelium (reviewed by Cvekl and Tamm91). Migrating mesenchymal cells either undergo mesenchymal-to-epithelial transition and differentiate into corneal endothelium or differentiate into keratocytes, which produce the extracellular matrix of the corneal stroma. In Porcnlox/Y;Wnt1-Cre;Rx3-Cre eyes, the cornea separates from the lens (Figure 7, D and N), suggesting that maturation of the corneal endothelium proceeds normally. However, similar to Porcnhet eyes at E13.5 (Figure 2), we observed a thinning of the cornea in Porcnlox/Y;Wnt1-Cre;Rx3-Cre eyes, caused by a severe decrease in cell number (Figure 7, M and N). The corneal epithelium is correctly specified in Porcnlox/Y;Wnt1-Cre;Rx3-Cre eyes, because PAX6 and cytokeratin-12 are robustly expressed at E15.5 (Figure 7, O–R). This is consistent with previous studies indicating that Wnt–β-catenin activity is not required during differentiation of the corneal epithelium.97,98 In the corneal mesenchyme, expression of LEF1 is markedly down-regulated, indicating reduced Wnt–β-catenin activity (Figure 7, S and T). However, similar to expression at E12.5, the Wnt–β-catenin target PITX2 is not affected by Porcn depletion; in Porcnlox/Y;Wnt1-Cre;Rx3-Cre eyes, PITX2 is expressed in the hypocellular corneal mesenchyme at E15.5 (Figure 7, U and V). This suggests that differentiation of the hypocellular corneal mesenchyme at this age proceeds normally, despite reduced LEF1 expression. Furthermore, Rho GTPases can act as effectors of noncanonical Wnt signaling to regulate migration of the developing neural crest (reviewed by Mayor and Theveneau99). Specifically, the GTPase CDC42 is robustly expressed in the corneal mesenchyme, starting at E11.5, and regulates neural crest migration and/or proliferation during corneal morphogenesis and wound healing.100–103 We observed that CDC42 was strongly expressed throughout the corneal mesenchyme in control eyes at E15.5, but was decreased in the Porcnlox/Y;Wnt1-Cre;Rx3-Cre cornea (Figure 7, W and X). Taken together, our observations suggest that Porcn deficiency results in corneal hypoplasia due to defects in proliferation, survival, and/or migration of the periocular mesenchyme.
Discussion
Our present findings show that Porcn depletion during optic cup morphogenesis leads to closure defects of the optic fissure and eyelids, a hypocellular cornea, and a range of RPE defects. Unless Porcn is completely inactivated in most of the ocular and extraocular tissues, these defects are highly variable and less penetrant. This is reminiscent of ocular abnormalities found in female FDH patients, who exhibit mosaic deletion of PORCN due to stochastic X-chromosome inactivation. Approximately 40% of FDH patients exhibit ophthalmological findings, including microphthalmia, anophthalmia, coloboma (of the eyelid, iris, choroid, retina, and optic nerve), aniridia, and hypopigmentation.1,2,16 Here, we have shown that deletion of Porcn in multiple tissues mimics several aspects of FDH with high incidence, and our analysis provides insight into some of the mechanisms by which Porcn regulates eye development.
Porcn Deficiency Causes RPE Abnormalities Characteristic of Atypical Colobomata and Aniridia
Porcn deficiency leads to loss of pigmentation in the optic cup periphery, and we identified tissue gaps or iris hypoplasia as one possible cause. These gaps could be thought of as atypical iris colobomata, because they arise outside of the inferonasal quadrant harboring the optic fissure. Atypical colobomata can occur independently from a closure defect in the optic fissure and are also associated with anterior segment disorders such as aniridia and Axenfeld–Rieger syndrome (reviewed by Chang et al84). Circumferential pigment defects are accompanied by tissue hypoplasia in Porcnlox/Y;Wnt1-Cre;Rx3-Cre eyes, reminiscent of aniridia. These abnormalities are consistent with a role of Wnt–β-catenin signaling in differentiation of RPE, iris epithelium, and ciliary body in the optic cup.24,27,30 Deletion of β-catenin in the optic cup periphery attenuates growth and patterning of the ciliary margin, accompanied by a shortened optic cup.24,30 Similarly, combined disruption of the secreted Wnt modulators sFRP-1 and sFRP-2 (which are required here for extracellular spreading and availability of Wnt proteins) leads to identical defects.19,78
A considerable number of Porcnlox/Y;Wnt1-Cre;Rx3-Cre eyes exhibit severe transdifferentiation involving major parts of the proximal RPE; to date, this has not been described in humans. Previous studies, including our own, have demonstrated that a complex of β-catenin–TCF/LEF directly transactivates enhancers of the RPE key regulatory genes Mitf and Otx2; thus, deletion of β-catenin in the RPE leads directly to a loss of MITF and OTX2, which are strictly required for RPE development.20,21,25,29 Our present findings provide further evidence that it is the signaling function of β-catenin that is required to promote RPE differentiation. Interestingly, our findings also suggest that Wnt proteins secreted from multiple tissues are required for Wnt–β-catenin activation in the RPE, because Porcn depletion in periocular mesenchyme or optic neuroepithelium or lens alone is not sufficient to cause transdifferentiation of RPE into retina.
We observed that, in rare cases, transdifferentiation involving large regions of the posterior RPE resulted in extreme RPE defects during optic cup formation (Figure 3H), possibly because of an earlier onset of Porcn inactivation. It is conceivable that this might later lead to severe microphthalmia; however, we could not explore this because of perinatal lethality. Furthermore, Porcn depletion before the optic vesicle stage could result in extreme microphthalmia or anophthalmia. Noncanonical Wnt signaling is required before optic vesicle formation in zebrafish and frog, either to suppress canonical Wnt pathways and/or to promote eye-specific gene expression41,43,44 (reviewed by Fuhrmann104), and mutation of PCP effector genes such as Fuz, Int, or Wdpcp results in microphthalmia or anophthalmia in mouse.45–47 Thus, the occurrence of FDH with severe microphthalmia or anophthalmia could be explained by an early role of noncanonical Wnt signaling during eye development.
Porcn Regulates Closure of the Optic Fissure
Similar to FDH in humans, in mouse the loss or reduction of PORCN results in a failure of the optic fissure to close. We found that the neuroepithelium in the ventral optic cup and underlying periocular mesenchyme differentiated properly up to E12.5, because several genes critical for closure of the optic fissure (eg, Vax2, Pax2, Mitf, and Pitx2) were normally expressed. Maintenance of PITX2 expression is transiently dependent on Wnt–β-catenin signaling,89 and so the normal expression pattern of PITX2 in Porcnlox/Y;Wnt1-Cre;Rx3-Cre mesenchyme was unexpected. This suggests that the temporal requirement for Wnt–β-catenin activity may have passed already. Furthermore, neural crest–specific inactivation of β-catenin causes abnormalities in the optic stalk and eye positioning; however, it is not clear whether the optic fissure closes properly.89 Further studies are needed to determine whether the closure defects results from impaired Wnt–β-catenin activity in the mesenchyme.
Disruption of the Wnt receptor Frizzled-5 (encoded by Fz5) during early embryonic development occasionally causes coloboma, and the incidence of coloboma increases when one allele of the closely related Fz8 is additionally removed.77,81 These triallelic Fz5/Fz8 mutants exhibit retinal neurogenesis defects that are attributed to abnormal formation of retinal apical junctions and defective HES1 expression, consistent with a role of Fz5 and Fz8 in neuronal polarity and possibly noncanonical Wnt signaling during neural development.77,105 However, it has not been determined whether apical junction formation is already impaired during closure of the optic fissure in triallelic Fz5/Fz8 mutants.77 Porcnlox/Y;Wnt1-Cre;Rx3-Cre embryos do not exhibit obvious defects in retinal neurogenesis and HES1 expression, and apicobasal polarity appears to be normal in the ventral optic cup. One possibility is that FZ5 and FZ8 are activated by ligands independently of Wnt proteins. On the other hand, there is increasing evidence that interference with noncanonical Wnt signaling can result in ectopic activation of Wnt–β-catenin signaling,52,106–109 and disruption of the canonical Wnt antagonists Dkk-1 and Axin-2 results in coloboma110 (A.A. and S.F., unpublished data). It is possible, therefore, that colobomata in triallelic Fz5/Fz8 mutants result from ectopic activation of Wnt–β-catenin signaling.
Disruption of the Wnt coreceptor LRP6 causes severe coloboma and dorsoventral patterning defects; specifically, the dorsal domain of the optic cup is dramatically reduced, as shown by loss of Tbx5 and expansion of Vax2 expression.82 By contrast, in Porcnlox/Y;Wnt1-Cre;Rx3-Cre embryos, colobomata are less severe, characterized usually by a small gap ventrally, and dorsoventral patterning appears largely normal. Although disruption of Porcn is expected to interfere with all Wnt pathways, this difference could be explained by unexpected, PORCN-independent secretion of some Wnt proteins. Another possibility is that Porcn deletion in our Porcnlox/Y;Wnt1-Cre;Rx3-Cre embryos is not complete. Interestingly, recent studies have identified novel, highly context-dependent functions of LRP6; although it is required to activate Wnt–β-catenin signaling, LRP6 modulates the noncanonical Wnt/PCP pathway during mouse heart morphogenesis and neural tube closure.106,107 These and other studies show that the outcomes of LRP6 and Wnt signaling are highly context-dependent and can involve antagonistic or coregulative action of both Wnt–β-catenin and noncanonical Wnt pathways. Thus, it is possible that Lrp6 mutants may exhibit gain of function of noncanonical Wnt signaling during closure of the optic fissure, and we hypothesize that this could exacerbate defects in optic cup morphogenesis.
Defects in Eyelid Closure and Corneal Morphogenesis on Porcn Disruption
FDH can manifest with coloboma of the eyelids, and our observations in Porcn mutants are consistent with a role of Wnt signaling during eyelid morphogenesis and closure.13 LEF1 expression in the eyelid mesenchyme and eyelid epithelium is decreased, indicating compromised Wnt–β-catenin signaling. Defects can manifest as permanent open eyelids or delayed closure, and disruption of several Wnt–β-catenin and noncanonical pathway genes (eg, Tcf3, Dkk2, Lrp6, Vangl2, Fz3, Fz6, Celsr1, and Ptk7) causes eyelid closure abnormalities.95,98 Surprisingly, the actual role of Wnt signaling in eyelid closure is not well understood. A detailed analysis in mouse using in vivo imaging in combination with genetic and laser ablation revealed that epidermal cells intercalate and generate a force towing the surrounding epidermis over the eye.94 These movements appear similar to convergent extension movements in gastrulation, which are mediated by noncanonical Wnt signaling. It is therefore possible that localized actions of both Wnt–β-catenin and noncanonical Wnt signaling participate in growth and fusion of eyelids.
We found that Porcn deficiency leads to severe hypoplasia of the corneal mesenchyme in Porcnlox/Y;Wnt1-Cre;Rx3-Cre eyes. PITX2 is expressed in the hypocellular cornea, suggesting that the remaining mesenchymal cells may differentiate normally. Other processes of periocular mesenchyme development that could be affected by Porcn deficiency at this stage are survival, proliferation, and migration. Such defects may explain the abnormally reduced primary vitreous body in Porcn-mutant embryos. The Rho GTPase CDC42 can act downstream of noncanonical Wnt signaling and has been implicated in migration and proliferation of cranial mesenchyme during development and wound healing of corneal endothelium.100–103 We observed decreased expression of CDC42 in the hypocellular corneal mesenchyme in Porcnlox/Y;Wnt1-Cre;Rx3-Cre embryos. Further studies are needed to determine whether the decrease in CDC42 expression is a result of abnormal noncanonical Wnt signaling. In addition, the decrease in LEF1 expression suggests that Wnt–β-catenin signaling may be affected, therefore, we cannot exclude that distinct Wnt pathways participate in regulating morphogenesis of the cornea.
In summary, we have presented a mouse model that shows high penetrance of ocular defects reminiscent of those found in FDH. Our results demonstrate that Porcn is required in multiple tissues to regulate distinct processes during embryonic eye development. We also identified a novel role for PORCN/Wnt signaling in corneal morphogenesis. Our results show that Wnt proteins from multiple tissues mediate critical interactions among periocular mesenchyme, surface ectoderm, and optic cup neuroectoderm, consistent with activity of potential Wnt pathway readouts (LEF1, AXIN-2, CDC42). Further studies are needed to determine the actual molecular and cellular mechanisms underlying the ocular defects caused by Porcn deficiency.
Acknowledgments
We thank Nadean Brown for providing antibodies, Ed Levine for critical reading of the manuscript, and members of the Fuhrmann and Levine laboratories for technical support and helpful comments.
Footnotes
Supported by NIH grants EY014954 (S.F.), R01-EY12505 (M.J.), and R21-OD010559 (L.C.M.), NIH Core Grant P30-EY014800 (Department of Ophthalmology & Visual Sciences, University of Utah), and in part by an unrestricted grant from Research to Prevent Blindness, Inc. (New York, NY; Department of Ophthalmology & Visual Sciences, University of Utah).
Disclosures: None declared.
Current address of M.P.C., Department of Human Genetics, University of Utah, Salt Lake City, UT.
Supplemental Data
Cre-mediated recombination in the optic cup at E12.5 using the ROSAR26LacZ reporter line. Expression of β-galactosidase in sagittal sections of the optic cup of Porcnlox/Y;Wnt1-Cre embryos shows expression in the mesenchyme (A, arrow), in retina, RPE, and lens (arrow) of Porcnlox/Y;Rx3-Cre embryos (B), and in ocular and extraocular tissues in Porcnlox/Y;Wnt1-Cre;Rx3-Cre embryos (C). Scale bar = 100 μm.
Normal development of retinal progenitor cells in Porcnlox/Y;Wnt1-Cre;Rx3-Cre eyes at E15.5. A and B: The transcription factor OTX2 is normally expressed in photoreceptor precursors and retinal progenitors as well as in the RPE (A), and this expression pattern is not changed in Porcnlox/Y;Wnt1-Cre;Rx3-Cre eyes (B). C and D: BRN-3 is present in differentiating ganglion cells in wild-type (WT) retina (C), and BRN-3 expression appears normal in Porcnlox/Y;Wnt1-Cre;Rx3-Cre retina (D). E and F: The homeodomain transcription factor VSX2 labels retinal progenitors in a similar pattern in WT (E) and Porcnlox/Y;Wnt1-Cre;Rx3-Cre retina (F). G and H: The basic helix–loop–helix transcription factor HES1 is expressed in undifferentiated retinal progenitor cells (G). H: This expression pattern is not changed in the Porcnlox/Y;Wnt1-Cre;Rx3-Cre retina.
Apoptosis and proliferation are unaffected in Porcnlox/Y;Wnt1-Cre;Rx3-Cre eyes at E11.5. A and B: Cells labeled with phosphorylated histone H3 (PHH3) in the optic cup of sagittal sections of control (A) and Porcnlox/Y;Wnt1-Cre;Rx3-Cre eyes (B; arrows). C: Quantitative analysis of PHH3-labeled cells shows no significant difference between control and Porcnlox/Y;Wnt1-Cre;Rx3-Cre optic cups (P = 0.33; n = 6 eyes from 3 embryos per condition). D and E: Caspase-3–labeled cells in control (D) and Porcnlox/Y;Wnt1-Cre;Rx3-Cre optic cup (E; arrows). F: Quantitative analysis of caspase-3–labeled cells shows no significant difference between control and Porcnlox/Y;Wnt1-Cre;Rx3-Cre eyes (P = 0.25; n = 6 eyes from 3 embryos per condition). Scale bar = 100 μm.
References
- 1.Temple I.K., MacDowall P., Baraitser M., Atherton D.J. Focal dermal hypoplasia (Goltz syndrome) J Med Genet. 1990;27:180–187. doi: 10.1136/jmg.27.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goltz R.W., Peterson W.C., Gorlin R.J., Ravits H.G. Focal dermal hypoplasia. Arch Dermatol. 1962;86:708–717. doi: 10.1001/archderm.1962.01590120006002. [DOI] [PubMed] [Google Scholar]
- 3.Wang X., Sutton V.R., Peraza-Llanes J.O., Yu Z., Rosetta R., Kou Y.C., Eble T.N., Patel A., Thaller C., Fang P., Van den Veyver I.B. Mutations in X-linked PORCN, a putative regulator of Wnt signaling, cause focal dermal hypoplasia. Nat Genet. 2007;39:836–838. doi: 10.1038/ng2057. [DOI] [PubMed] [Google Scholar]
- 4.Grzeschik K.H., Bornholdt D., Oeffner F., König A., del Carmen Boente M., Enders H., Fritz B., Hertl M., Grasshoff U., Höfling K., Oji V., Paradisi M., Schuchardt C., Szalai Z., Tadini G., Traupe H., Happle R. Deficiency of PORCN, a regulator of Wnt signaling, is associated with focal dermal hypoplasia. Nat Genet. 2007;39:833–835. doi: 10.1038/ng2052. [DOI] [PubMed] [Google Scholar]
- 5.Proffitt K.D., Virshup D.M. Precise regulation of porcupine activity is required for physiological Wnt signaling. J Biol Chem. 2012;287:34167–34178. doi: 10.1074/jbc.M112.381970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka K., Okabayashi K., Asashima M., Perrimon N., Kadowaki T. The evolutionarily conserved porcupine gene family is involved in the processing of the Wnt family. Eur J Biochem. 2000;267:4300–4311. doi: 10.1046/j.1432-1033.2000.01478.x. [DOI] [PubMed] [Google Scholar]
- 7.Galli L.M., Barnes T.L., Secrest S.S., Kadowaki T., Burrus L.W. Porcupine-mediated lipid-modification regulates the activity and distribution of Wnt proteins in the chick neural tube. Development. 2007;134:3339–3348. doi: 10.1242/dev.02881. [DOI] [PubMed] [Google Scholar]
- 8.Covey T.M., Kaur S., Tan Ong T., Proffitt K.D., Wu Y., Tan P., Virshup D.M. PORCN moonlights in a Wnt-independent pathway that regulates cancer cell proliferation. PLoS One. 2012;7:e34532. doi: 10.1371/journal.pone.0034532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Najdi R., Proffitt K., Sprowl S., Kaur S., Yu J., Covey T.M., Virshup D.M., Waterman M.L. A uniform human Wnt expression library reveals a shared secretory pathway and unique signaling activities. Differentiation. 2012;84:203–213. doi: 10.1016/j.diff.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clevers H., Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Liu W., Shaver T.M., Balasa A., Ljungberg M.C., Wang X., Wen S., Nguyen H., Van den Veyver I.B. Deletion of Porcn in mice leads to multiple developmental defects and models human focal dermal hypoplasia (Goltz syndrome) PLoS One. 2012;7:e32331. doi: 10.1371/journal.pone.0032331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biechele S., Cox B.J., Rossant J. Porcupine homolog is required for canonical Wnt signaling and gastrulation in mouse embryos. Dev Biol. 2011;355:275–285. doi: 10.1016/j.ydbio.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 13.Barrott J.J., Cash G.M., Smith A.P., Barrow J.R., Murtaugh L.C. Deletion of mouse Porcn blocks Wnt ligand secretion and reveals an ectodermal etiology of human focal dermal hypoplasia/Goltz syndrome. Proc Natl Acad Sci USA. 2011;108:12752–12757. doi: 10.1073/pnas.1006437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bornholdt D., Oeffner F., König A., Happle R., Alanay Y., Ascherman J., Benke P.J., Boente Mdel C., van der Burgt I., Chassaing N., Ellis I., Francisco C.R., Della Giovanna P., Hamel B., Has C., Heinelt K., Janecke A., Kastrup W., Loeys B., Lohrisch I., Marcelis C., Mehraein Y., Nicolas M.E., Pagliarini D., Paradisi M., Patrizi A., Piccione M., Piza-Katzer H., Prager B., Prescott K., Strien J., Utine G.E., Zeller M.S., Grzeschik K.H. PORCN mutations in focal dermal hypoplasia: coping with lethality. Hum Mutat. 2009;30:E618–E628. doi: 10.1002/humu.20992. [Erratum appeared in Hum Mutat 2009, 30:1472–1473] [DOI] [PubMed] [Google Scholar]
- 15.Biechele S., Adissu H.A., Cox B.J., Rossant J. Zygotic Porcn paternal allele deletion in mice to model human focal dermal hypoplasia. PLoS One. 2013;8:e79139. doi: 10.1371/journal.pone.0079139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L., Jin X., Zhao X., Liu D., Hu T., Li W., Jiang L., Dan H., Zeng X., Chen Q. Focal dermal hypoplasia: updates. Oral Dis. 2014;20:17–24. doi: 10.1111/odi.12083. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J., Fuhrmann S., Vetter M.L. A nonautonomous role for retinal Frizzled-5 in regulating hyaloid vitreous vasculature development. Invest Ophthalmol Vis Sci. 2008;49:5561–5567. doi: 10.1167/iovs.08-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burns C.J., Zhang J., Brown E.C., Van Bibber A.M., Van Es J., Clevers H., Ishikawa T.O., Taketo M.M., Vetter M.L., Fuhrmann S. Investigation of Frizzled-5 during embryonic neural development in mouse. Dev Dyn. 2008;237:1614–1626. doi: 10.1002/dvdy.21565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esteve P., Sandonis A., Ibanez C., Shimono A., Guerrero I., Bovolenta P. Secreted frizzled-related proteins are required for Wnt/beta-catenin signalling activation in the vertebrate optic cup. Development. 2011;138:4179–4184. doi: 10.1242/dev.065839. [DOI] [PubMed] [Google Scholar]
- 20.Westenskow P.D., McKean J.B., Kubo F., Nakagawa S., Fuhrmann S. Ectopic Mitf in the embryonic chick retina by co-transfection of beta-catenin and Otx2. Invest Ophthalmol Vis Sci. 2010;51:5328–5335. doi: 10.1167/iovs.09-5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Westenskow P., Piccolo S., Fuhrmann S. Beta-catenin controls differentiation of the retinal pigment epithelium in the mouse optic cup by regulating Mitf and Otx2 expression. Development. 2009;136:2505–2510. doi: 10.1242/dev.032136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agathocleous M., Iordanova I., Willardsen M.I., Xue X.Y., Vetter M.L., Harris W.A., Moore K.B. A directional Wnt/beta-catenin-Sox2-proneural pathway regulates the transition from proliferation to differentiation in the Xenopus retina. Development. 2009;136:3289–3299. doi: 10.1242/dev.040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuhrmann S., Riesenberg A.N., Mathiesen A.M., Brown E.C., Vetter M.L., Brown N.L. Characterization of a transient TCF/LEF-responsive progenitor population in the embryonic mouse retina. Invest Ophthalmol Vis Sci. 2009;50:432–440. doi: 10.1167/iovs.08-2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu X., Sun H., Klein W.H., Mu X. Beta-catenin is essential for lamination but not neurogenesis in mouse retinal development. Dev Biol. 2006;299:424–437. doi: 10.1016/j.ydbio.2006.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujimura N., Taketo M.M., Mori M., Korinek V., Kozmik Z. Spatial and temporal regulation of Wnt/beta-catenin signaling is essential for development of the retinal pigment epithelium. Dev Biol. 2009;334:31–45. doi: 10.1016/j.ydbio.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Cho S.H., Cepko C.L. Wnt2b/beta-catenin-mediated canonical Wnt signaling determines the peripheral fates of the chick eye. Development. 2006;133:3167–3177. doi: 10.1242/dev.02474. [DOI] [PubMed] [Google Scholar]
- 27.Fotaki V., Smith R., Pratt T., Price D.J. Foxg1 is required to limit the formation of ciliary margin tissue and Wnt/beta-catenin signalling in the developing nasal retina of the mouse. Dev Biol. 2013;380:299–313. doi: 10.1016/j.ydbio.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kubo F., Takeichi M., Nakagawa S. Wnt2b controls retinal cell differentiation at the ciliary marginal zone. Development. 2003;130:587–598. doi: 10.1242/dev.00244. [DOI] [PubMed] [Google Scholar]
- 29.Hägglund A.C., Berghard A., Carlsson L. Canonical Wnt/beta-catenin signalling is essential for optic cup formation. PLoS One. 2013;8:e81158. doi: 10.1371/journal.pone.0081158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu H., Xu S., Wang Y., Mazerolle C., Thurig S., Coles B.L., Ren J.C., Taketo M.M., van der Kooy D., Wallace V.A. Ciliary margin transdifferentiation from neural retina is controlled by canonical Wnt signaling. Dev Biol. 2007;308:54–67. doi: 10.1016/j.ydbio.2007.04.052. [DOI] [PubMed] [Google Scholar]
- 31.Ye X., Wang Y., Cahill H., Yu M., Badea T.C., Smallwood P.M., Peachey N.S., Nathans J. Norrin, Frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell. 2009;139:285–298. doi: 10.1016/j.cell.2009.07.047. [Erratum appeared in Cell 2010, 141:191] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kubo F., Takeichi M., Nakagawa S. Wnt2b inhibits differentiation of retinal progenitor cells in the absence of Notch activity by downregulating the expression of proneural genes. Development. 2005;132:2759–2770. doi: 10.1242/dev.01856. [DOI] [PubMed] [Google Scholar]
- 33.Das A.V., Bhattacharya S., Zhao X., Hegde G., Mallya K., Eudy J.D., Ahmad I. The canonical Wnt pathway regulates retinal stem cells/progenitors in concert with Notch signaling. Dev Neurosci. 2008;30:389–409. doi: 10.1159/000178017. [DOI] [PubMed] [Google Scholar]
- 34.Koso H., Ouchi Y., Tabata Y., Aoki Y., Satoh S., Arai K., Watanabe S. SSEA-1 marks regionally restricted immature subpopulations of embryonic retinal progenitor cells that are regulated by the Wnt signaling pathway. Dev Biol. 2006;292:265–276. doi: 10.1016/j.ydbio.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 35.Meyers J.R., Hu L., Moses A., Kaboli K., Papandrea A., Raymond P.A. beta-catenin/Wnt signaling controls progenitor fate in the developing and regenerating zebrafish retina. Neural Dev. 2012;7:30. doi: 10.1186/1749-8104-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bharti K., Gasper M., Ou J., Brucato M., Clore-Gronenborn K., Pickel J., Arnheiter H. A regulatory loop involving PAX6, MITF, and WNT signaling controls retinal pigment epithelium development. PLoS Genet. 2012;8:e1002757. doi: 10.1371/journal.pgen.1002757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinfeld J., Steinfeld I., Coronato N., Hampel M.L., Layer P.G., Araki M., Vogel-Höpker A. RPE specification in the chick is mediated by surface ectoderm-derived BMP and Wnt signalling. Development. 2013;140:4959–4969. doi: 10.1242/dev.096990. [DOI] [PubMed] [Google Scholar]
- 38.Rasmussen J.T., Deardorff M.A., Tan C., Rao M.S., Klein P.S., Vetter M.L. Regulation of eye development by Frizzled signaling in Xenopus. Proc Natl Acad Sci U S A. 2001;98:3861–3866. doi: 10.1073/pnas.071586298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y., Nathans J. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development. 2007;134:647–658. doi: 10.1242/dev.02772. [DOI] [PubMed] [Google Scholar]
- 40.Green J.L., Kuntz S.G., Sternberg P.W. Ror receptor tyrosine kinases: orphans no more. Trends Cell Biol. 2008;18:536–544. doi: 10.1016/j.tcb.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cavodeassi F., Carreira-Barbosa F., Young R.M., Concha M.L., Allende M.L., Houart C., Tada M., Wilson S.W. Early stages of zebrafish eye formation require the coordinated activity of Wnt11, Fz5, and the Wnt/beta-catenin pathway. Neuron. 2005;47:43–56. doi: 10.1016/j.neuron.2005.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heisenberg C.P., Tada M., Rauch G.J., Saúde L., Concha M.L., Geisler R., Stemple D.L., Smith J.C., Wilson S.W. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- 43.Kibardin A., Ossipova O., Sokol S.Y. Metastasis-associated kinase modulates Wnt signaling to regulate brain patterning and morphogenesis. Development. 2006;133:2845–2854. doi: 10.1242/dev.02445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maurus D., Héligon C., Bürger-Schwärzler A., Brändli A.W., Kühl M. Noncanonical Wnt-4 signaling and EAF2 are required for eye development in Xenopus laevis. EMBO J. 2005;24:1181–1191. doi: 10.1038/sj.emboj.7600603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gray R.S., Abitua P.B., Wlodarczyk B.J., Szabo-Rogers H.L., Blanchard O., Lee I., Weiss G.S., Liu K.J., Marcotte E.M., Wallingford J.B., Finnell R.H. The planar cell polarity effector Fuz is essential for targeted membrane trafficking, ciliogenesis and mouse embryonic development. Nat Cell Biol. 2009;11:1225–1232. doi: 10.1038/ncb1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeng H., Hoover A.N., Liu A. PCP effector gene Inturned is an important regulator of cilia formation and embryonic development in mammals. Dev Biol. 2010;339:418–428. doi: 10.1016/j.ydbio.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Cui C., Chatterjee B., Lozito T.P., Zhang Z., Francis R.J., Yagi H., Swanhart L.M., Sanker S., Francis D., Yu Q., San Agustin J.T., Puligilla C., Chatterjee T., Tansey T., Liu X., Kelley M.W., Spiliotis E.T., Kwiatkowski A.V., Tuan R., Pazour G.J., Hukriede N.A., Lo C.W. Wdpcp, a PCP protein required for ciliogenesis, regulates directional cell migration and cell polarity by direct modulation of the actin cytoskeleton. PLoS Biol. 2013;11:e1001720. doi: 10.1371/journal.pbio.1001720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Summerhurst K., Stark M., Sharpe J., Davidson D., Murphy P. 3D representation of Wnt and Frizzled gene expression patterns in the mouse embryo at embryonic day 11.5 (Ts19) Gene Expr Patterns. 2008;8:331–348. doi: 10.1016/j.gep.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu H., Mohamed O., Dufort D., Wallace V.A. Characterization of Wnt signaling components and activation of the Wnt canonical pathway in the murine retina. Dev Dyn. 2003;227:323–334. doi: 10.1002/dvdy.10315. [DOI] [PubMed] [Google Scholar]
- 50.Grocott T., Johnson S., Bailey A.P., Streit A. Neural crest cells organize the eye via TGF-beta and canonical Wnt signalling. Nat Commun. 2011;2:265. doi: 10.1038/ncomms1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Teraoka M.E., Paschaki M., Muta Y., Ladher R.K. Rostral paraxial mesoderm regulates refinement of the eye field through the bone morphogenetic protein (BMP) pathway. Dev Biol. 2009;330:389–398. doi: 10.1016/j.ydbio.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 52.Kestler H.A., Kühl M. From individual Wnt pathways towards a Wnt signalling network. Philos Trans R Soc Lond B Biol Sci. 2008;363:1333–1347. doi: 10.1098/rstb.2007.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Furuta Y., Lagutin O., Hogan B.L., Oliver G.C. Retina- and ventral forebrain-specific Cre recombinase activity in transgenic mice. Genesis. 2000;26:130–132. [PubMed] [Google Scholar]
- 54.Danielian P.S., Muccino D., Rowitch D.H., Michael S.K., McMahon A.P. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- 55.Swindell E.C., Bailey T.J., Loosli F., Liu C., Amaya-Manzanares F., Mahon K.A., Wittbrodt J., Jamrich M. Rx-Cre, a tool for inactivation of gene expression in the developing retina. Genesis. 2006;44:361–363. doi: 10.1002/dvg.20225. [DOI] [PubMed] [Google Scholar]
- 56.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 57.Lustig B., Jerchow B., Sachs M., Weiler S., Pietsch T., Karsten U., van de Wetering M., Clevers H., Schlag P.M., Birchmeier W., Behrens J. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Furuta Y., Behringer R.R. Recent innovations in tissue-specific gene modifications in the mouse. Birth Defects Res C Embryo Today. 2005;75:43–57. doi: 10.1002/bdrc.20036. [DOI] [PubMed] [Google Scholar]
- 59.Bumsted K.M., Barnstable C.J. Dorsal retinal pigment epithelium differentiates as neural retina in the microphthalmia (mi/mi) mouse. Invest Ophthalmol Vis Sci. 2000;41:903–908. [PubMed] [Google Scholar]
- 60.Nguyen M., Arnheiter H. Signaling and transcriptional regulation in early mammalian eye development: a link between FGF and MITF. Development. 2000;127:3581–3591. doi: 10.1242/dev.127.16.3581. [DOI] [PubMed] [Google Scholar]
- 61.Martinez-Morales J.R., Signore M., Acampora D., Simeone A., Bovolenta P. Otx genes are required for tissue specification in the developing eye. Development. 2001;128:2019–2030. doi: 10.1242/dev.128.11.2019. [DOI] [PubMed] [Google Scholar]
- 62.Fuhrmann S., Zou C., Levine E.M. Retinal pigment epithelium development, plasticity, and tissue homeostasis. Exp Eye Res. 2014;123:141–150. doi: 10.1016/j.exer.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nishida A., Furukawa A., Koike C., Tano Y., Aizawa S., Matsuo I., Furukawa T. Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nat Neurosci. 2003;6:1255–1263. doi: 10.1038/nn1155. [DOI] [PubMed] [Google Scholar]
- 64.Liu H., Thurig S., Mohamed O., Dufort D., Wallace V.A. Mapping canonical Wnt signaling in the developing and adult retina. Invest Ophthalmol Vis Sci. 2006;47:5088–5097. doi: 10.1167/iovs.06-0403. [DOI] [PubMed] [Google Scholar]
- 65.Evans A.L., Gage P.J. Expression of the homeobox gene Pitx2 in neural crest is required for optic stalk and ocular anterior segment development. Hum Mol Genet. 2005;14:3347–3359. doi: 10.1093/hmg/ddi365. [DOI] [PubMed] [Google Scholar]
- 66.Fuhrmann S., Levine E.M., Reh T.A. Extraocular mesenchyme patterns the optic vesicle during early eye development in the embryonic chick. Development. 2000;127:4599–4609. doi: 10.1242/dev.127.21.4599. [DOI] [PubMed] [Google Scholar]
- 67.Lupo G., Gestri G., O'Brien M., Denton R.M., Chandraratna R.A., Ley S.V., Harris W.A., Wilson S.W. Retinoic acid receptor signaling regulates choroid fissure closure through independent mechanisms in the ventral optic cup and periocular mesenchyme. Proc Natl Acad Sci U S A. 2011;108:8698–8703. doi: 10.1073/pnas.1103802108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fu J., Ivy Yu H.M., Maruyama T., Mirando A.J., Hsu W. Gpr177/mouse Wntless is essential for Wnt-mediated craniofacial and brain development. Dev Dyn. 2011;240:365–371. doi: 10.1002/dvdy.22541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ikeya M., Lee S.M., Johnson J.E., McMahon A.P., Takada S. Wnt signalling required for expansion of neural crest and CNS progenitors. Nature. 1997;389:966–970. doi: 10.1038/40146. [DOI] [PubMed] [Google Scholar]
- 70.Brault V., Moore R., Kutsch S., Ishibashi M., Rowitch D.H., McMahon A.P., Sommer L., Boussadia O., Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- 71.Yamaguchi T.P., Bradley A., McMahon A.P., Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- 72.Carpenter A.C., Rao S., Wells J.M., Campbell K., Lang R.A. Generation of mice with a conditional null allele for Wntless. Genesis. 2010;48:554–558. doi: 10.1002/dvg.20651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fu J., Jiang M., Mirando A.J., Yu H.M., Hsu W. Reciprocal regulation of Wnt and Gpr177/mouse Wntless is required for embryonic axis formation. Proc Natl Acad Sci U S A. 2009;106:18598–18603. doi: 10.1073/pnas.0904894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu J., Chia J., Canning C.A., Jones C.M., Bard F.A., Virshup D.M. WLS retrograde transport to the endoplasmic reticulum during Wnt secretion. Dev Cell. 2014;29:277–291. doi: 10.1016/j.devcel.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 75.Chiquet B.T., Blanton S.H., Burt A., Ma D., Stal S., Mulliken J.B., Hecht J.T. Variation in WNT genes is associated with non-syndromic cleft lip with or without cleft palate. Hum Mol Genet. 2008;17:2212–2218. doi: 10.1093/hmg/ddn121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.He F., Chen Y. Wnt signaling in lip and palate development. Front Oral Biol. 2012;16:81–90. doi: 10.1159/000337619. [DOI] [PubMed] [Google Scholar]
- 77.Liu C., Bakeri H., Li T., Swaroop A. Regulation of retinal progenitor expansion by Frizzled receptors: implications for microphthalmia and retinal coloboma. Hum Mol Genet. 2012;21:1848–1860. doi: 10.1093/hmg/ddr616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Esteve P., Sandonis A., Cardozo M., Malapeira J., Ibañez C., Crespo I., Marcos S., Gonzalez-Garcia S., Toribio M.L., Arribas J., Shimono A., Guerrero I., Bovolenta P. SFRPs act as negative modulators of ADAM10 to regulate retinal neurogenesis. Nat Neurosci. 2011;14:562–569. doi: 10.1038/nn.2794. [DOI] [PubMed] [Google Scholar]
- 79.Geeraets R. An electron microscopic study of the closure of the optic fissure in the golden hamster. Am J Anat. 1976;145:411–431. doi: 10.1002/aja.1001450402. [DOI] [PubMed] [Google Scholar]
- 80.Hero I. Optic fissure closure in the normal cinnamon mouse. An ultrastructural study. Invest Ophthalmol Vis Sci. 1990;31:197–216. [PubMed] [Google Scholar]
- 81.Liu C., Nathans J. An essential role for Frizzled 5 in mammalian ocular development. Development. 2008;135:3567–3576. doi: 10.1242/dev.028076. [DOI] [PubMed] [Google Scholar]
- 82.Zhou C.J., Molotkov A., Song L., Li Y., Pleasure D.E., Pleasure S.J., Wang Y.Z. Ocular coloboma and dorsoventral neuroretinal patterning defects in Lrp6 mutant eyes. Dev Dyn. 2008;237:3681–3689. doi: 10.1002/dvdy.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gregory-Evans C.Y., Williams M.J., Halford S., Gregory-Evans K. Ocular coloboma: a reassessment in the age of molecular neuroscience. J Med Genet. 2004;41:881–891. doi: 10.1136/jmg.2004.025494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chang L., Blain D., Bertuzzi S., Brooks B.P. Uveal coloboma: clinical and basic science update. Curr Opin Ophthalmol. 2006;17:447–470. doi: 10.1097/01.icu.0000243020.82380.f6. [DOI] [PubMed] [Google Scholar]
- 85.Lee C.S., May N.R., Fan C.M. Transdifferentiation of the ventral retinal pigmented epithelium to neural retina in the growth arrest specific gene 1 mutant. Dev Biol. 2001;236:17–29. doi: 10.1006/dbio.2001.0280. [DOI] [PubMed] [Google Scholar]
- 86.Tang K., Xie X., Park J.I., Jamrich M., Tsai S., Tsai M.J. COUP-TFs regulate eye development by controlling factors essential for optic vesicle morphogenesis. Development. 2010;137:725–734. doi: 10.1242/dev.040568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee J., Lee B.K., Gross J.M. Bcl6a function is required during optic cup formation to prevent p53-dependent apoptosis and colobomata. Hum Mol Genet. 2013;22:3568–3582. doi: 10.1093/hmg/ddt211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Laemle L.K., Puszkarczuk M., Feinberg R.N. Apoptosis in early ocular morphogenesis in the mouse. Brain Res Dev Brain Res. 1999;112:129–133. doi: 10.1016/s0165-3806(98)00153-9. [DOI] [PubMed] [Google Scholar]
- 89.Zacharias A.L., Gage P.J. Canonical Wnt/beta-catenin signaling is required for maintenance but not activation of Pitx2 expression in neural crest during eye development. Dev Dyn. 2010;239:3215–3225. doi: 10.1002/dvdy.22459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gage P.J., Suh H., Camper S.A. Dosage requirement of Pitx2 for development of multiple organs. Development. 1999;126:4643–4651. doi: 10.1242/dev.126.20.4643. [DOI] [PubMed] [Google Scholar]
- 91.Cvekl A., Tamm E.R. Anterior eye development and ocular mesenchyme: new insights from mouse models and human diseases. Bioessays. 2004;26:374–386. doi: 10.1002/bies.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kuracha M.R., Siefker E., Licht J.D., Govindarajan V. Spry1 and Spry2 are necessary for eyelid closure. Dev Biol. 2013;383:227–238. doi: 10.1016/j.ydbio.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang J., Dattilo L.K., Rajagopal R., Liu Y., Kaartinen V., Mishina Y., Deng C.X., Umans L., Zwijsen A., Roberts A.B., Beebe D.C. FGF-regulated BMP signaling is required for eyelid closure and to specify conjunctival epithelial cell fate. Development. 2009;136:1741–1750. doi: 10.1242/dev.034082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Heller E., Kumar K.V., Grill S.W., Fuchs E. Forces generated by cell intercalation tow epidermal sheets in mammalian tissue morphogenesis. Dev Cell. 2014;28:617–632. doi: 10.1016/j.devcel.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu C.I., Hoffman J.A., Shy B.R., Ford E.M., Fuchs E., Nguyen H., Merrill B.J. Function of Wnt/beta-catenin in counteracting Tcf3 repression through the Tcf3-beta-catenin interaction. Development. 2012;139:2118–2129. doi: 10.1242/dev.076067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li G., Gustafson-Brown C., Hanks S.K., Nason K., Arbeit J.M., Pogliano K., Wisdom R.M., Johnson R.S. c-Jun is essential for organization of the epidermal leading edge. Dev Cell. 2003;4:865–877. doi: 10.1016/s1534-5807(03)00159-x. [DOI] [PubMed] [Google Scholar]
- 97.Mukhopadhyay M., Gorivodsky M., Shtrom S., Grinberg A., Niehrs C., Morasso M.I., Westphal H. Dkk2 plays an essential role in the corneal fate of the ocular surface epithelium. Development. 2006;133:2149–2154. doi: 10.1242/dev.02381. [Erratum appeared in Development 2006, 133:2447 and Development 2006, 133:2595] [DOI] [PubMed] [Google Scholar]
- 98.Gage P.J., Qian M., Wu D., Rosenberg K.I. The canonical Wnt signaling antagonist DKK2 is an essential effector of PITX2 function during normal eye development. Dev Biol. 2008;317:310–324. doi: 10.1016/j.ydbio.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mayor R., Theveneau E. The role of the non-canonical Wnt-planar cell polarity pathway in neural crest migration. Biochem J. 2014;457:19–26. doi: 10.1042/BJ20131182. [DOI] [PubMed] [Google Scholar]
- 100.Fuchs S., Herzog D., Sumara G., Büchmann-Møller S., Civenni G., Wu X., Chrostek-Grashoff A., Suter U., Ricci R., Relvas J.B., Brakebusch C., Sommer L. Stage-specific control of neural crest stem cell proliferation by the small Rho GTPases Cdc42 and Rac1. Cell Stem Cell. 2009;4:236–247. doi: 10.1016/j.stem.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 101.Liu Y., Jin Y., Li J., Seto E., Kuo E., Yu W., Schwartz R.J., Blazo M., Zhang S.L., Peng X. Inactivation of Cdc42 in neural crest cells causes craniofacial and cardiovascular morphogenesis defects. Dev Biol. 2013;383:239–252. doi: 10.1016/j.ydbio.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 102.Takatsuka A., Yagi R., Koike M., Oneyama C., Nada S., Schmedt C., Uchiyama Y., Okada M. Ablation of Csk in neural crest lineages causes corneal anomaly by deregulating collagen fibril organization and cell motility. Dev Biol. 2008;315:474–488. doi: 10.1016/j.ydbio.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 103.Mitchell D.C., Bryan B.A., Liu J.P., Liu W.B., Zhang L., Qu J., Zhou X., Liu M., Li D.W. Developmental expression of three small GTPases in the mouse eye. Mol Vis. 2007;13:1144–1153. [PMC free article] [PubMed] [Google Scholar]
- 104.Fuhrmann S. Wnt signaling in eye organogenesis. Organogenesis. 2008;4:60–67. doi: 10.4161/org.4.2.5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Slater P.G., Ramirez V.T., Gonzalez-Billault C., Varela-Nallar L., Inestrosa N.C. Frizzled-5 receptor is involved in neuronal polarity and morphogenesis of hippocampal neurons. PLoS One. 2013;8:e78892. doi: 10.1371/journal.pone.0078892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Allache R., Lachance S., Guyot M.C., De Marco P., Merello E., Justice M.J., Capra V., Kibar Z. Novel mutations in Lrp6 orthologs in mouse and human neural tube defects affect a highly dosage-sensitive Wnt non-canonical planar cell polarity pathway. Hum Mol Genet. 2014;23:1687–1699. doi: 10.1093/hmg/ddt558. [Erratum appeared in Hum Mol Genet 2014, 23:4185] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bryja V., Andersson E.R., Schambony A., Esner M., Bryjová L., Biris K.K., Hall A.C., Kraft B., Cajanek L., Yamaguchi T.P., Buckingham M., Arenas E. The extracellular domain of Lrp5/6 inhibits noncanonical Wnt signaling in vivo. Mol Biol Cell. 2009;20:924–936. doi: 10.1091/mbc.E08-07-0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gray J.D., Kholmanskikh S., Castaldo B.S., Hansler A., Chung H., Klotz B., Singh S., Brown A.M., Ross M.E. LRP6 exerts non-canonical effects on Wnt signaling during neural tube closure. Hum Mol Genet. 2013;22:4267–4281. doi: 10.1093/hmg/ddt277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zilber Y., Babayeva S., Seo J.H., Liu J.J., Mootin S., Torban E. The PCP effector Fuzzy controls cilial assembly and signaling by recruiting Rab8 and Dishevelled to the primary cilium. Mol Biol Cell. 2013;24:555–565. doi: 10.1091/mbc.E12-06-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lieven O., Rüther U. The Dkk1 dose is critical for eye development. Dev Biol. 2011;355:124–137. doi: 10.1016/j.ydbio.2011.04.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cre-mediated recombination in the optic cup at E12.5 using the ROSAR26LacZ reporter line. Expression of β-galactosidase in sagittal sections of the optic cup of Porcnlox/Y;Wnt1-Cre embryos shows expression in the mesenchyme (A, arrow), in retina, RPE, and lens (arrow) of Porcnlox/Y;Rx3-Cre embryos (B), and in ocular and extraocular tissues in Porcnlox/Y;Wnt1-Cre;Rx3-Cre embryos (C). Scale bar = 100 μm.
Normal development of retinal progenitor cells in Porcnlox/Y;Wnt1-Cre;Rx3-Cre eyes at E15.5. A and B: The transcription factor OTX2 is normally expressed in photoreceptor precursors and retinal progenitors as well as in the RPE (A), and this expression pattern is not changed in Porcnlox/Y;Wnt1-Cre;Rx3-Cre eyes (B). C and D: BRN-3 is present in differentiating ganglion cells in wild-type (WT) retina (C), and BRN-3 expression appears normal in Porcnlox/Y;Wnt1-Cre;Rx3-Cre retina (D). E and F: The homeodomain transcription factor VSX2 labels retinal progenitors in a similar pattern in WT (E) and Porcnlox/Y;Wnt1-Cre;Rx3-Cre retina (F). G and H: The basic helix–loop–helix transcription factor HES1 is expressed in undifferentiated retinal progenitor cells (G). H: This expression pattern is not changed in the Porcnlox/Y;Wnt1-Cre;Rx3-Cre retina.
Apoptosis and proliferation are unaffected in Porcnlox/Y;Wnt1-Cre;Rx3-Cre eyes at E11.5. A and B: Cells labeled with phosphorylated histone H3 (PHH3) in the optic cup of sagittal sections of control (A) and Porcnlox/Y;Wnt1-Cre;Rx3-Cre eyes (B; arrows). C: Quantitative analysis of PHH3-labeled cells shows no significant difference between control and Porcnlox/Y;Wnt1-Cre;Rx3-Cre optic cups (P = 0.33; n = 6 eyes from 3 embryos per condition). D and E: Caspase-3–labeled cells in control (D) and Porcnlox/Y;Wnt1-Cre;Rx3-Cre optic cup (E; arrows). F: Quantitative analysis of caspase-3–labeled cells shows no significant difference between control and Porcnlox/Y;Wnt1-Cre;Rx3-Cre eyes (P = 0.25; n = 6 eyes from 3 embryos per condition). Scale bar = 100 μm.